Application of ECIS to Assess FCCP-Induced Changes of MSC Micromotion and Wound Healing Migration
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Measurement of O2 Consumption
2.3. Impedance Measurement
2.4. Detection of Micromotion
2.5. ECIS Wound Healing Assay
2.6. Statistical Analysis
3. Results
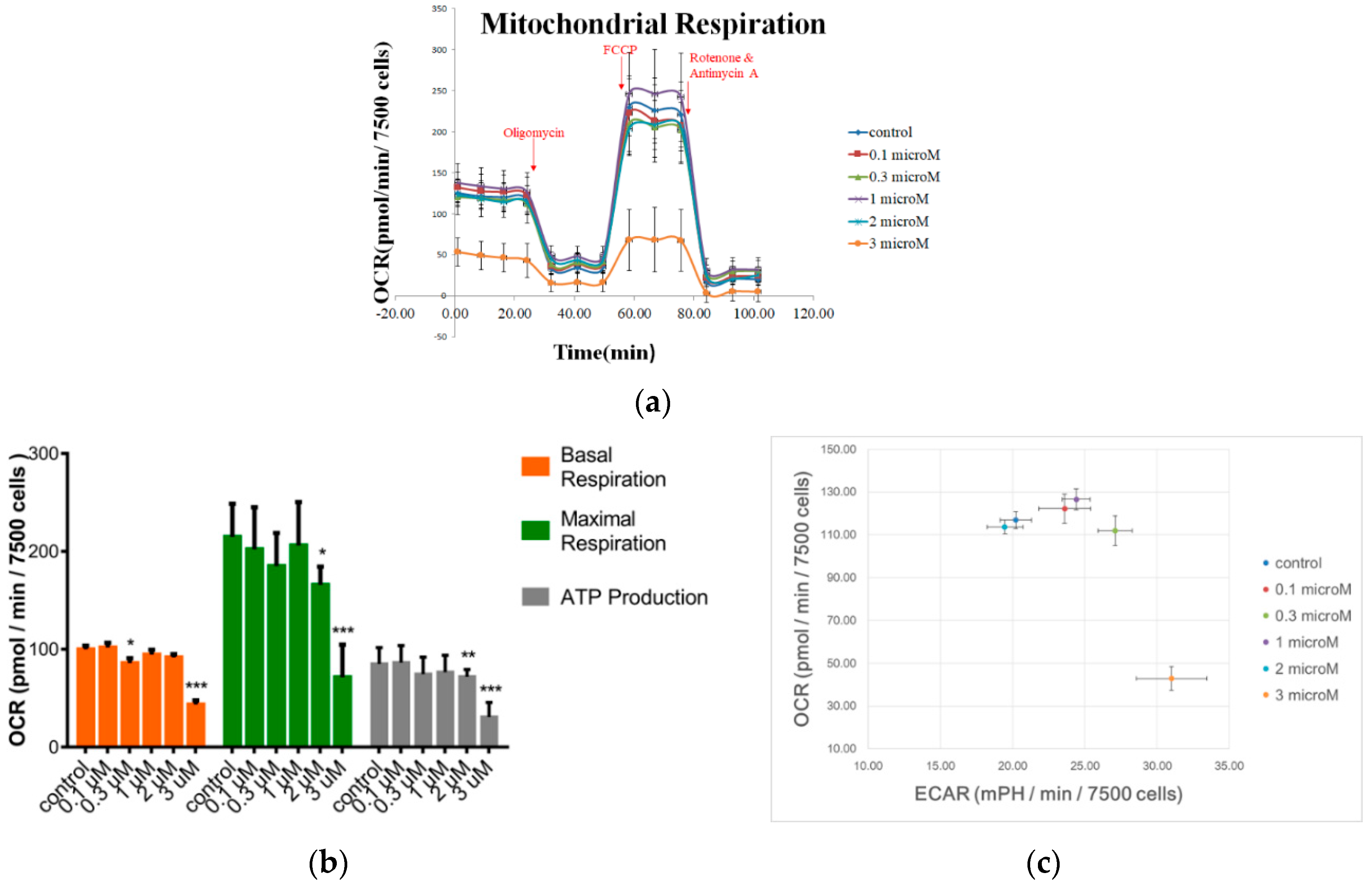
3.1. Reduction of hMSC Mitochondrial Respiration in Response to FCCP
3.2. Real-Time Monitoring of hMSC Attachment and Spreading
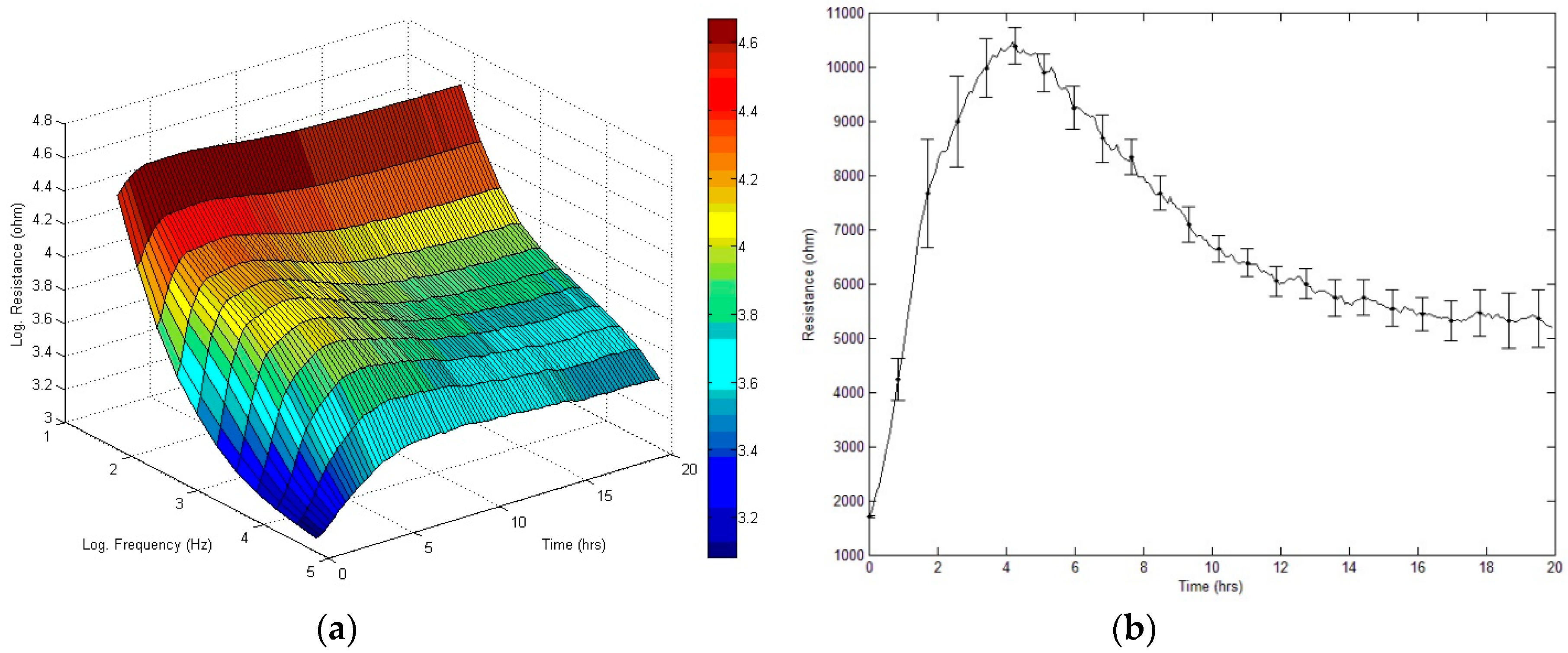
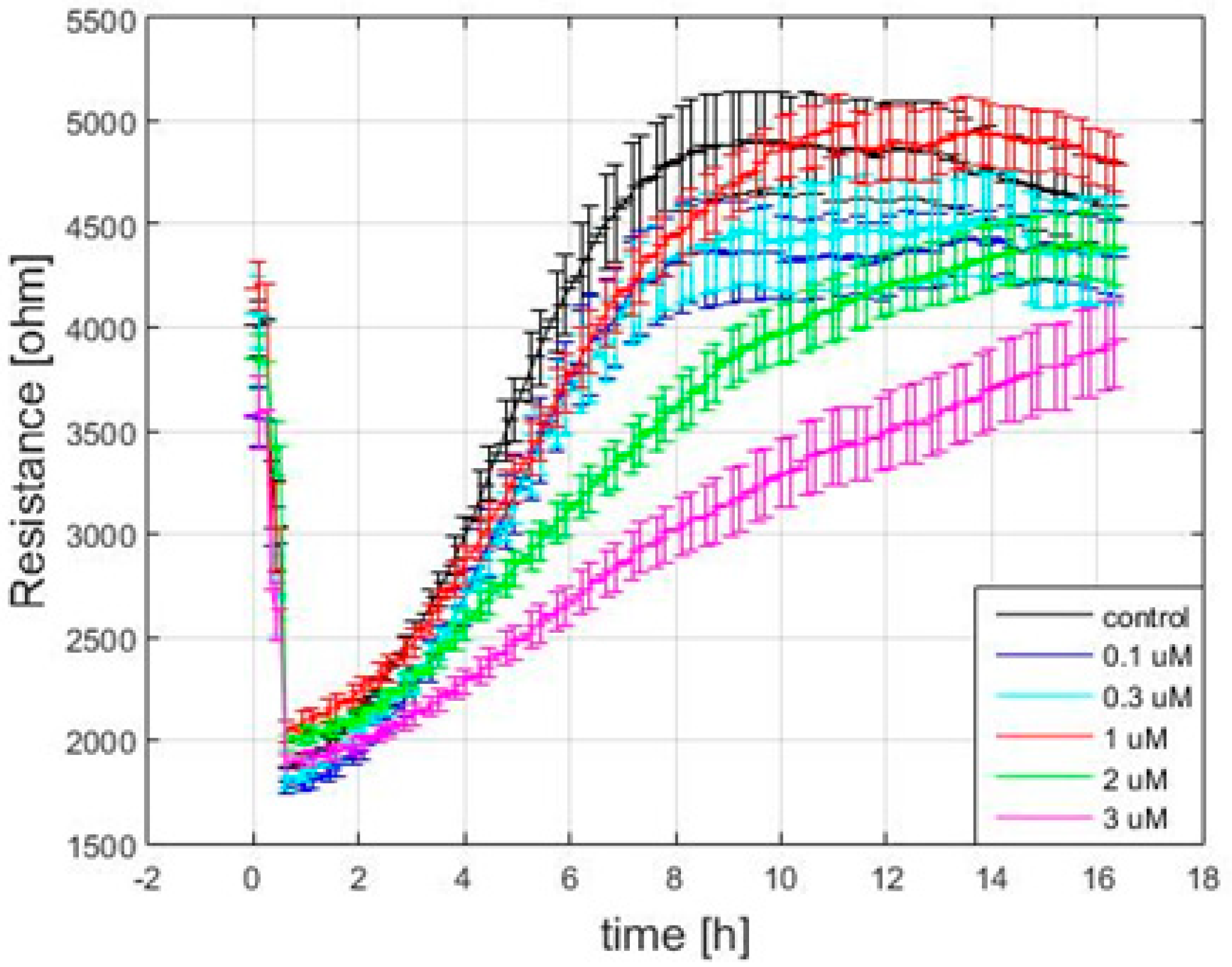
3.3. Effect of FCCP on the Time Course of Overall Resistance
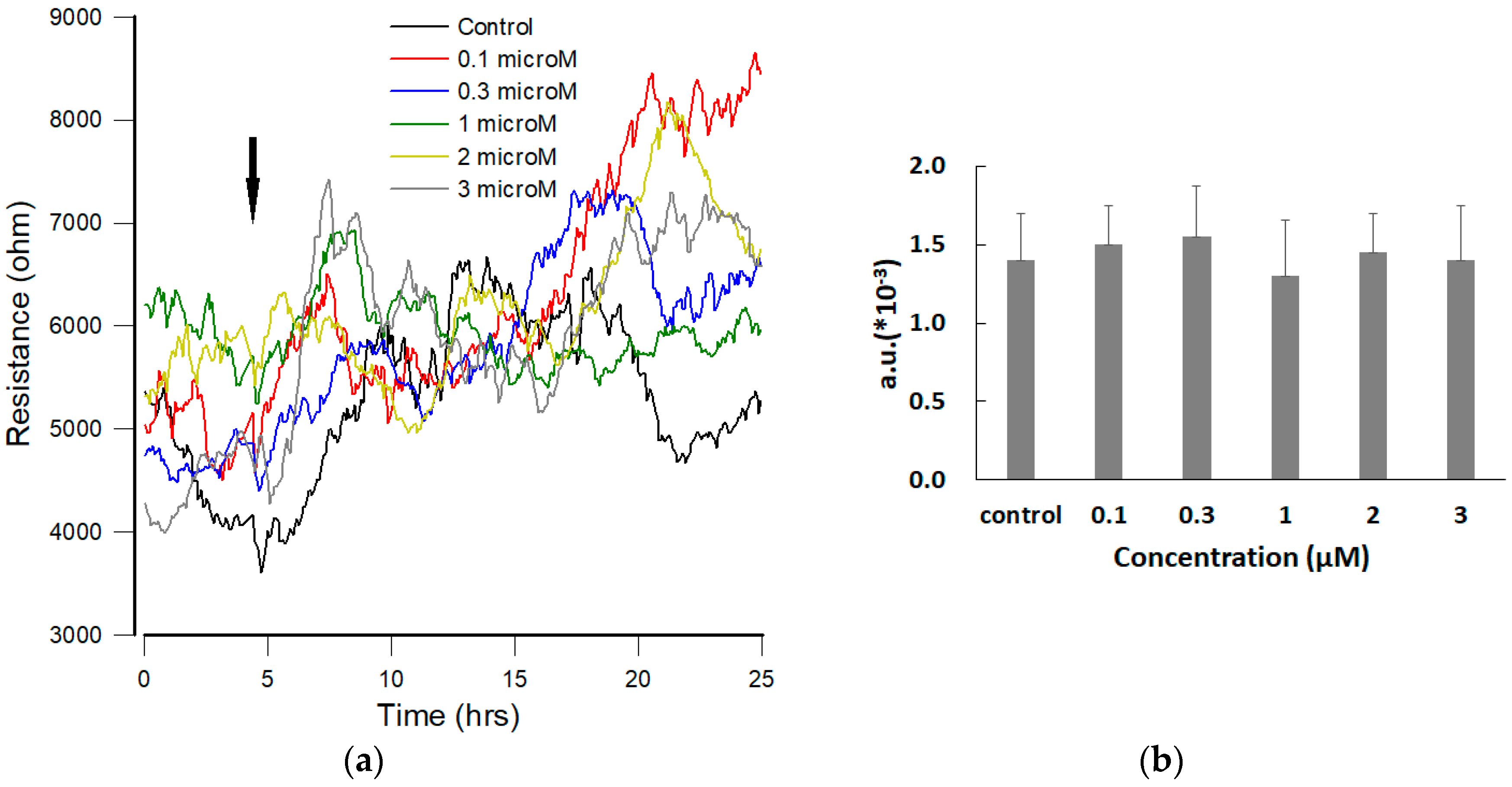
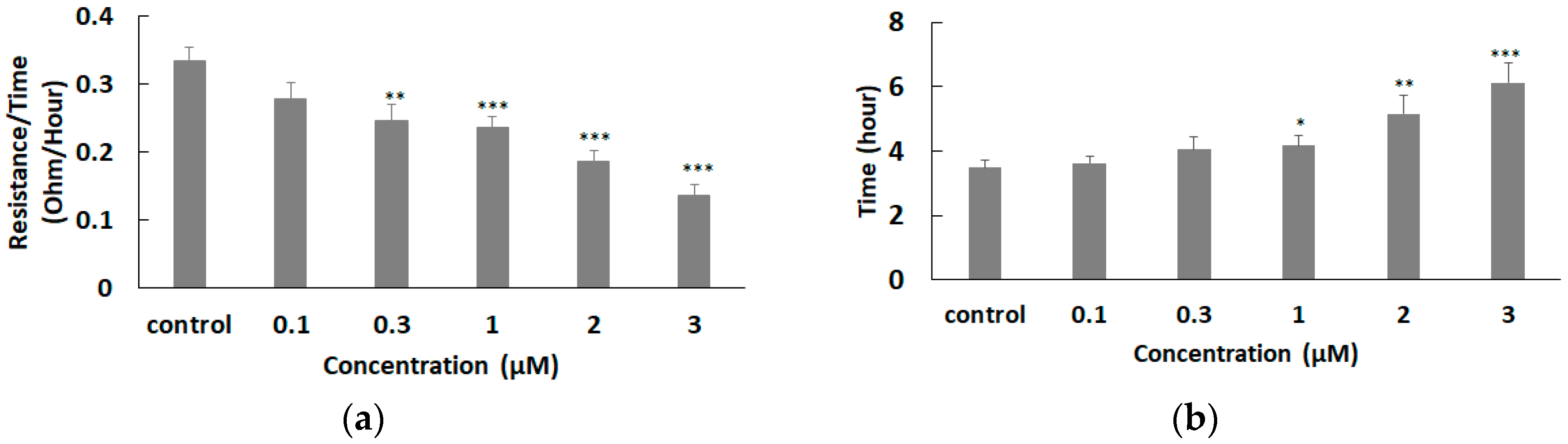
3.4. Effect of FCCP on hMSC Micromotion
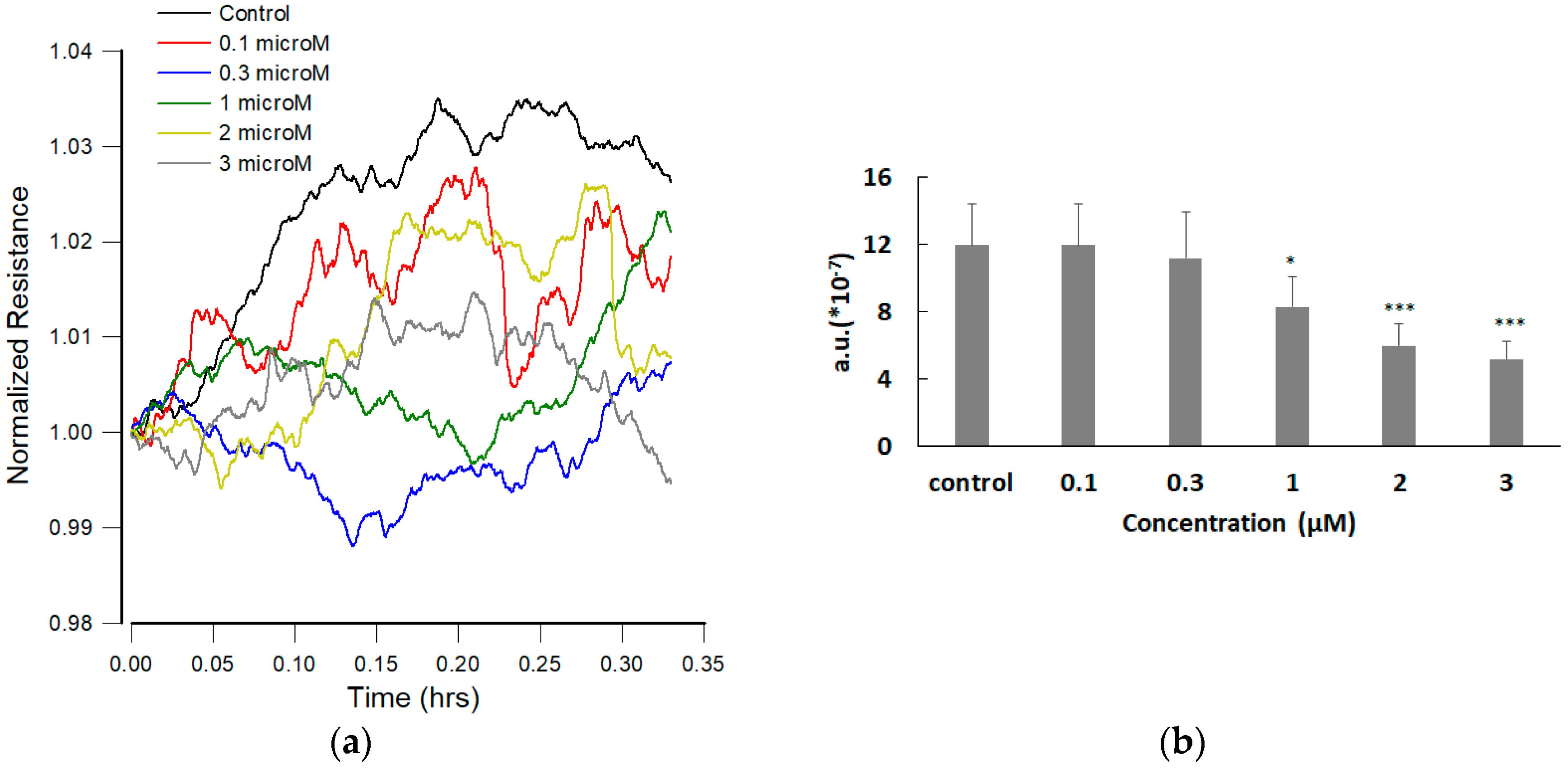
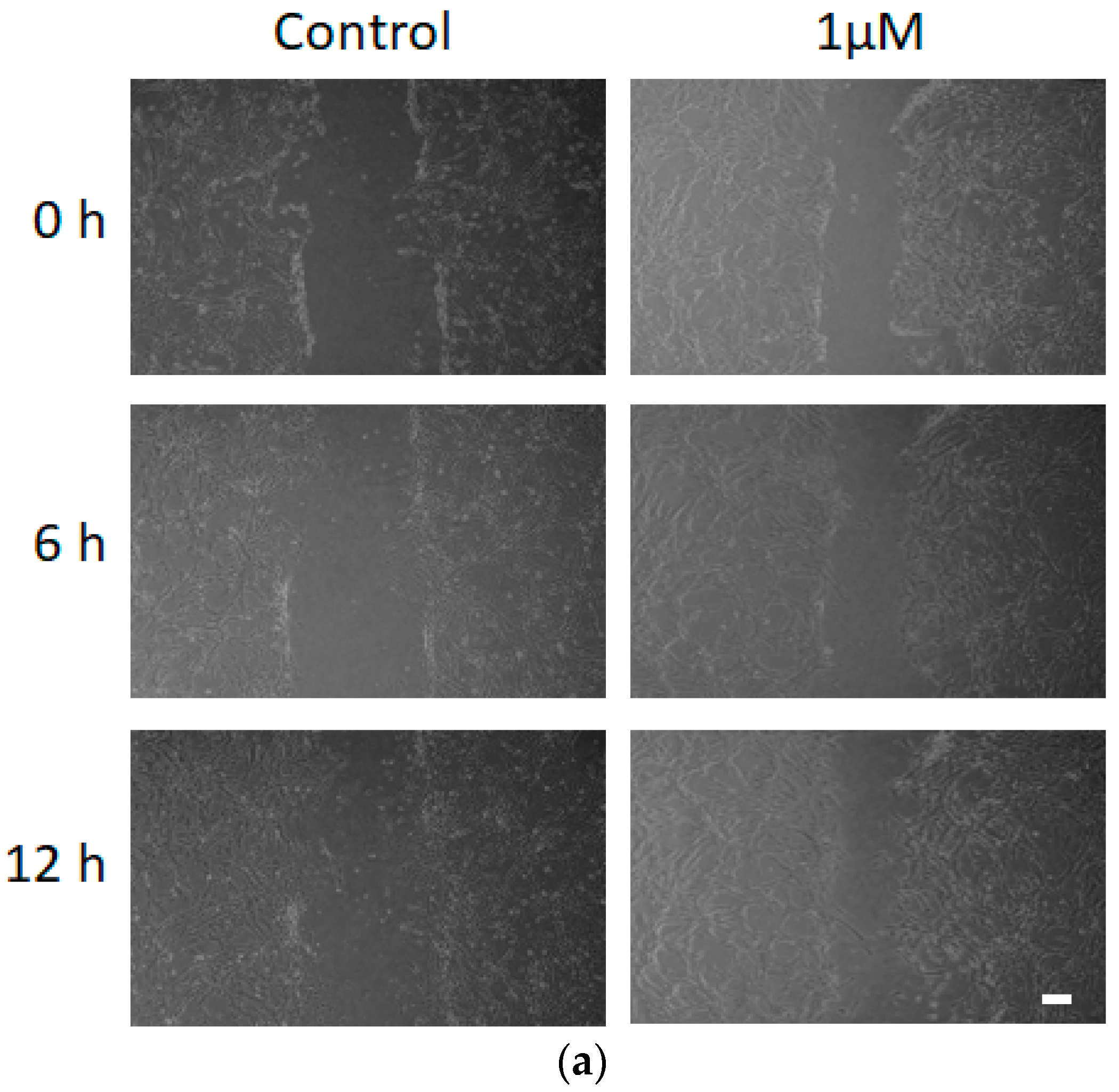
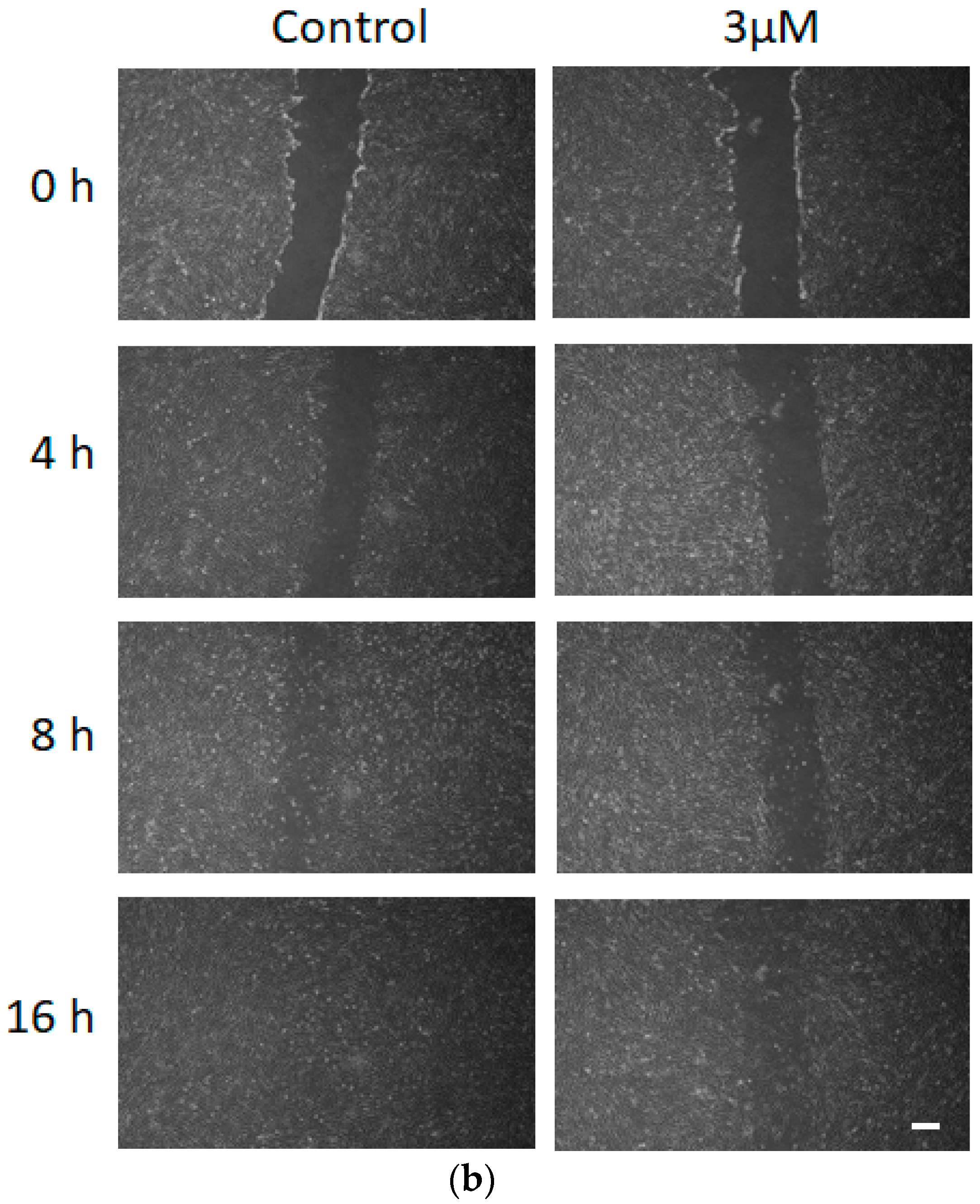
3.5. Effect of FCCP on hMSC Wound Healing Migration
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—Current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef] [PubMed]
