Kelvin–Voigt Parameters Reconstruction of Cervical Tissue-Mimicking Phantoms Using Torsional Wave Elastography
Abstract
1. Introduction
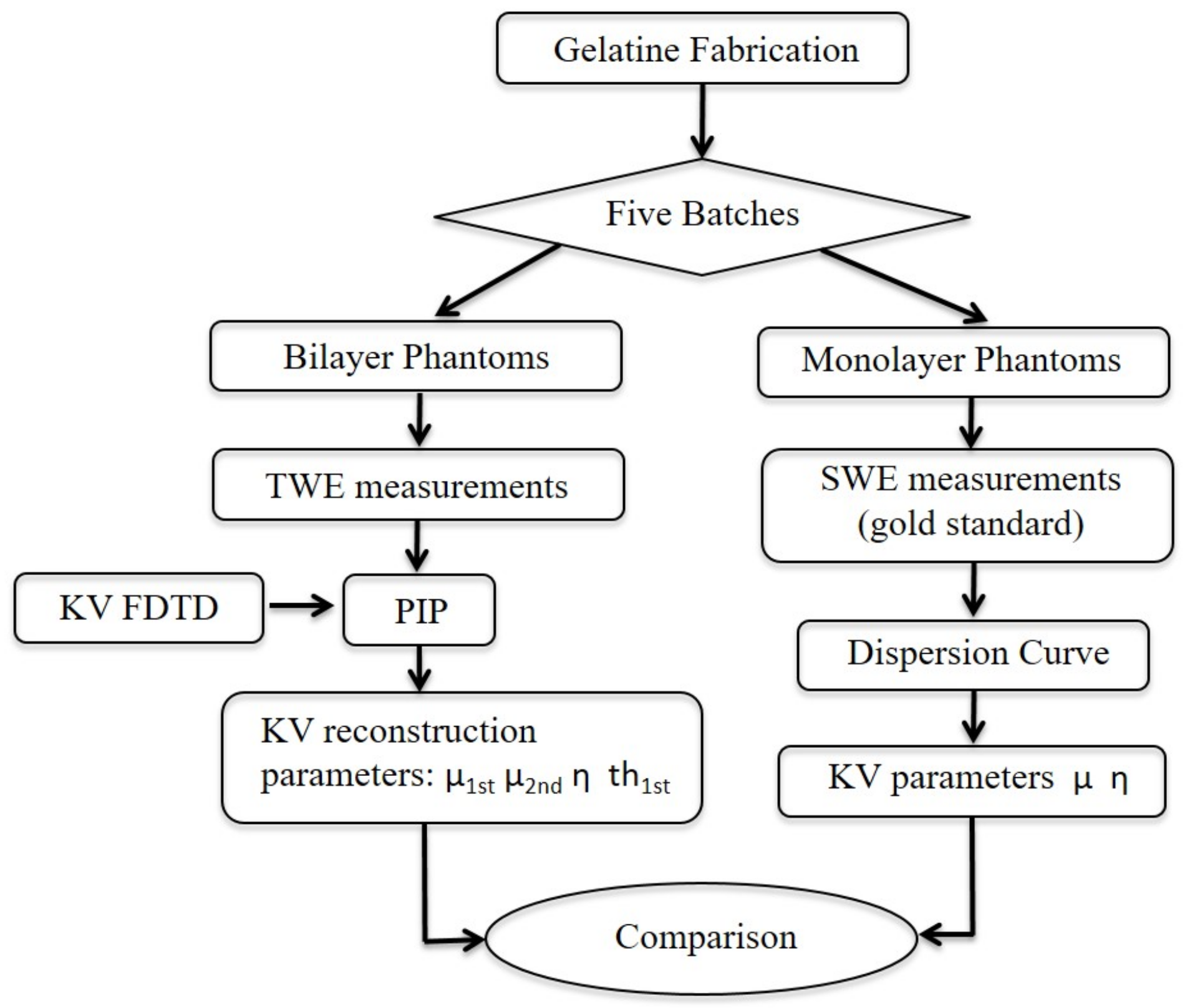
2. Materials and Methods
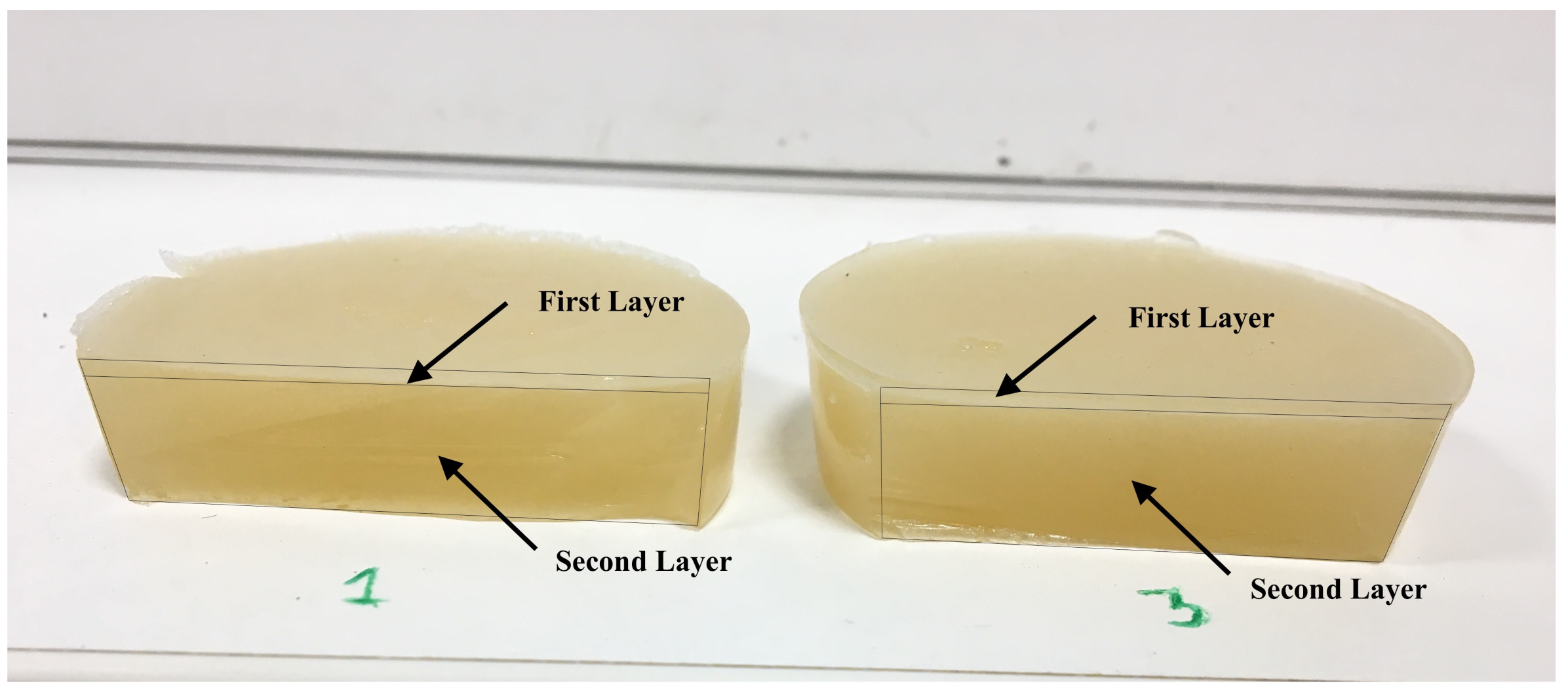
2.1. Tissue-Mimicking Phantom Fabrication
- Weigh and prepare each of the components listed in Table 1.
- Add the K-sorbate to the distilled water and begin mixing for five min.
- Add the surfactant and keep mixing for another 5 min.
- Add the oil with the previous solution and mix manually at a rate that minimized the formation of air bubbles and the formation of large clumps.
- Heat the combined solution at a rate of approximately 1 °C per minute.
- Gradually add the gelatine powder to the combined solution.
- Allow 5–10 min to verify that the gelatine is well mixed.
- Cover and heat the solution to 85 °C at a rate of approximately 1 °C per minute.
- Hold the solution between 85 °C and 90 °C for 90 min.
- Cool the mixture from 85 °C to 40 °C at a rate of 1 °C per minute.
- Add the formalin and mix the solution for 5 min.
- Pour the mixture into the round molds (5 cm in diameter) until the thickness of the connective layer is reached. A thickness of 15 mm has been considered to avoid interference in the measurements due to reflections with the bottom of the gelatin.
- Wait until the mixture reaches 37 °C–38 °C.
- While the first batch is getting cold, repeat steps 1 to 11 with the ingredients needed to fabricate the first layer.
- When both batches reach the temperature of 37 °C–38 °C, carefully pour the second batch (first layer) on the first batch using a syringe. According to the dimensions of the mold, the volume of gelatine necessary to reach the required thickness is calculated.
