Precision and Limits of Detection for Selected Commercially Available, Low-Cost Carbon Dioxide and Methane Gas Sensors
Abstract
1. Introduction
2. Methods
2.1. Sensor Selection
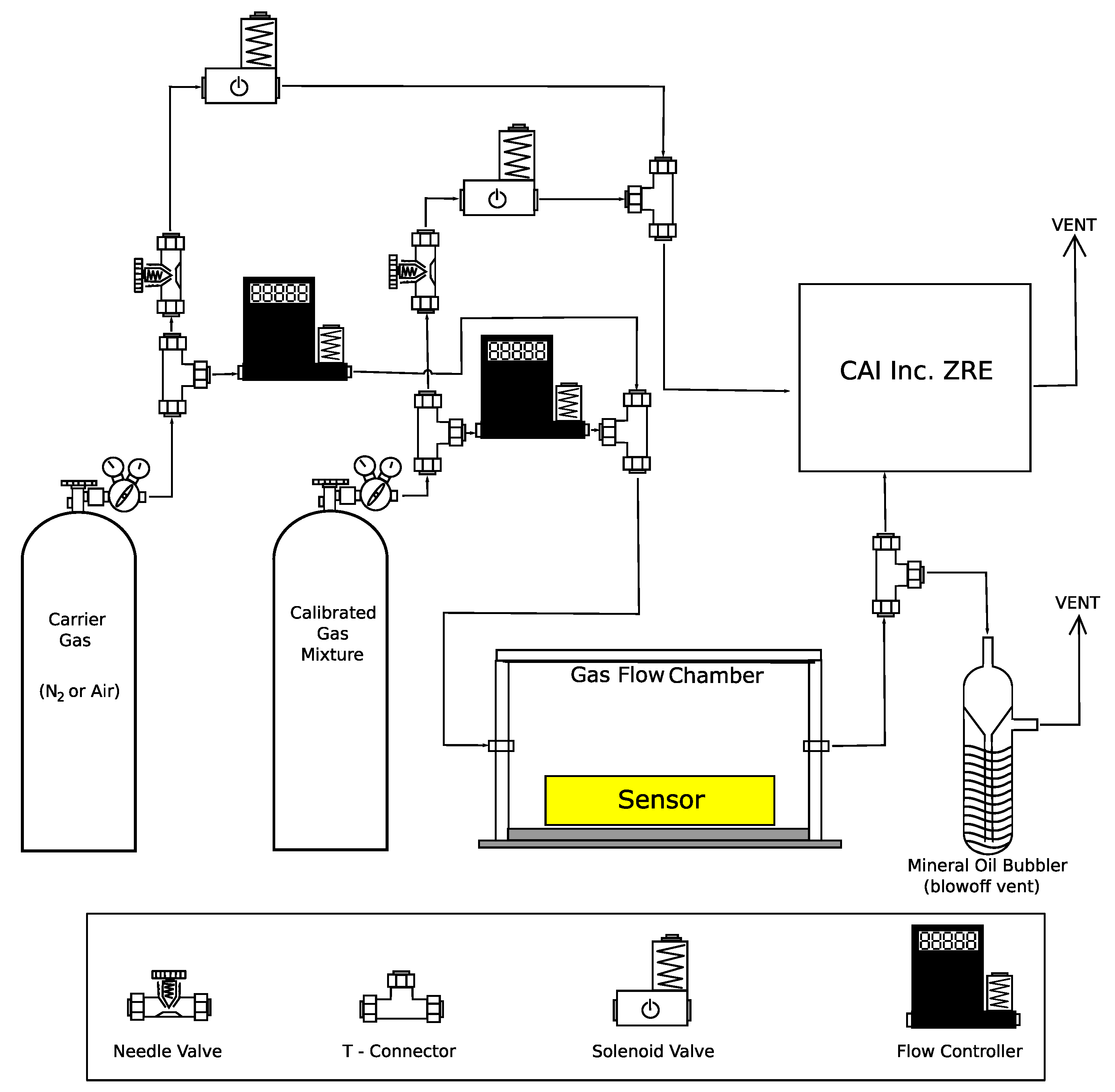
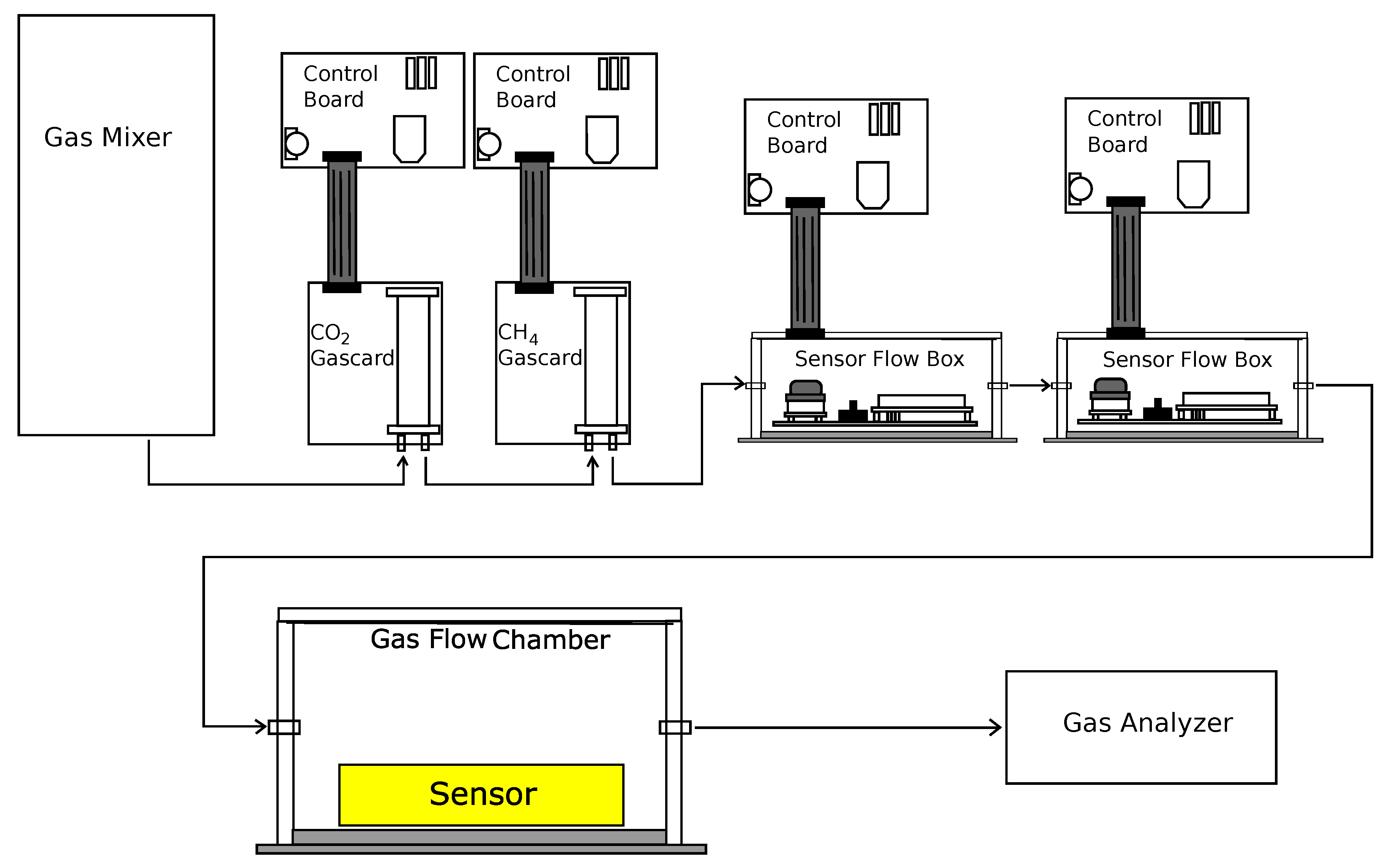
2.2. Analyte Preparation and Sensor Testing
2.3. Sensor Output and Data Collection
2.4. Calibration and Fitting Procedures
2.5. Precision and Baseline Noise Tests
2.6. Limit of Detection Determination
3. Results and Discussion
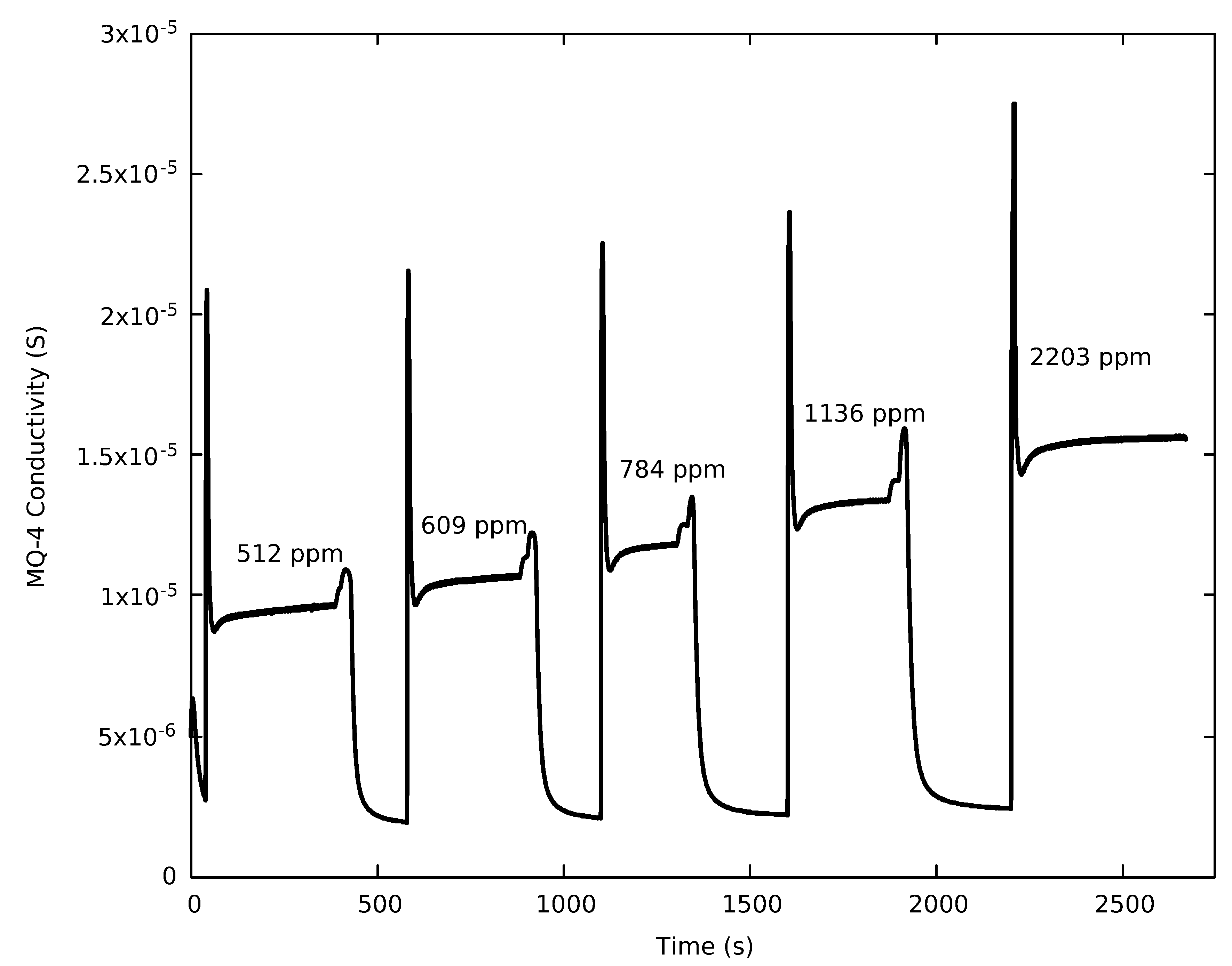
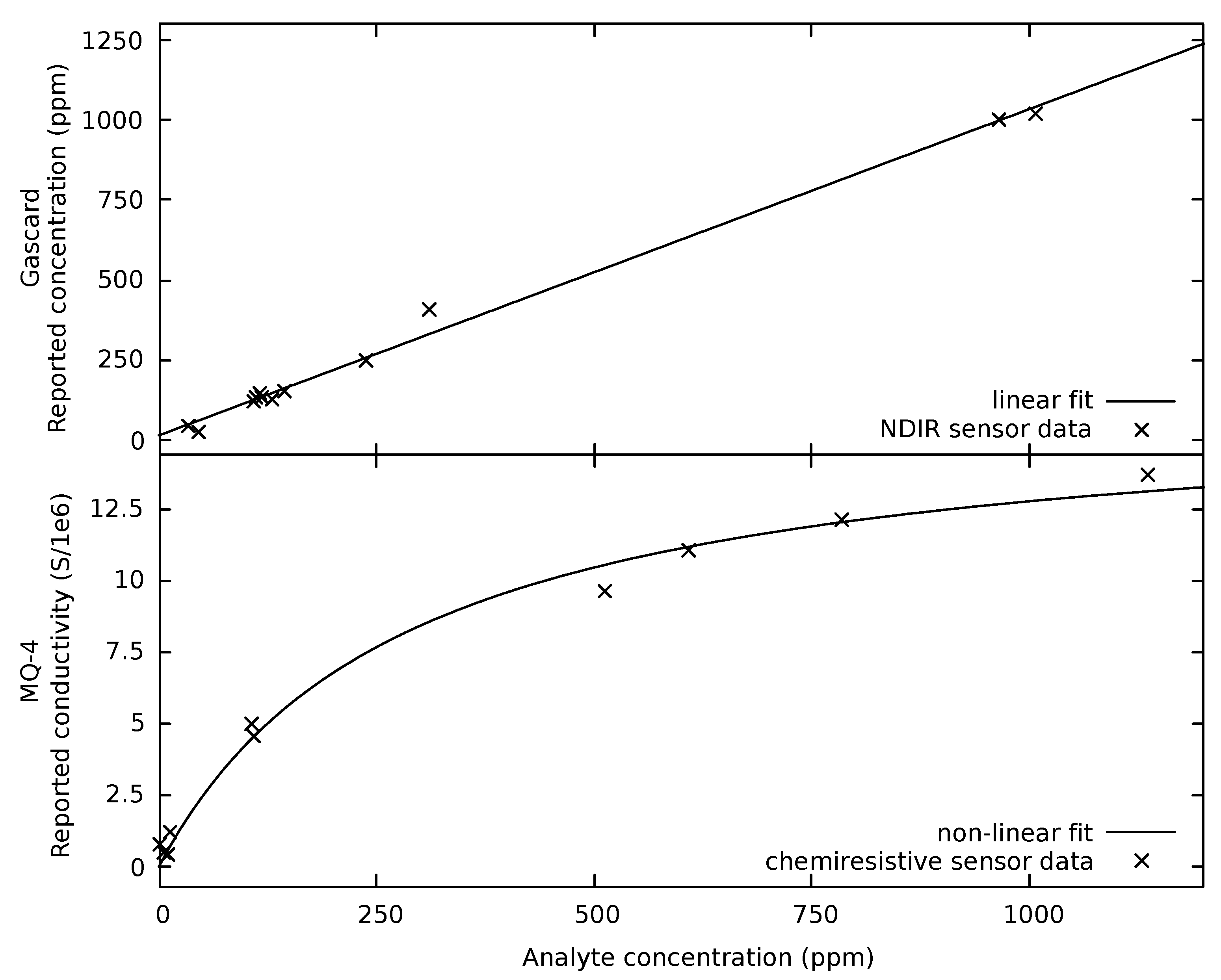
3.1. Sensor Response
3.2. Calibration Result
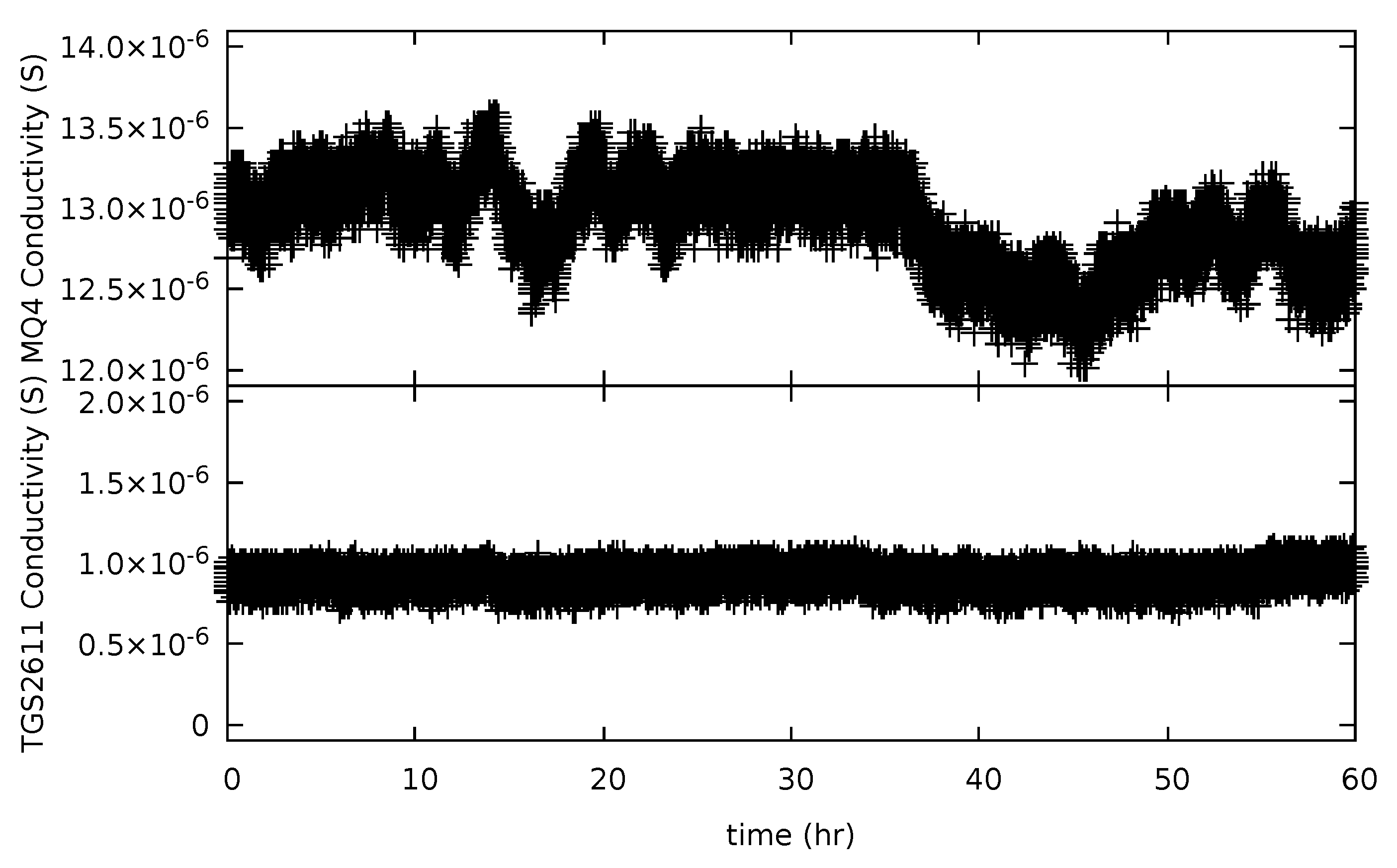
3.3. Precision and Baseline Noise Tests
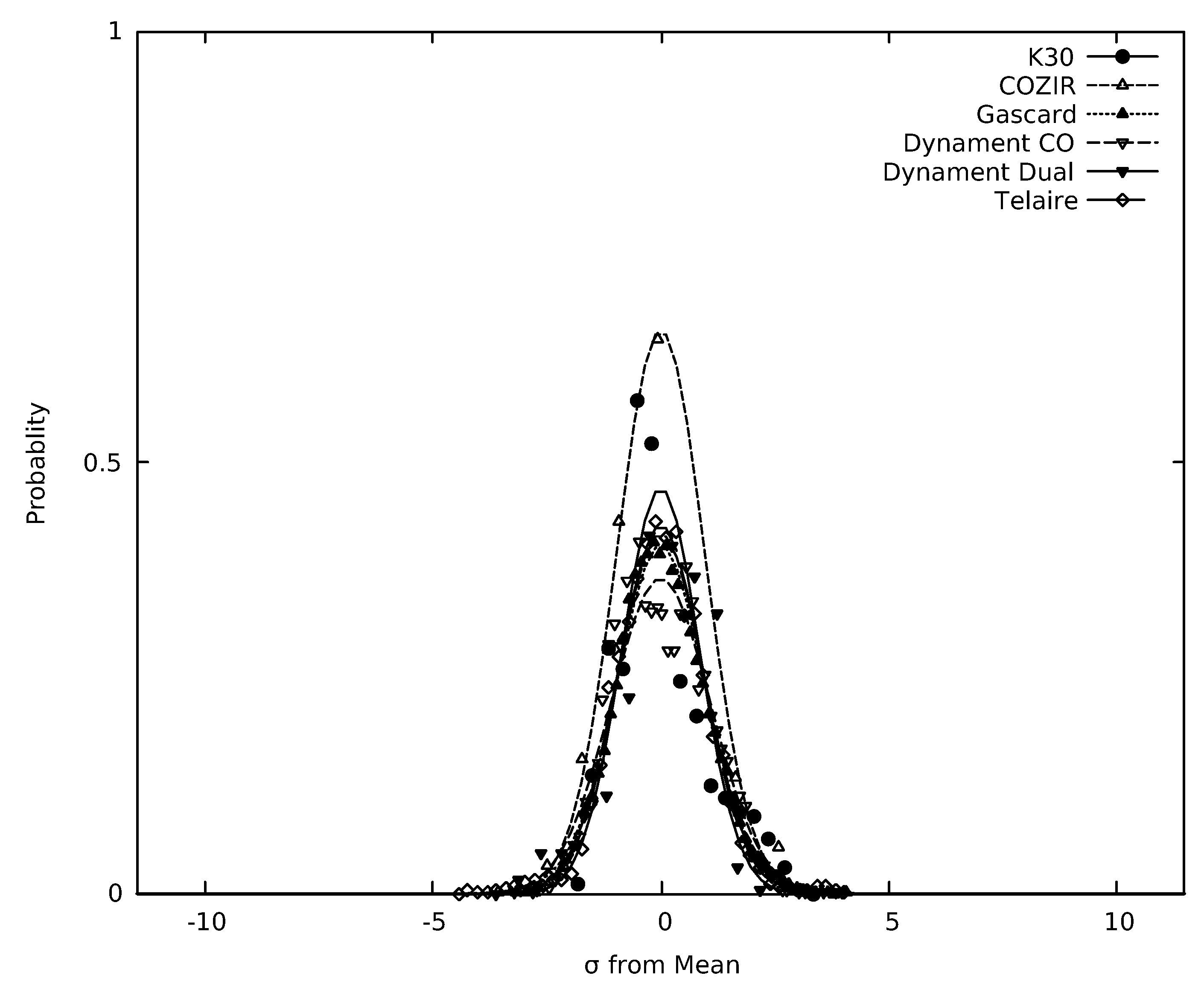
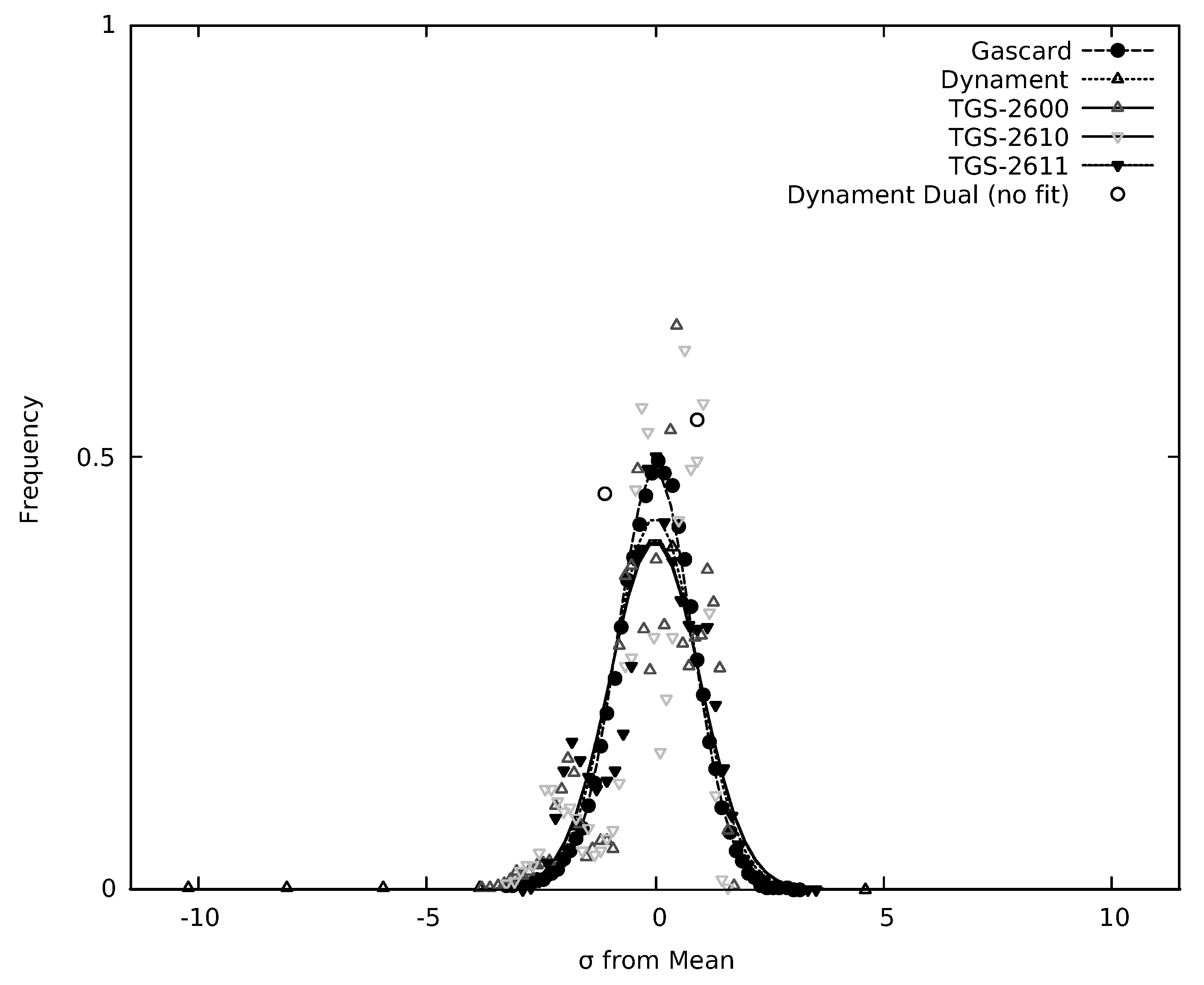
3.4. Limits of Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CO2 | Carbon dioxide |
| CH4 | Methane |
| NDIR | Nondispersive Infrared |