- De Lucas, B.; Perez, L.M.; Galvez, B.G. Importance and regulation of adult stem cell migration. J. Cell. Mol. Med. 2018, 22, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Tian, Y.; Yang, C.; Ma, X.; Wang, X.; Pei, J.; Qian, A. Mesenchymal stem cell migration during bone formation and bone diseases therapy. Int. J. Mol. Sci. 2018, 19, 2343. [Google Scholar] [CrossRef]
- De Becker, A.; Riet, I.V. Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World J. Stem Cells 2016, 8, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Choi, G.E.; Oh, J.Y.; Lee, H.J.; Kim, J.S.; Chae, C.W.; Choi, D.; Han, H.J. Succinate promotes stem cell migration through the gpr91-dependent regulation of drp1-mediated mitochondrial fission. Sci. Rep. 2017, 7, 12582. [Google Scholar] [CrossRef]
- Ciria, M.; Garcia, N.A.; Ontoria-Oviedo, I.; Gonzalez-King, H.; Carrero, R.; De La Pompa, J.L.; Montero, J.A.; Sepulveda, P. Mesenchymal stem cell migration and proliferation are mediated by hypoxia-inducible factor-1alpha upstream of notch and sumo pathways. Stem Cells Dev. 2017, 26, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Mas-Bargues, C.; Sanz-Ros, J.; Roman-Dominguez, A.; Ingles, M.; Gimeno-Mallench, L.; El Alami, M.; Vina-Almunia, J.; Gambini, J.; Vina, J.; Borras, C. Relevance of oxygen concentration in stem cell culture for regenerative medicine. Int. J. Mol. Sci. 2019, 20, 1195. [Google Scholar] [CrossRef] [PubMed]