- Leave the phantom to solidify at room temperature for 2 h before being stored in the refrigerator.
- Remove the phantom from the refrigerator and left at room temperature for 6 h.
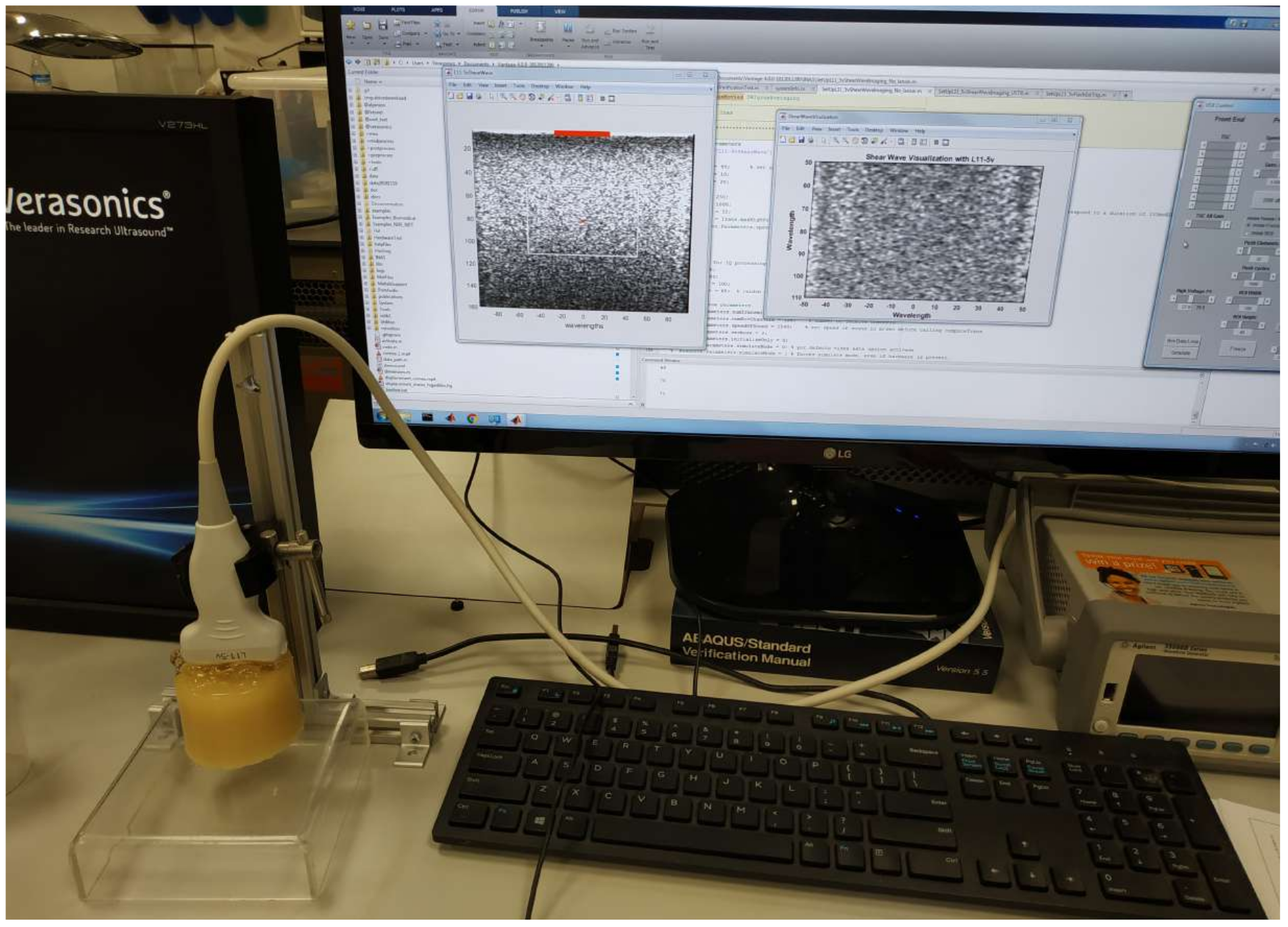
2.2. SWE Characterization of the Phantoms
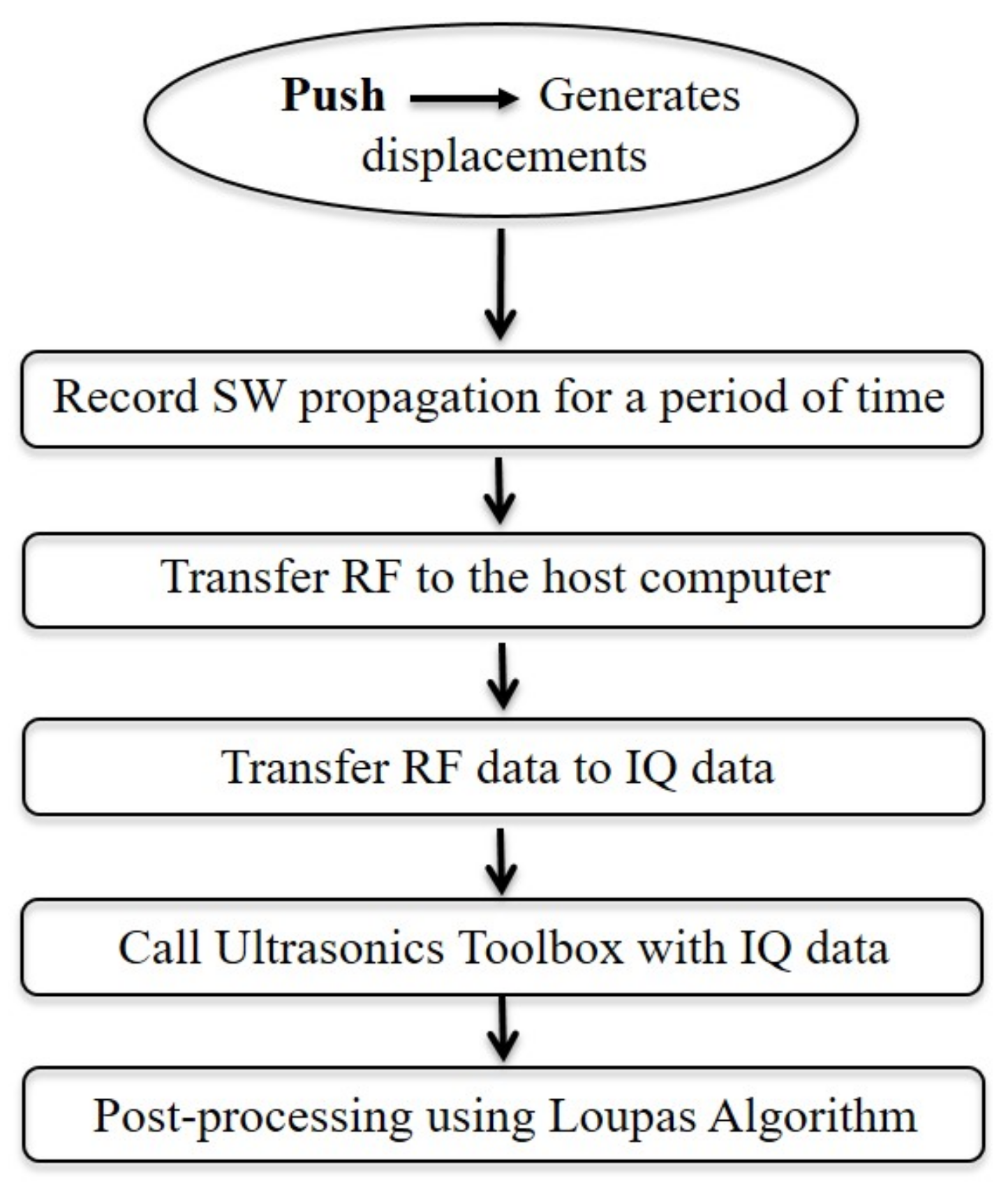
2.2.1. Tissue Motion Estimation
- Generate the push sequence (Create displacements).
- Transmit shear waves according to the push sequence.
- Record shear wave propagation during a period of time.
- Transfer the recorded RF to the host computer, then transform RF data to IQ data.
- Call the Ultrasound Toolbox (USTB) with the IQ data and post-process using Loupas 2D autocorrelator.