| RMSE | Root-mean-squared error |
References
- Keith, L.H.; Crummett, W.; Deegan, J.; Libby, R.A.; Taylor, J.K.; Wentler, G. Principles of Environmental Analysis. Anal. Chem. 1983, 55, 2210–2218. [Google Scholar] [CrossRef]
- Yang, Z.; Li, N.; Becerik-Gerber, B.; Orosz, M. A Systematic Approach to Occupancy Modeling in Ambient Sensor-Rich Buildings. Simulation 2014, 90, 960–977. [Google Scholar] [CrossRef]
- Chung, W.Y.; Lee, S.C. A Selective AQS System with Artificial Neural Network in Automobile. Sens. Actuators B Chem. 2008, 130, 258–263. [Google Scholar] [CrossRef]
- Won, W.; Lee, K.S. Nonlinear Observer with Adaptive Grid Allocation for a Fixed-Bed Adsorption Process. Comput. Chem. Eng. 2012, 46, 69–77. [Google Scholar] [CrossRef]
- Yi, P.; Xiao, L.; Zhang, Y. Remote Real-Time Monitoring System for Oil and Gas Well Based on Wireless Sensor Networks. In Proceedings of the 2010 International Conference on Mechanic Automation and Control Engineering, Wuhan, China, 26–28 June 2010; pp. 2427–2429. [Google Scholar] [CrossRef]