- Pattappa, G.; Heywood, H.K.; De Bruijn, J.D.; Lee, D.A. The metabolism of human mesenchymal stem cells during proliferation and differentiation. J. Cell. Physiol. 2011, 226, 2562–2570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Marsboom, G.; Toth, P.T.; Rehman, J. Mitochondrial respiration regulates adipogenic differentiation of human mesenchymal stem cells. PLoS ONE 2013, 8, e77077. [Google Scholar] [CrossRef] [PubMed]
- Khacho, M.; Slack, R.S. Mitochondrial activity in the regulation of stem cell self-renewal and differentiation. Curr. Opin. Cell. Biol. 2017, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wanet, A.; Arnould, T.; Najimi, M.; Renard, P. Connecting mitochondria, metabolism, and stem cell fate. Stem Cells Dev. 2015, 24, 1957–1971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Khvorostov, I.; Hong, J.S.; Oktay, Y.; Vergnes, L.; Nuebel, E.; Wahjudi, P.N.; Setoguchi, K.; Wang, G.; Do, A.; et al. Ucp2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J. 2011, 30, 4860–4873. [Google Scholar] [CrossRef] [PubMed]
- Folmes, C.D.; Nelson, T.J.; Martinez-Fernandez, A.; Arrell, D.K.; Lindor, J.Z.; Dzeja, P.P.; Ikeda, Y.; Perez-Terzic, C.; Terzic, A. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011, 14, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Teslaa, T.; Teitell, M.A. Pluripotent stem cell energy metabolism: An update. EMBO J. 2014, 34, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.L.; Graos, M.; Rodrigues, A.S.; Anjo, S.I.; Carvalho, R.A.; Oliveira, P.J.; Arenas, E.; Ramalho-Santos, J. Inhibition of mitochondrial complex iii blocks neuronal differentiation and maintains embryonic stem cell pluripotency. PLoS ONE 2013, 8, e82095. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Lindgren, A.G.; Srivastava, A.S.; Clark, A.T.; Banerjee, U. Mitochondrial function controls proliferation and early differentiation potential of embryonic stem cells. Stem Cells 2011, 29, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.J.; Yoon, S.H.; Do, J.T. Mitochondrial dynamics in stem cells and differentiation. Int. J. Mol. Sci. 2018, 19, 3893. [Google Scholar] [CrossRef]