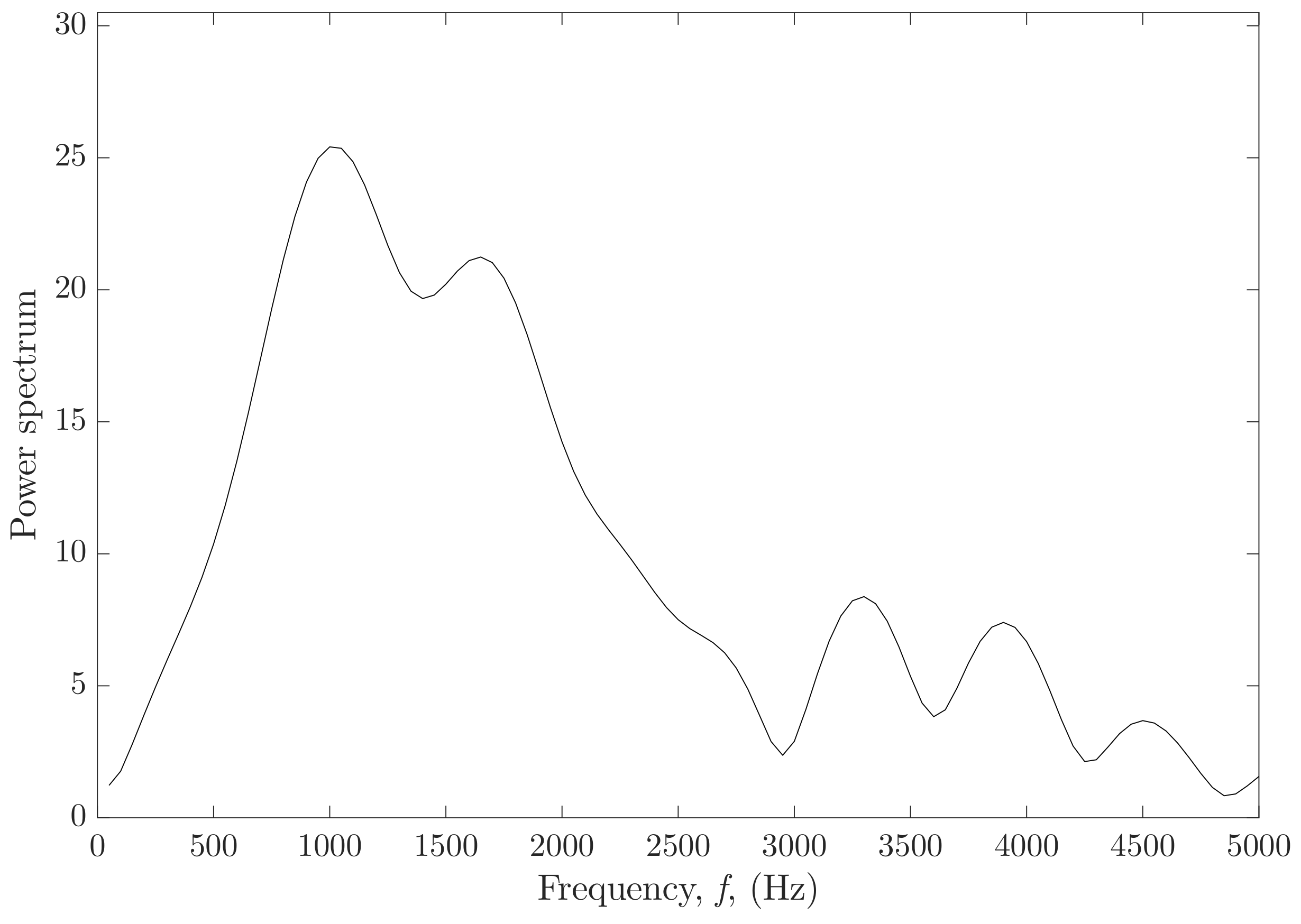
2.2.2. Dispersion Shear Wave Speed Curve
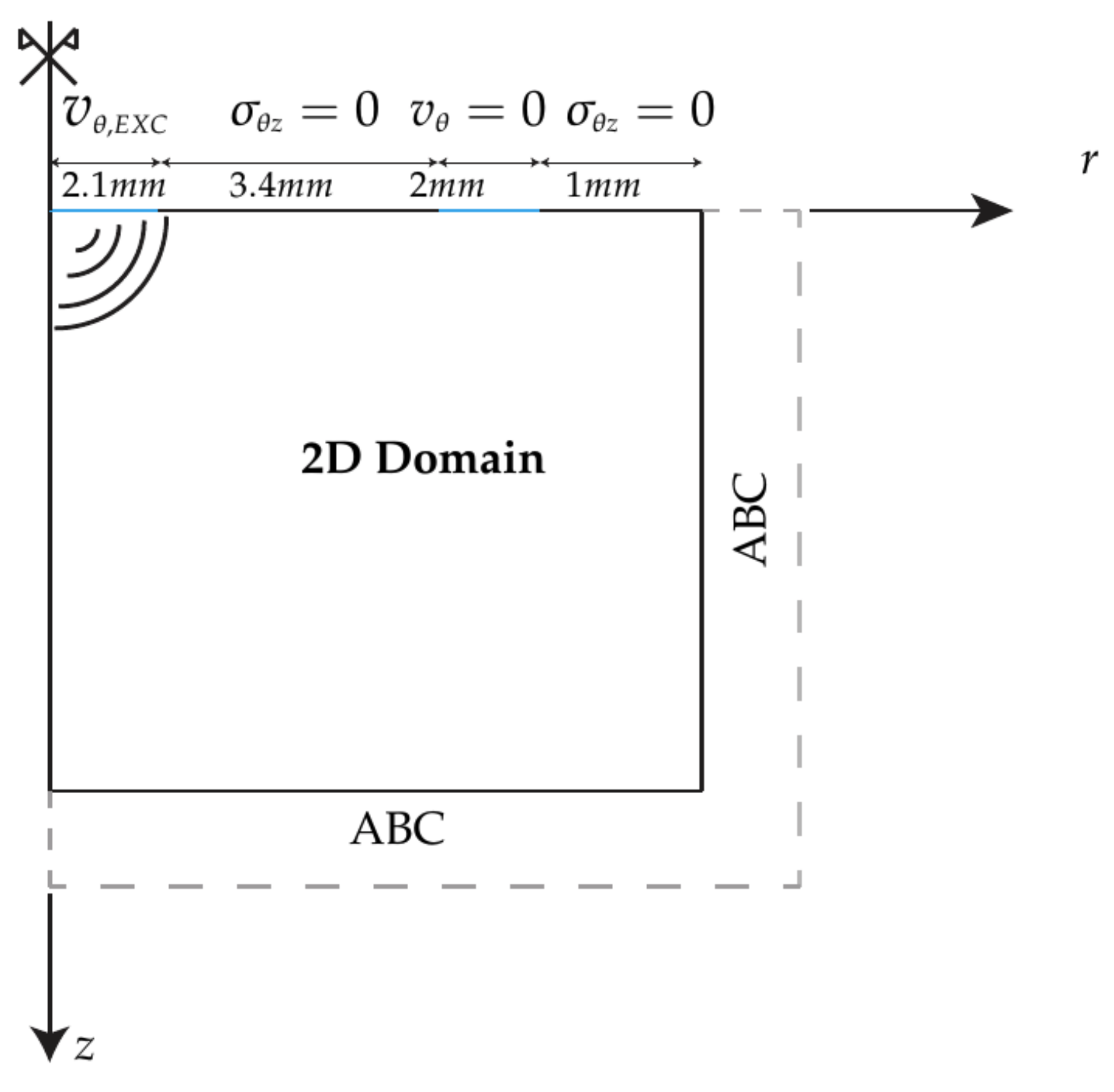
2.3. TWE Characterization of the Phantoms
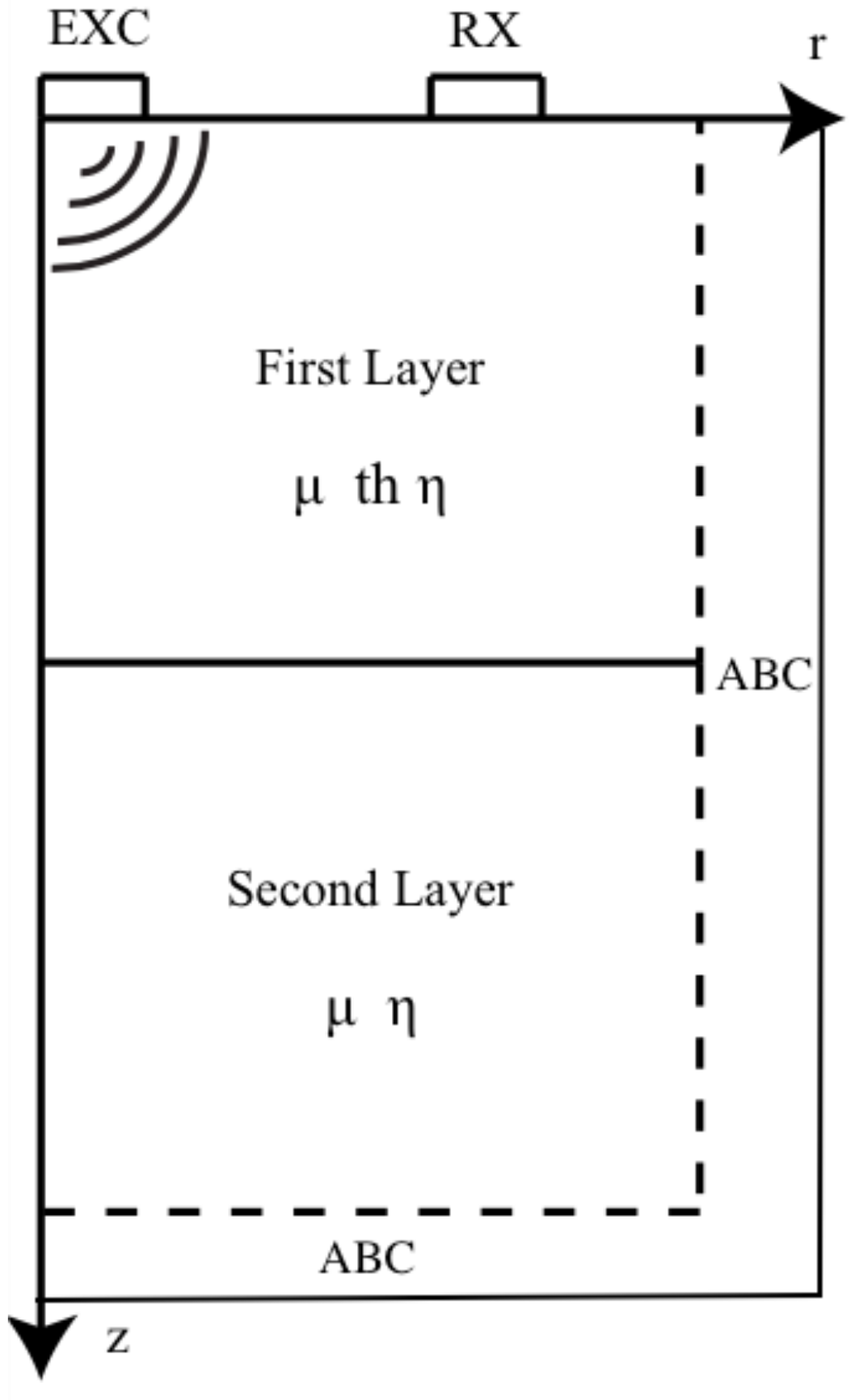
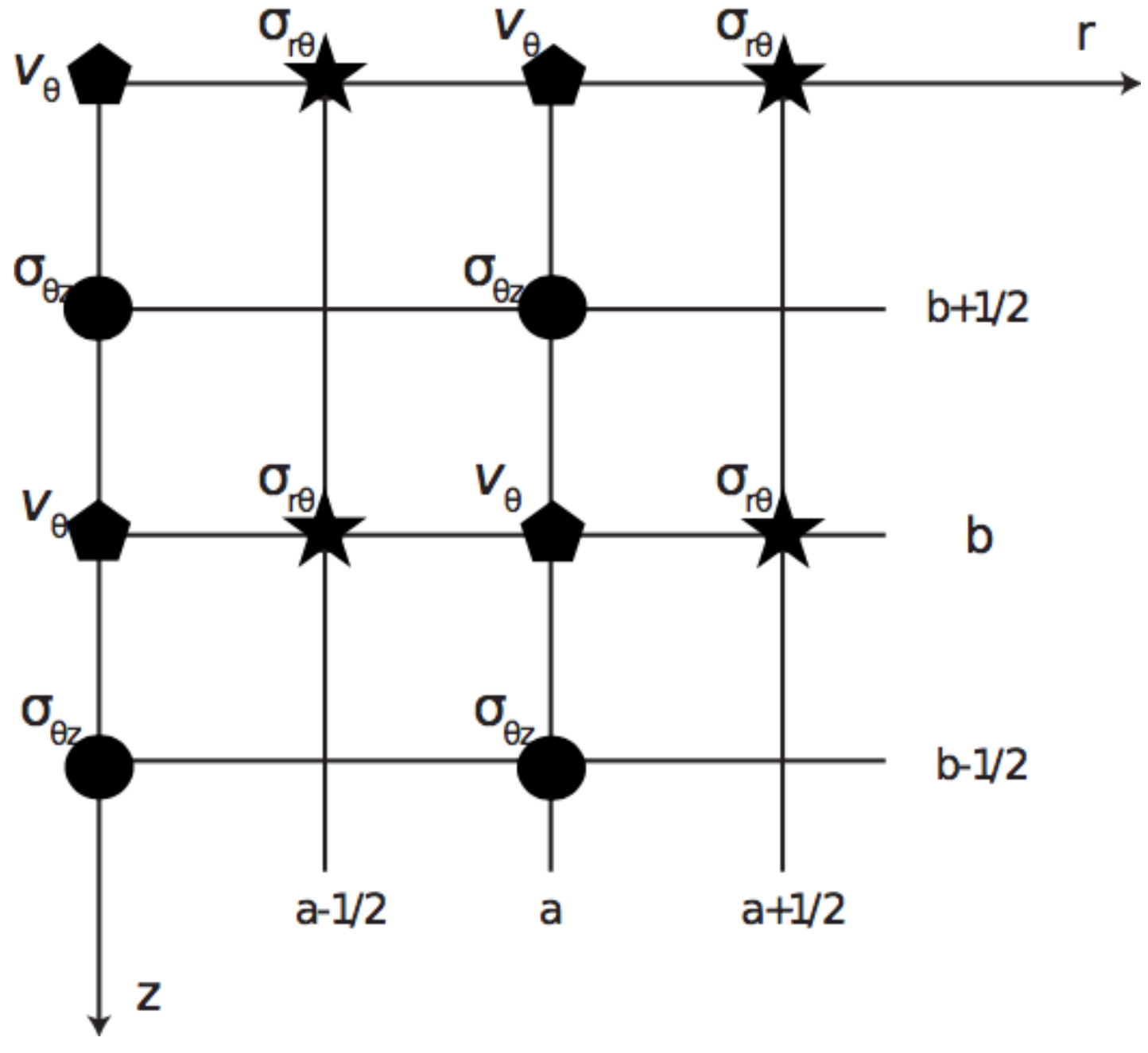
2.4. Kelvin-Voigt FDTD Propagation Model
2.5. Viscoelastic Parameters Reconstruction from SWE Measurements
2.6. Probabilistic Inverse Problem (PIP)
3. Results
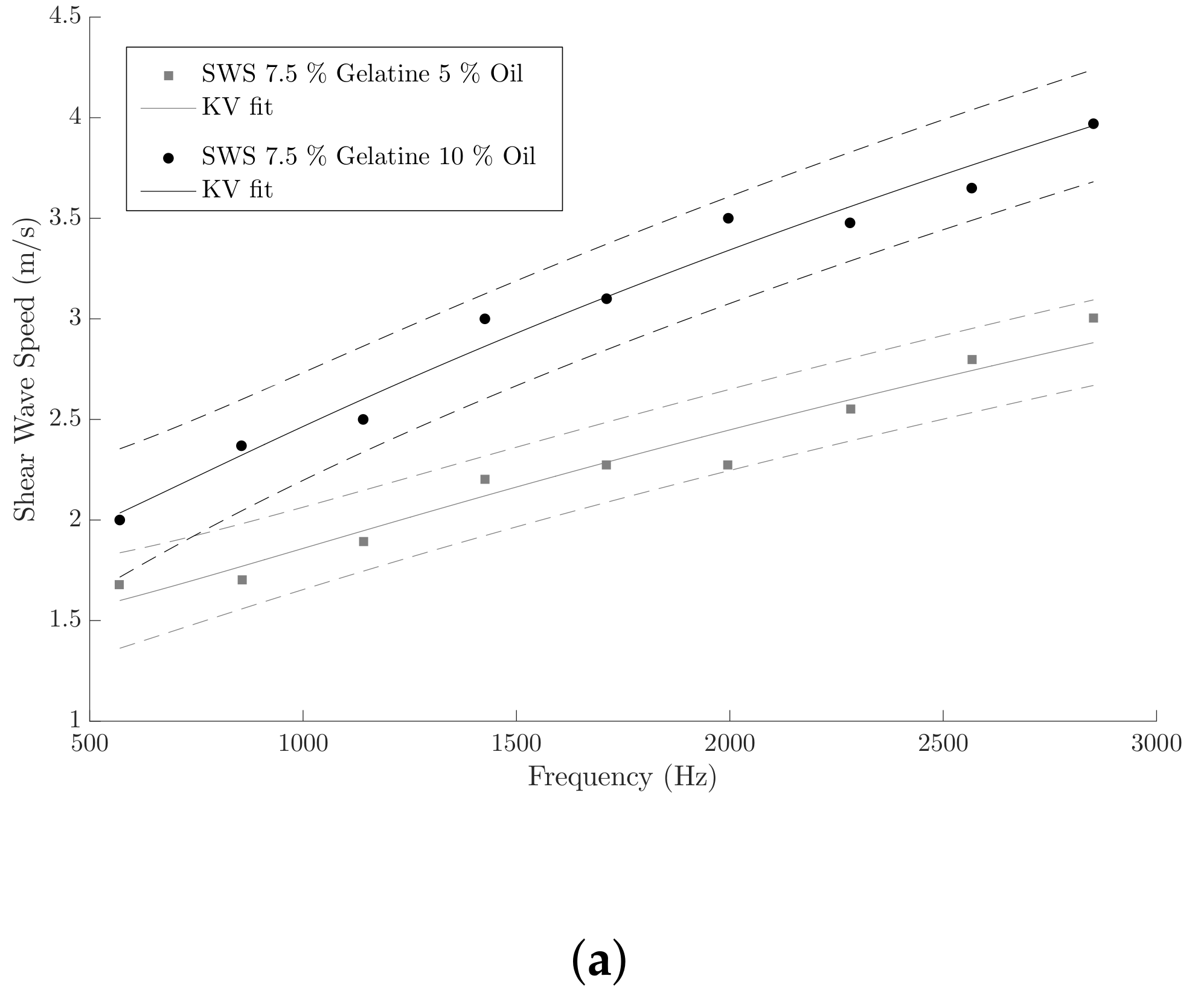
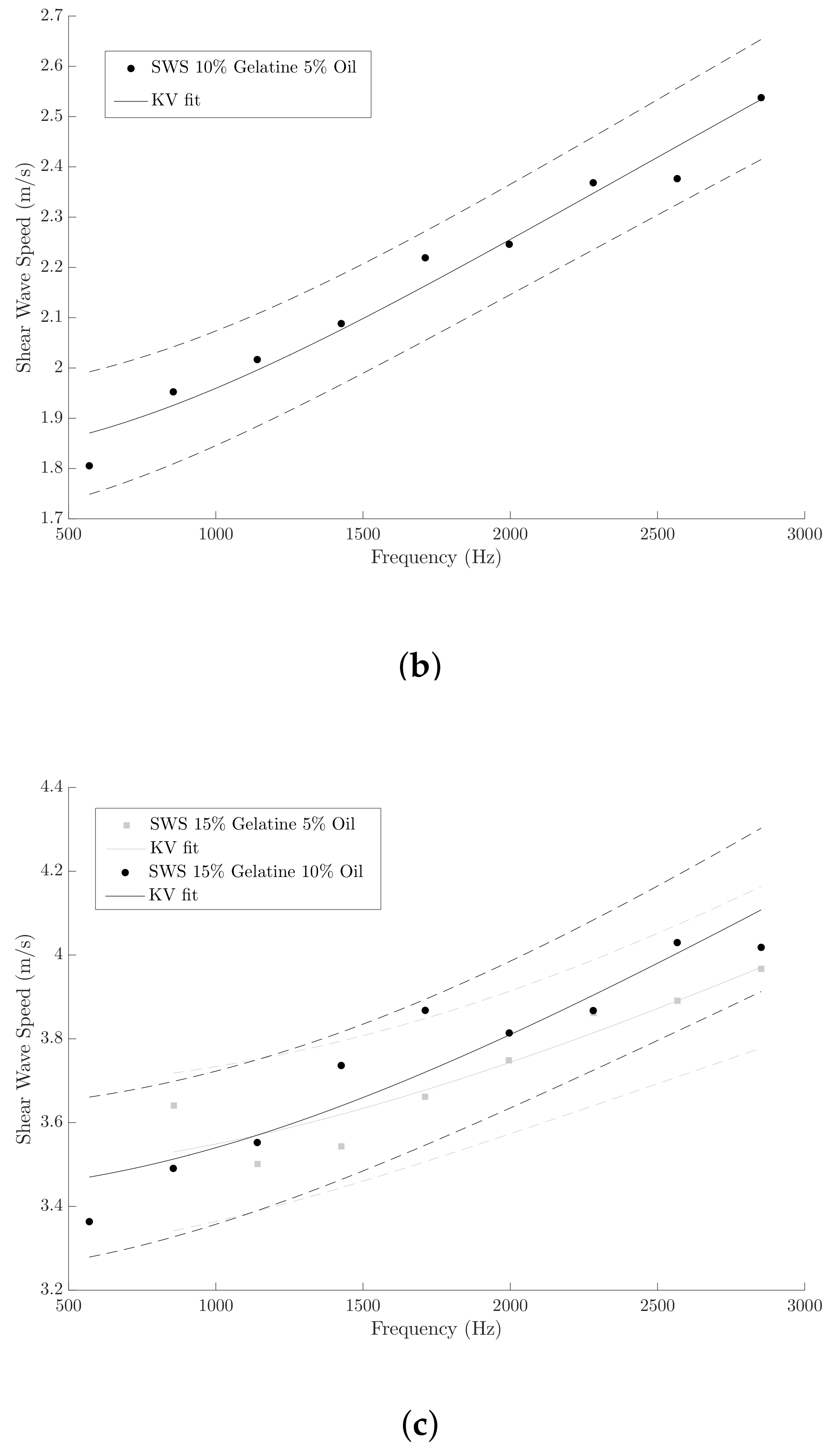
3.1. KV Parameters Reconstruction from SWE Measurements
3.2. KV Parameters Reconstruction from TWE Measurements
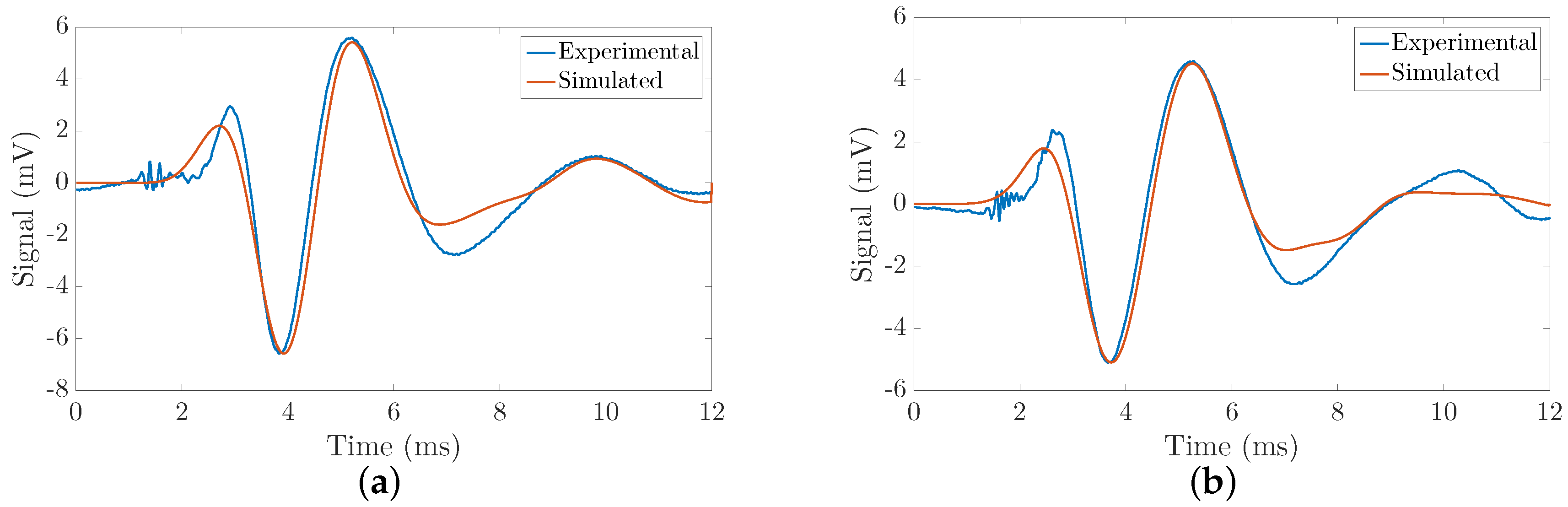
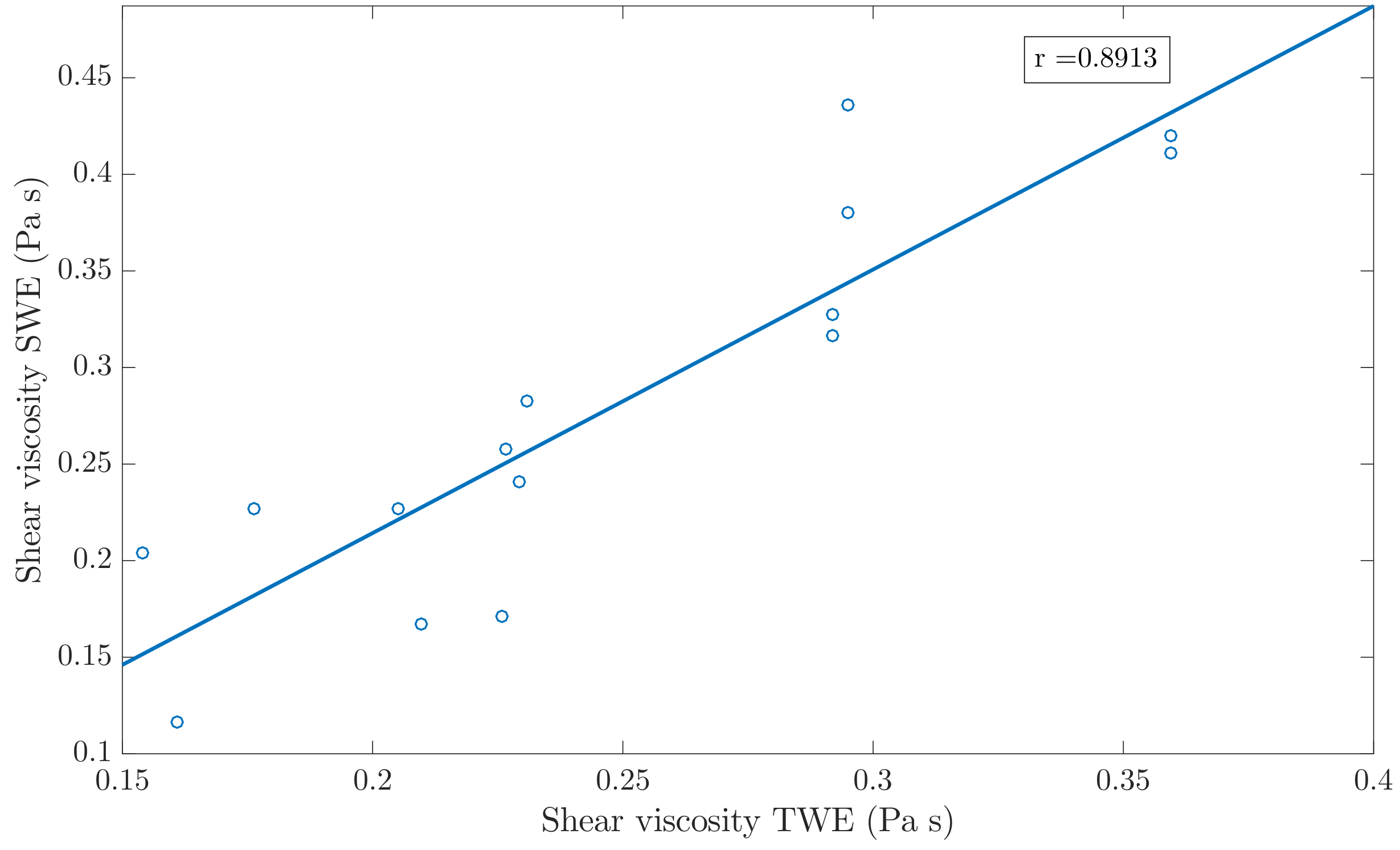
3.3. Validation of the Kelvin-Voigt Parameters Reconstruction Method
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TWE | Torsional Wave Elastography |
| PIP | Probabilistic Inverse Problem |
| KV | Kelvin-Voigt |
| FDTD | Finite Difference Time Domain |
| ABC | Absorbing Boundary Conditions |
| SWE | Shear Wave Elastography |
| TOF | Time-Of-Flight |
| ARF | Acoustic Radiation Force |
| MTL | Multiple Tracking Location |
| IQ | In-Phase and Quadrature |
| USTB | Ultrasound Toolbox |
| FFT | Fast Fourier Transform |
| PLA | Polylactic Acid |
References
- Myers, K.M.; Feltovich, H.; Mazza, E.; Vink, J.; Bajka, M.; Wapner, R.J.; Hall, T.J.; House, M. The mechanical role of the cervix in pregnancy. J. Biomech. 2015, 48, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Faris, I.; Callejas, A. Histobiomechanical remodeling of the cervix during pregnancy: Proposed framework. Math. Probl. Eng. 2019, 2019, 5957432. [Google Scholar] [CrossRef]
- Timmons, B.; Akins, M.; Mahendroo, M. Cervical remodeling during pregnancy and parturition. Trends Endocrinol. Metab. 2010, 21, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Aït-Belkacem, D.; Hessabi, M.; Gennisson, J.L.; Grangé, G.; Goffinet, F.; Lecarpentier, E.; Cabrol, D.; Tanter, M.; Tsatsaris, V. Assessment of the cervix in pregnant women using shear wave elastography: A feasibility study. Ultrasound Med. Biol. 2015, 41, 2789–2797. [Google Scholar] [CrossRef] [PubMed]
- Peralta, L.; Molina, F.S.; Melchor, J.; Gómez, L.F.; Massó, P.; Florido, J.; Rus, G. Transient elastography to assess the cervical ripening during pregnancy: A preliminary study. Ultraschall Medizin-Eur. J. Ultrasound 2017, 38, 395–402. [Google Scholar] [CrossRef]
- Feltovich, H.; Hall, T.J.; Berghella, V. Beyond cervical length: Emerging technologies for assessing the pregnant cervix. Am. J. Obstet. Gynecol. 2012, 207, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.; Hennessy, E.M.; Myles, J.; Johnson, S.J.; Draper, E.S.; Costeloe, K.L.; Marlow, N. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: The EPICure studies. BMJ 2012, 345, e7961. [Google Scholar] [CrossRef]