- Somov, A.; Baranov, A.; Spirjakin, D.; Spirjakin, A.; Sleptsov, V.; Passerone, R. Deployment and Evaluation of a Wireless Sensor Network for Methane Leak Detection. Sens. Actuators A Phys. 2013, 202, 217–225. [Google Scholar] [CrossRef]
- Pering, T.; Tamburello, G.; McGonigle, A.; Aiuppa, A.; Cannata, A.; Giudice, G.; Patanè, D. High Time Resolution Fluctuations in Volcanic Carbon Dioxide Degassing from Mount Etna. J. Volcanol. Geotherm. Res. 2014, 270, 115–121. [Google Scholar] [CrossRef]
- Black, R.; Mick Meyer, C.; Yates, A.; Zweiten, L.V.; Mueller, J. Formation of Artefacts While Sampling Emissions of PCDD/PCDF from Open Burning of Biomass. Chemosphere 2012, 88, 352–357. [Google Scholar] [CrossRef]
- Guohua, H.; Lvye, W.; Yanhong, M.; Lingxia, Z. Study of Grass Carp (Ctenopharyngodon Idellus) Quality Predictive Model Based on Electronic Nose. Sens. Actuators B Chem. 2012, 166–167, 301–308. [Google Scholar] [CrossRef]
- Karunanithi, S.; Din, N.M.; Hakimie, H.; Hua, C.K.; Omar, R.C.; Yee, T.C. Performance of Labscale Solar Powered Wireless Landfill Monitoring System. In Proceedings of the 2009 3rd International Conference on Energy and Environment (ICEE), Malacca, Malaysia, 7–8 December 2009; pp. 443–448. [Google Scholar] [CrossRef]
- Shendell, D.G.; Therkorn, J.H.; Yamamoto, N.; Meng, Q.; Kelly, S.W.; Foster, C.A. Outdoor Near-Roadway, Community and Residential Pollen, Carbon Dioxide and Particulate Matter Measurements in the Urban Core of an Agricultural Region in Central CA. Atmos. Environ. 2012, 50, 103–111. [Google Scholar] [CrossRef]
- Blasing, T. Recent Greenhouse Gas Concentrations; Technical Report; U. S. Department of Energy, CDIAC: Washington, DC, USA, 2016.
- Dlugokencky, E. Trends in Atmospheric Methane; Technical Report; NOAA/ESRL: Boulder, CO, USA, 2016.
