1. Introduction
To optimize nitrogen (N) fertilizer application, it is necessary to match the N supply to the N demand [
1,
2]. A potentially very effective approach would be the rapid and frequent on-farm assessment of crop N status that permits rapid adjustment of the N supply [
3,
4,
5]. Proximal optical sensors are a broad group of non-destructive monitoring tools that can be used to assess crop N status [
5,
6,
7]. One particularly promising group of proximal optical sensors are leaf chlorophyll meters.
Chlorophyll meters are relatively simple proximal optical sensors that indirectly assess relative leaf chlorophyll content by measuring the differential absorbance and transmittance of different radiation wavelengths by the leaf [
3,
7,
8]. Given that leaf chlorophyll content is usually related to crop N content [
6,
9,
10], these measurements can be used to assess crop N status [
3,
7]. Three commercially-available meters, with different characteristics, such as the wavelengths used, are the SPAD-502 meter (Konica-Minolta, Tokyo, Japan), atLEAF+ sensor (FT Green LLC, Wilmington, DE, USA), and MC-100 chlorophyll meter (Apogee Instruments, Inc., Logan, UT, USA) [
5,
7,
11]. The SPAD-502 measures absorbance at 650 nm (red) and 940 nm (NIR), the atLEAF+ at 660 nm and 940 nm, and the MC-100 at 653 nm and 931 nm. Using the two absorbance values, these three meters calculate a dimensionless numerical value, which is related to chlorophyll content [
11]. There are also differences in price between these meters; the atLEAF+ sensor is almost 10 times cheaper than the SPAD-502 and the MC-100 meters. The major practical advantages of chlorophyll meters as indicators of crop N status are that they are easy to use, do not require any particular training, and they make measurements very rapidly, with no or very little data processing [
3,
4,
7,
12].
Chlorophyll meter measurements do not directly indicate crop N status, so interpretation is required [
7]. Two broad approaches have been proposed to interpret chlorophyll meter measurements to assess crop N status. One approach is the use of so-called “reference plots” [
13,
14]. This approach divides the measured values of the crop by those from a well-fertilized reference plot that has no N limitation [
15]. This is considered to isolate the effect of relative N status from other confounding factors that are common to both areas [
16], which could greatly facilitate the adoption of chlorophyll meters on farms. However, this approach is considered to be impractical for commercial fertigated vegetable crops, given: (1) the additional cost of having separate fertigation sectors for reference plots, and (2) the implicit assumption of sensor saturation may not apply when luxury N uptake occurs, as has been reported for some vegetable species [
5,
11].
Another approach to interpret chlorophyll meter measurements, for the assessment of crop N status, is the use of absolute sufficiency values based on direct measurement. The sufficiency value is an absolute value, below which the crop is deficient and responds to additional N fertilizer [
3,
17], and above which yield is not affected [
3] and the immediate N supply may be excessive [
5]. Sufficiency values provide information on whether adjustments in N fertilization are required when absolute measurements deviate from sufficiency values [
18].
To determine sufficiency values, the nitrogen nutrition index (
NNI) can be used [
7,
14]. The
NNI is an effective and established indicator of crop N status [
19] that relates the actual crop N content to the critical crop N content (i.e., the minimum N content necessary to achieve maximum growth of a crop) [
20]. Values of
NNI = 1 correspond to optimal N nutrition [
19]. Sufficiency values of chlorophyll meter measurements are derived from the relationship between crop
NNI and chlorophyll meter measurements by solving the relationship for
NNI = 1 [
7,
17]. Chlorophyll meter sufficiency values have been determined for fresh tomato [
4,
21], and cucumber [
17,
22]. Sufficiency values are not available for most vegetable species, including important crops such as sweet pepper. Additionally, sufficiency values should be determined for specific agricultural systems and regions.
In Southeast (SE) Spain, the greenhouse-based intensive vegetable production system consists of approximately 40,000 ha of relatively simple plastic greenhouses, most of which are concentrated in the province of Almeria [
23,
24]. Nitrate (NO
3−) leaching from this system [
25] is associated with considerable aquifer NO
3− contamination [
26]. Frequent monitoring of these fertigated vegetable crops with chlorophyll meters is a promising approach to optimize crop N management, which would reduce N fertilizer use, thereby contributing to reduced aquifer NO
3− contamination. In Almeria, sweet pepper is either the most or second most important crop, depending on the year, occupying approximately 8000 ha each year [
27]. Globally, sweet pepper is grown on 1.9 million hectares [
28].