- Blencowe, H.; Cousens, S.; Chou, D.; Oestergaard, M.; Say, L.; Moller, A.B.; Kinney, M.; Lawn, J. The Born Too Soon Preterm Birth Action Group. Born too soon: The global epidemiology of 15 million preterm births. Reprod. Health 2013, 10, S2. [Google Scholar] [CrossRef]
- Althabe, F. Born Too Soon: The Global Action Report on Preterm Birth; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Feltovich, H.; Hall, T. Quantitative imaging of the cervix: Setting the bar. Ultrasound Obstet. Gynecol. 2013, 41, 121–128. [Google Scholar] [CrossRef]
- Carlson, L.C.; Feltovich, H.; Palmeri, M.L.; Dahl, J.J.; Munoz del Rio, A.; Hall, T.J. Estimation of shear wave speed in the human uterine cervix. Ultrasound Obstet. Gynecol. 2014, 43, 452–458. [Google Scholar] [CrossRef]
- Carlson, L.C.; Feltovich, H.; Palmeri, M.L.; del Rio, A.M.; Hall, T.J. Statistical analysis of shear wave speed in the uterine cervix. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Andrade, E.; Aurioles-Garibay, A.; Garcia, M.; Korzeniewski, S.J.; Schwartz, A.G.; Ahn, H.; Martinez-Varea, A.; Yeo, L.; Chaiworapongsa, T.; Hassan, S.S.; et al. Effect of depth on shear-wave elastography estimated in the internal and external cervical os during pregnancy. J. Perinat. Med. 2014, 42, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Carlson, L.C.; Hall, T.J.; Rosado-Mendez, I.M.; Palmeri, M.L.; Feltovich, H. Detection of changes in cervical softness using shear wave speed in early versus late pregnancy: An in vivo cross-sectional study. Ultrasound Med. Biol. 2018, 44, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Fahey, B.J.; Palmeri, M.L.; Trahey, G.E. Frame rate considerations for real-time abdominal acoustic radiation force impulse imaging. Ultrason. Imaging 2006, 28, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, M.L.; Nightingale, K.R. On the thermal effects associated with radiation force imaging of soft tissue. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Valtorta, D.; Mazza, E. Dynamic measurement of soft tissue viscoelastic properties with a torsional resonator device. Med. Image Anal. 2005, 9, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Hadj Henni, A.; Schmitt, C.; Trop, I.; Cloutier, G. Shear wave induced resonance elastography of spherical masses with polarized torsional waves. Appl. Phys. Lett. 2012, 100, 133702. [Google Scholar] [CrossRef] [PubMed]
- Yengul, S.S.; Barbone, P.E.; Madore, B. Dispersion in Tissue-Mimicking Gels Measured with Shear Wave Elastography and Torsional Vibration Rheometry. Ultrasound Med. Biol. 2019, 45, 586–604. [Google Scholar] [CrossRef]
- Melchor, J.; Rus, G. Torsional ultrasonic transducer computational design optimization. Ultrasonics 2014, 54, 1950–1962. [Google Scholar] [CrossRef]