- Zhang, H.; Menzies, K.J.; Auwerx, J. The role of mitochondria in stem cell fate and aging. Development 2018, 145, dev143420. [Google Scholar] [CrossRef]
- Paluch, E.K.; Raz, E. The role and regulation of blebs in cell migration. Curr. Opin. Cell Biol. 2013, 25, 582–590. [Google Scholar] [CrossRef]
- Giaever, I.; Keese, C.R. Micromotion of mammalian cells measured electrically. Proc. Natl. Acad. Sci. USA 1991, 88, 7896–7900. [Google Scholar] [CrossRef]
- Giaever, I.; Keese, C.R. A morphological biosensor for mammalian cells. Nature 1993, 366, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Keese, C.R.; Wegener, J.; Walker, S.R.; Giaever, I. Electrical wound-healing assay for cells in vitro. Proc. Natl. Acad. Sci. USA 2004, 101, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Gamal, W.; Borooah, S.; Smith, S.; Underwood, I.; Srsen, V.; Chandran, S.; Bagnaninchi, P.; Dhillon, B. Real-time quantitative monitoring of hipsc-based model of macular degeneration on electric cell-substrate impedance sensing microelectrodes. Biosens. Bioelectron. 2015, 71, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-M.; Keese, C.R.; Giaever, I. Monitoring motion of confluent cells in tissue culture. Exp. Cell Res. 1993, 204, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Opp, D.; Wafula, B.; Lim, J.; Huang, E.; Lo, J.-C.; Lo, C.-M. Use of electric cell-substrate impedance sensing to assess in vitro cytotoxicity. Biosens. Bioelectron. 2009, 24, 2625–2629. [Google Scholar] [CrossRef] [PubMed]
- Lovelady, D.C.; Friedman, J.; Patel, S.; Rabson, D.A.; Lo, C.-M. Detecting effects of low levels of cytochalasin b in 3t3 fibroblast cultures by analysis of electrical noise obtained from cellular micromotion. Biosens. Bioelectron. 2009, 24, 2250–2254. [Google Scholar] [CrossRef] [PubMed]
- Lang, O.; Kohidai, L.; Wegener, J. Label-free profiling of cell dynamics: A sequence of impedance-based assays to estimate tumor cell invasiveness in vitro. Exp. Cell Res. 2017, 359, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.P.; Batsaikhan, B.; Huang, H.M.; Wang, J.Y. Application of electric cell-substrate impedance sensing to investigate the cytotoxic effects of andrographolide on u-87 mg glioblastoma cell migration and apoptosis. Sensors 2019, 19, 2275. [Google Scholar] [CrossRef]