- Turner, A.J.; Jacob, D.J.; Benmergui, J.; Wofsy, S.C.; Maasakkers, J.D.; Butz, A.; Hasekamp, O.; Biraud, S.C. A Large Increase in U.S. Methane Emissions over the Past Decade Inferred from Satellite Data and Surface Observations. Geophys. Res. Lett. 2016, 43, 2218–2224. [Google Scholar] [CrossRef]
- Bamberger, I.; Stieger, J.; Buchmann, N.; Eugster, W. Spatial Variability of Methane: Attributing Atmospheric Concentrations to Emissions. Environ. Pollut. 2014, 190, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Dlugokencky, E.; Tans, P. Trends in Atmospheric Carbon Dioxide; Technical Report; NOAA/ESRL: Boulder, CO, USA, 2016.
- Wetchakun, K.; Samerjai, T.; Tamaekong, N.; Liewhiran, C.; Siriwong, C.; Kruefu, V.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Semiconducting Metal Oxides as Sensors for Environmentally Hazardous Gases. Sens. Actuators B Chem. 2011, 160, 580–591. [Google Scholar] [CrossRef]
- Neri, G. First Fifty Years of Chemoresistive Gas Sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Albert, K.J.; Lewis, N.S.; Schauer, C.L.; Sotzing, G.A.; Stitzel, S.E.; Vaid, T.P.; Walt, D.R. Cross-Reactive Chemical Sensor Arrays. Chem. Rev. 2000, 100, 2595–2626. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Prudenziati, M.; Morten, B. Thick-Film Sensors: An Overview. Sens. Actuators 1986, 10, 65–82. [Google Scholar] [CrossRef]
- Coblentz Society, Inc. Evaluated Infrared Reference Spectra. In NIST Chemistry WebBook; Lindstrom, P.J., Mallard, W.G., Eds.; Number 69 in NIST Standard Reference Database; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1996. [Google Scholar]
- Sekhar, P.K.; Kysar, J.; Brosha, E.L.; Kreller, C.R. Development and Testing of an Electrochemical Methane Sensor. Sens. Actuators B Chem. 2016, 228, 162–167. [Google Scholar] [CrossRef]
- Karpov, E.C.; Karpov, C.F.; Suchkov, C.; Mironov, S.; Baranov, A.; Sleptsov, V.; Calliari, L. Energy Efficient Planar Catalytic Sensor for Methane Measurement. Sens. Actuators A Phys. 2013, 194, 176–180. [Google Scholar] [CrossRef]
- Chiu, S.W.; Tang, K.T. Towards a Chemiresistive Sensor-Integrated Electronic Nose: A Review. Sensors 2013, 13, 14214–14247. [Google Scholar] [CrossRef]
- Eugster, W.; Kling, G.W. Performance of a Low-Cost Methane Sensor for Ambient Concentration Measurements in Preliminary Studies. Atmos. Meas. Tech. 2012, 5, 1925–1934. [Google Scholar] [CrossRef]
- Van den Bossche, M.; Rose, N.T.; Wekker, S.F.J.D. Potential of a Low-Cost Gas Sensor for Atmospheric Methane Monitoring. Sens. Actuators B Chem. 2017, 238, 501–509. [Google Scholar] [CrossRef]
- Williams, T.; Kelley, C. Gnuplot 5.0: An Interactive Plotting Program. Available online: http://www.gnuplot.info/ (accessed on 17 July 2019).