- Callejas, A.; Gomez, A.; Melchor, J.; Riveiro, M.; Massó, P.; Torres, J.; López-López, M.; Rus, G. Performance study of a torsional wave sensor and cervical tissue characterization. Sensors 2017, 17, 2078. [Google Scholar] [CrossRef]
- Akins, M.L.; Luby-Phelps, K.; Bank, R.A.; Mahendroo, M. Cervical softening during pregnancy: Regulated changes in collagen cross-linking and composition of matricellular proteins in the mouse. Biol. Reprod. 2011, 84, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Kitamura, K.; Mori, Y.; Hirakawa, S. The change in solubility of type I collagen in human uterine cervix in pregnancy at term. Biochem. Med. 1979, 21, 262–270. [Google Scholar] [CrossRef]
- Zork, N.M.; Myers, K.M.; Yoshida, K.; Cremers, S.; Jiang, H.; Ananth, C.V.; Wapner, R.J.; Kitajewski, J.; Vink, J. A systematic evaluation of collagen cross-links in the human cervix. Am. J. Obstetrics Gynecol. 2015, 212, 321-e1. [Google Scholar] [CrossRef] [PubMed]
- Blaskewicz, C.D.; Pudney, J.; Anderson, D.J. Structure and function of intercellular junctions in human cervical and vaginal mucosal epithelia. Biol. Reprod. 2011, 85, 97–104. [Google Scholar] [CrossRef]
- Rus, G.; Melchor, J. Logical Inference Framework for Experimental Design of Mechanical Characterization Procedures. Sensors 2018, 18, 2984. [Google Scholar] [CrossRef]
- Gomez, A.; Rus, G.; Saffari, N. Use of shear waves for diagnosis and ablation monitoring of prostate cancer: A feasibility study. J. Phys. Conf. Ser. 2016, 684, 012006. [Google Scholar] [CrossRef]
- Gómez Fernández, A.J. Transurethral Shear Wave Elastography for Prostate Cancer. Ph.D. Thesis, UCL (University College London), London, UK, 2018. [Google Scholar]
- Tarantola, A. Inverse Problem Theory and Methods for Model Parameter Estimation; SIAM: Paris, France, 2005; Volume 89. [Google Scholar]
- Melchor, J.; Muñoz, R.; Rus, G. Torsional Ultrasound Sensor Optimization for Soft Tissue Characterization. Sensors 2017, 17, 1402. [Google Scholar] [CrossRef] [PubMed]
- Oestreicher, H.L. Field and impedance of an oscillating sphere in a viscoelastic medium with an application to biophysics. J. Acoustical Soc. Am. 1951, 23, 707–714. [Google Scholar] [CrossRef]
- Yamakoshi, Y.; Sato, J.; Sato, T. Ultrasonic imaging of internal vibration of soft tissue under forced Vibration. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1990, 37, 45–53. [Google Scholar] [CrossRef]
- Holm, S.; Sinkus, R. A unifying fractional wave equation for compressional and shear waves. J. Acoust. Soc. Am. 2010, 127, 542–548. [Google Scholar] [CrossRef]