- Ferrick, D.A.; Neilson, A.; Beeson, C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov. Today 2008, 13, 268–274. [Google Scholar] [CrossRef]
- Starkov, A.A. “Mild” uncoupling of mitochondria. Biosci. Rep. 1997, 17, 273–279. [Google Scholar] [CrossRef]
- Kane, M.S.; Paris, A.; Codron, P.; Cassereau, J.; Procaccio, V.; Lenaers, G.; Reynier, P.; Chevrollier, A. Current mechanistic insights into the cccp-induced cell survival response. Biochem. Pharm. 2018, 148, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Mlejnek, P.; Dolezel, P. Loss of mitochondrial transmembrane potential and glutathione depletion are not sufficient to account for induction of apoptosis by carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone in human leukemia k562 cells. Chem. Biol. Interact. 2015, 239, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, S.; Qualls, C.W., Jr.; Tyler, R.D.; Witherspoon, S.M.; Benavides, G.R.; Yoon, L.W.; Dold, K.; Brown, R.H.; Sangiah, S.; Morgan, K.T. Effects of minimally toxic levels of carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (fccp), elucidated through differential gene expression with biochemical and morphological correlations. Toxicol. Sci. 2003, 73, 348–361. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, S.-P.; Lee, Y.-W.; Wu, L.-Y.; Tung, T.-H.; Gomez, S.; Lo, C.-M.; Wang, J.-Y. Application of ECIS to Assess FCCP-Induced Changes of MSC Micromotion and Wound Healing Migration. Sensors 2019, 19, 3210. https://doi.org/10.3390/s19143210
Chiu S-P, Lee Y-W, Wu L-Y, Tung T-H, Gomez S, Lo C-M, Wang J-Y. Application of ECIS to Assess FCCP-Induced Changes of MSC Micromotion and Wound Healing Migration. Sensors. 2019; 19(14):3210. https://doi.org/10.3390/s19143210
Chicago/Turabian StyleChiu, Sheng-Po, Yu-Wei Lee, Ling-Yi Wu, Tse-Hua Tung, Sofia Gomez, Chun-Min Lo, and Jia-Yi Wang. 2019. "Application of ECIS to Assess FCCP-Induced Changes of MSC Micromotion and Wound Healing Migration" Sensors 19, no. 14: 3210. https://doi.org/10.3390/s19143210
APA StyleChiu, S.-P., Lee, Y.-W., Wu, L.-Y., Tung, T.-H., Gomez, S., Lo, C.-M., & Wang, J.-Y. (2019). Application of ECIS to Assess FCCP-Induced Changes of MSC Micromotion and Wound Healing Migration. Sensors, 19(14), 3210. https://doi.org/10.3390/s19143210