- Barsan, N.; Schweizer-Berberich, M.; Göpel, W. Fundamental and Practical Aspects in the Design of Nanoscaled SnO2 Gas Sensors: A Status Report. Fresenius’ J. Anal. Chem. 1999, 365, 287–304. [Google Scholar] [CrossRef]
- Ahlers, S.; Müller, G.; Doll, T. A Rate Equation Approach to the Gas Sensitivity of Thin Film Metal Oxide Materials. Sens. Actuators B Chem. 2005, 107, 587–599. [Google Scholar] [CrossRef]
- Long, G.L.; Winefordner, J.D. Limit of Detection A Closer Look at the IUPAC Definition. Anal. Chem. 1983, 55, 712A–724A. [Google Scholar] [CrossRef]
- Currie, L.A. Detection: International Update, and Some Emerging Di-Lemmas Involving Calibration, the Blank, and Multiple Detection Decisions. Chemom. Intell. Lab. Syst. 1997, 31, 151–181. [Google Scholar] [CrossRef]
- Mocak, J.; Bond, A.M.; Mitchell, S.; Scollary, G. A Statistical Overview of Standard (IUPAC and ACS) and New Procedures for Determining the Limits of Detection and Quantification: Application to Voltammetric and Stripping Techniques (Technical Report). Pure Appl. Chem. 2009, 69, 297. [Google Scholar] [CrossRef]
- Benkstein, K.D.; Rogers, P.H.; Montgomery, C.B.; Jin, C.; Raman, B.; Semancik, S. Analytical Capabilities of Chemiresistive Microsensor Arrays in a Simulated Martian Atmosphere. Sens. Actuators B Chem. 2014, 197, 280–291. [Google Scholar] [CrossRef]
- Martin, C.R.; Zeng, N.; Karion, A.; Dickerson, R.R.; Ren, X.; Turpie, B.N.; Weber, K.J. Evaluation and environmental correction of ambient CO2 measurements from a low-cost NDIR sensor. Atmos. Meas. Tech. 2017, 10, 2383–2395. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, Y.; Jiang, B. A One Ppm NDIR Methane Gas Sensor with Single Frequency Filter Denoising Algorithm. Sensors 2012, 12, 12729–12740. [Google Scholar] [CrossRef]
| Carbon Dioxide | Methane | Balance Gas |
|---|---|---|
| 3000 ppm | 3000 ppm | nitrogen |
| 100 ppm | 100 ppm | nitrogen |
| 0 ppm | 20 ppm | nitrogen |
| Sensor | (ppm) | RMSE | IUPAC | Corrected | |
|---|---|---|---|---|---|
| Carbon Dioxide | K-30 SE-0018 | 1.91 | 0.219 | 5.7 | 27.1 |
| COZIR AMB GC-020 | 14.1 | 0.304 | 42.3 | 80.6 | |
| Gascard CO2 | 2.12 | 0.223 | 6.4 | 32.1 | |
| MSH-DP/HC/CO2 | 86.4 | 0.197 | 260 | 254 | |
| MSH-P/CO2 | 17.6 | 0.217 | 52.8 | 68.0 | |
| Telaire T6615 | 4.42 | 0.185 | 13.3 | 31.1 | |
| CH4/Hydrocarbon | MQ-4 | 26.7 | 80.0 | 82.0 | |
| Gascard CH4 | 35.7 | 0.222 | 110 | 151 | |
| MSH-P/HC | 3.54 | 0.152 | 10.6 | 170 | |
| TGS-2600 | 36.1 | 0.225 | 110 | 117 | |
| TGS-2610 | 37.1 | 0.237 | 111 | 113 | |
| TGS-2611 | 5.4 | 0.208 | 16.3 | 16.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honeycutt, W.T.; Ley, M.T.; Materer, N.F. Precision and Limits of Detection for Selected Commercially Available, Low-Cost Carbon Dioxide and Methane Gas Sensors. Sensors 2019, 19, 3157. https://doi.org/10.3390/s19143157
Honeycutt WT, Ley MT, Materer NF. Precision and Limits of Detection for Selected Commercially Available, Low-Cost Carbon Dioxide and Methane Gas Sensors. Sensors. 2019; 19(14):3157. https://doi.org/10.3390/s19143157
Chicago/Turabian StyleHoneycutt, Wesley T., M. Tyler Ley, and Nicholas F. Materer. 2019. "Precision and Limits of Detection for Selected Commercially Available, Low-Cost Carbon Dioxide and Methane Gas Sensors" Sensors 19, no. 14: 3157. https://doi.org/10.3390/s19143157
APA StyleHoneycutt, W. T., Ley, M. T., & Materer, N. F. (2019). Precision and Limits of Detection for Selected Commercially Available, Low-Cost Carbon Dioxide and Methane Gas Sensors. Sensors, 19(14), 3157. https://doi.org/10.3390/s19143157