- Dunmire, B.; Kucewicz, J.C.; Mitchell, S.B.; Crum, L.A.; Sekins, K.M. Characterizing an agar/gelatin phantom for image guided dosing and feedback control of high-intensity focused ultrasound. Ultrasound Med. Biol. 2013, 39, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Peralta, L.; Rus, G.; Bochud, N.; Molina, F. Assessing viscoelasticity of shear wave propagation in cervical tissue by multiscale computational simulation. J. Biomech. 2015, 48, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Patton, D.L.; Thwin, S.S.; Meier, A.; Hooton, T.M.; Stapleton, A.E.; Eschenbach, D.A. Epithelial cell layer thickness and immune cell populations in the normal human vagina at different stages of the menstrual cycle. Am. J. Obstetrics Gynecol. 2000, 183, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Catheline, S.; Gennisson, J.L.; Delon, G.; Fink, M.; Sinkus, R.; Abouelkaram, S.; Culioli, J. Measurement of viscoelastic properties of homogeneous soft solid using transient elastography: An inverse problem approach. J. Acoustical Soc. Am. 2004, 116, 3734–3741. [Google Scholar] [CrossRef] [PubMed]
- Bercoff, J.; Tanter, M.; Fink, M. Supersonic shear imaging: A new technique for soft tissue elasticity mapping. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, R.M.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303. [Google Scholar] [CrossRef]
- Ophir, J.; Céspedes, I.; Ponnekanti, H.; Yazdi, Y.; Li, X. Elastography: A quantitative method for imaging the elasticity of biological tissues. Ultrason. Imaging 1991, 13, 111–134. [Google Scholar] [CrossRef]
- Luc Gennisson, J.; Deffieux, T.; Fink, M.; Tanter, M. Ultrasound elastography: Principles and techniques. Diagn. Interv. Imaging 2013, 94, 487–495. [Google Scholar] [CrossRef]
- Deng, Y.; Rouze, N.C.; Palmeri, M.L.; Nightingale, K. Ultrasonic Shear Wave Elasticity Imaging (SWEI) Sequencing and Data Processing Using a Verasonics Research Scanner. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 64, 164–176. [Google Scholar] [CrossRef]
- Palmeri, M.L.; McAleavey, S.A.; Trahey, G.E.; Nightingale, K. Ultrasonic tracking of acoustic radiation force-induced displacements in homogeneous media. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 53, 1300–1313. [Google Scholar] [CrossRef]
- Deffieux, T.; luc Gennisson, J.; Larrat, B.; Fink, M.; Tanter, M. The variance of quantitative estimates in shear wave imaging: Theory and experiments. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 2390–2410. [Google Scholar] [CrossRef] [PubMed]
- Loupas, T.; Powers, J.; Gill, R.W. An axial velocity estimator for ultrasound blood flow imaging, based on a full evaluation of the Doppler equation by means of a two-dimensional autocorrelation approach. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1995, 42, 672–688. [Google Scholar] [CrossRef]
- Rodriguez-Molares, A.; Rindal, O.M.H.; Bernard, O.; Liebgott, H.; Austeng, A.; Lovstakken, L. The ultrasound toolbox. In Proceedings of the 2017 IEEE International Ultrasonics Symposium (IUS), Washington, DC, USA, 6–9 September 2017; p. 1. [Google Scholar]
- Chen, S.; Urban, M.W.; Pislaru, C.; Kinnick, R.; Zheng, Y.; Yao, A.; Greenleaf, J.F. Shearwave dispersion ultrasound vibrometry (SDUV) for measuring tissue elasticity and viscosity. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Mitri, F.G.; Urban, M.W.; Fatemi, M.; Greenleaf, J.F. Shear wave dispersion ultrasonic vibrometry for measuring prostate shear stiffness and viscosity: An in vitro pilot study. IEEE Trans. Biomed. Eng. 2011, 58, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Patent, I. Dispositivo emisor de ondas ultrasónicas de torsión y transductor que lo comprende. Patent WO2017009516A1, 16 July 2015. [Google Scholar]
- Orescanin, M.; Wang, Y.; Insana, M.F. 3-D FDTD simulation of shear waves for evaluation of complex modulus imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Olver, P.J. Introduction to Partial Differential Equations; Springer: New York, NY, USA, 2014. [Google Scholar]
- Rodríguez, J.M.M. Mechanics of Nonlinear Ultrasound in Tissue. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 2016. [Google Scholar]
- Cox, R.T. The algebra of probable inference. Am. J. Phys. 1963, 31, 66–67. [Google Scholar] [CrossRef]
- Tanter, M.; Touboul, D.; Gennisson, J.L.; Bercoff, J.; Fink, M. High-resolution quantitative imaging of cornea elasticity using supersonic shear imaging. IEEE Trans. Med. Imaging 2009, 28, 1881–1893. [Google Scholar] [CrossRef] [PubMed]



| Tissue-Mimicking Phantom Constituents | |
|---|---|
| Ingredient | Supplier, Type |
| Gelatine | Fisher Chemical, Gelatine General purpose grade |
| Formalin (0.24 cc) | Sigma Aldrich, Formaldehyde sol. wt in |
| K-Sorbate (1.62 g) | Alfa Aesar, Potassium sorbate, |
| Lubricating Oil | 50501 TDI 5W40 |
| Surfactant (0.5 g) | Sodium dodecyl sulfate, ACS reagent, ≥99% |
| (100 mL) | Laboratory distilled water |
| Phantoms | Layer | Gelatine (%) | Oil (%) | Th (mm) |
|---|---|---|---|---|
| 1 | First layer | 7.5 | 5 | 1 |
| Second layer | 15 | 5 | 15 | |
| 2 | First layer | 10 | 5 | 1 |
| Second layer | 15 | 5 | 15 | |
| 3 | First layer | 7.5 | 5 | 1 |
| Second layer | 10 | 5 | 15 | |
| 4 | First layer | 7.5 | 10 | 1 |
| Second layer | 15 | 10 | 15 | |
| 5 | First layer | 7.5 | 5 | 0.5 |
| Second layer | 15 | 5 | 15 |
| Number of elements | 128 |
| Pitch (mm) | 0.3 |
| Elevation focus (mm) | 18 |
| Sensitivity (dB) | −52 ± 3 |
| Parameter | |
|---|---|
| Push frequency (MHz) | 4.8 |
| Track frequency (MHz) | 5.6 |
| Push duration (cycles) | 1000 |
| Pulse repetition interval (μs) | 100 |
| Excitation Voltage (V) | 28 |
| Focal distance (mm) | 20 |
| Parameter | Description | Value |
|---|---|---|
| r spatial step | 75 | |
| z spatial step | 75 | |
| time interval | 1 | |
| total time of simulation | 12 ms | |
| number of ABC elements | 100 |
| Medium | (m/s) | Z s)) | |
|---|---|---|---|
| Phantom | 1000 | 2 | 2000 |
| PLA | 1180 | 200 | 236,000 |
| Kelvin-Voigt Model | |
|---|---|
| Parameter | Search Range |
| 0–20 kPa | |
| 0–50 kPa | |
| 0–5 Pa s | |
| 0–2000 |
| TWE | SWE | |||
|---|---|---|---|---|
| std (kPa) | std (Pa · s) | std (kPa) | std (Pa · s) | |
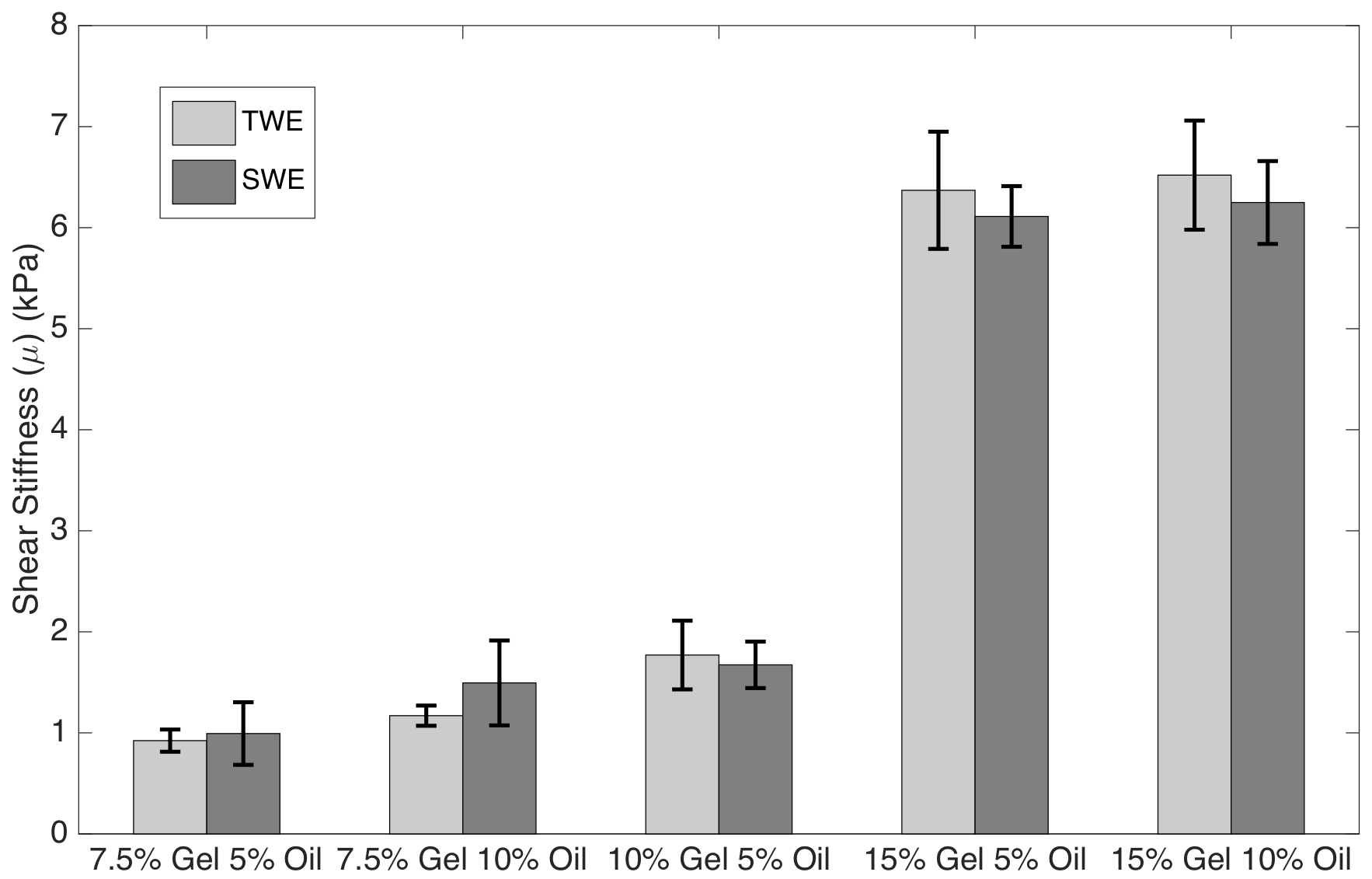
| 7.5% Gelatine 5% Oil | 0.923 ± 0.11 | 0.196 ± 0.04 | 0.993 ± 0.31 | 0.224 ± 0.02 |
| 7.5% Gelatine 10% Oil | 1.17 ± 0.10 | 0.30 ± 0.04 | 1.494 ± 0.42 | 0.420 ± 0.08 |
| 10% Gelatine 5% Oil | 1.77 ± 0.34 | 0.20 ± 0.03 | 1.673 ± 0.23 | 0.150 ± 0.03 |
| 15% Gelatine 5% Oil | 6.37 ± 0.58 | 0.21 ± 0.03 | 6.111 ± 0.30 | 0.269 ± 0.04 |
| 15% Gelatine 10% Oil | 6.52 ± 0.54 | 0.30 ± 0.04 | 6.249 ± 0.41 | 0.369 ± 0.05 |
| Phantoms | Layer | (kPa) | (Pa · s) | Th (mm) |
|---|---|---|---|---|
| 1 | First layer | 1.14 ± 0.15 | 0.20 ± 0.03 | 1.00 ± 0.12 |
| Second layer | 6.31 ± 0.62 | - | ||
| 2 | First layer | 1.43 ± 0.17 | 0.22 ± 0.02 | 0.79 ± 0.11 |
| Second layer | 6.02 ± 0.55 | - | ||
| 3 | First layer | 0.72 ± 0.045 | 0.19 ± 0.05 | 1.15 ± 0.38 |
| Second layer | 2.12 ± 0.52 | - | ||
| 4 | First layer | 1.17 ± 0.10 | 0.30 ± 0.04 | 0.87 ± 0.19 |
| Second layer | 6.52 ± 0.54 | - | ||
| 5 | First layer | 0.91 ± 0.16 | 0.20 ± 0.04 | 0.56 ± 0.23 |
| Second layer | 6.79 ± 0.57 | - |
| p-Value | ||
|---|---|---|
| Batch | ||
| 7.5% Gelatine 5% Oil | 0.365 | 0.1640 |
| 7.5% Gelatine 10% Oil | 0.134 | 0.041 * |
| 10% Gelatine 5% Oil | 0.262 | 0.0551 |
| 15% Gelatine 5% Oil | 0.274 | 0.0553 |
| 15% Gelatine 10% Oil | 0.263 | 0.067 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Callejas, A.; Gomez, A.; Faris, I.H.; Melchor, J.; Rus, G. Kelvin–Voigt Parameters Reconstruction of Cervical Tissue-Mimicking Phantoms Using Torsional Wave Elastography. Sensors 2019, 19, 3281. https://doi.org/10.3390/s19153281
Callejas A, Gomez A, Faris IH, Melchor J, Rus G. Kelvin–Voigt Parameters Reconstruction of Cervical Tissue-Mimicking Phantoms Using Torsional Wave Elastography. Sensors. 2019; 19(15):3281. https://doi.org/10.3390/s19153281
Chicago/Turabian StyleCallejas, Antonio, Antonio Gomez, Inas H. Faris, Juan Melchor, and Guillermo Rus. 2019. "Kelvin–Voigt Parameters Reconstruction of Cervical Tissue-Mimicking Phantoms Using Torsional Wave Elastography" Sensors 19, no. 15: 3281. https://doi.org/10.3390/s19153281
APA StyleCallejas, A., Gomez, A., Faris, I. H., Melchor, J., & Rus, G. (2019). Kelvin–Voigt Parameters Reconstruction of Cervical Tissue-Mimicking Phantoms Using Torsional Wave Elastography. Sensors, 19(15), 3281. https://doi.org/10.3390/s19153281