The objectives of the present work were: (i) to evaluate the sensitivity of three different chlorophyll meters to assess the crop N status of sweet pepper crops, and (ii) to calculate sufficiency values for each chlorophyll meter for maximum crop growth for four different phenological stages. This work was conducted in three different sweet pepper crops grown in different cropping years (2014–2015, 2016–2017, and 2017–2018) in a greenhouse. In each crop there was five different N treatments, ranging from very deficient to very excessive.
2. Materials and Methods
2.1. Experimental Site
Three sweet pepper (
Capsicum annuum, cultivar ‘Melchor’) crops were grown in soil in a plastic greenhouse, in conditions similar to commercial greenhouse vegetable production in SE Spain [
27]. The experimental work was conducted at the Experimental Station of the University of Almeria (36°51’N, 2°16’W and 92 m elevation). The greenhouse had polycarbonate walls and a roof of low-density polyethylene (LDPE) tri-laminated film (200 μm thickness), with transmittance to photosynthetically active radiation (PAR) of approximately 60%. It had no heating or artificial light, had passive ventilation (lateral side panels and flap roof windows), and an east–west orientation, with crop rows aligned north–south. The cropping area was 1300 m
2. The crops were grown in an “enarenado” soil, typical of those used for soil-grown greenhouse production in Almería [
25]. A more detailed description of the soil used is available in Padilla et al. [
29].
Above-ground drip irrigation was used for combined irrigation and mineral fertilizer application. Drip tape was arranged in paired lines, with 0.8 m spacing between lines within each pair of lines, 1.2 m spacing between adjacent pairs of lines, and 0.5 m spacing between drip emitters within drip lines, giving an emitter density of two emitters m
−2. The greenhouse was organized into 24 plots, measuring 6 m × 6 m; 20 plots were used in the current study. There were five N treatments with four replicate plots per treatment, arranged in a randomized block design. Each plot contained three paired lines of plants (six lines of plants in total), with 12 plants in each line, separated by a 0.5 m spacing. One plant was positioned 60 mm from and immediately adjacent to each dripper, giving a plant density of two plants m
−2 and 72 plants per replicate plot. Sheets of polyethylene film (250 µm thickness) buried up to 30 cm depth acted as a hydraulic barrier between plots [
30].
2.2. Experimental Design
The three sweet pepper crops were grown in different years. The first crop, in 2014–2015 (“the 2014 crop”) was transplanted on 12 August 2014 and ended on 29 January 2015 (cropping period of 170 days). The second crop, “the 2016 crop”, was transplanted on 19 July 2016 and ended on 24 March 2017 (cropping period of 248 days). The third crop, “the 2017 crop”, was transplanted on 21 July 2017 and ended on 20 February 2018 (cropping period of 214 days). The three crops were transplanted as 35-day old seedlings using the same cultivar.
In each crop, there were five treatments of different N concentration in the nutrient solution, applied by fertigation throughout the crops. In the 2014 crop, the N treatments commenced one day after transplanting (DAT), in the 2016 crop at nine DAT, and in the 2017 crop at 10 DAT. Plants were irrigated with water only (<0.04 mmol N L
−1) prior to commencing the N treatments. The N treatments were applied in every irrigation until the end of the crops. In each crop, the N treatments were very deficient (N1), deficient (N2), conventional (N3), excessive (N4), and very excessive (N5). The average mineral N (NO
3−–N + NH
4+–N) concentrations (mmol L
−1), applied in the nutrient solution, and the amounts (kg ha
−1) of N applied in each N treatment in each crop are presented in
Table 1. For all treatments, N was applied mostly as nitrate (NO
3−), the rest as ammonium (NH
4+); on average 88% of the N was applied as NO
3−. All other nutrients were applied in the nutrient solution to ensure they were not limiting.
The crops were managed following local practice. The crops were physically supported using a system of nylon cords placed horizontally along the side of the crop. Irrigation was scheduled to maintain soil matric potential (SMP) in the root zone, at 12 cm deep, within −15 to −25 kPa; one tensiometer (Irrometer, Co., Riverside, CA, USA) per plot was used to measure SMP. High temperature within the greenhouse was controlled by white-washing the plastic cladding with CaCO3 suspension.
2.3. Chlorophyll Meter Measurements
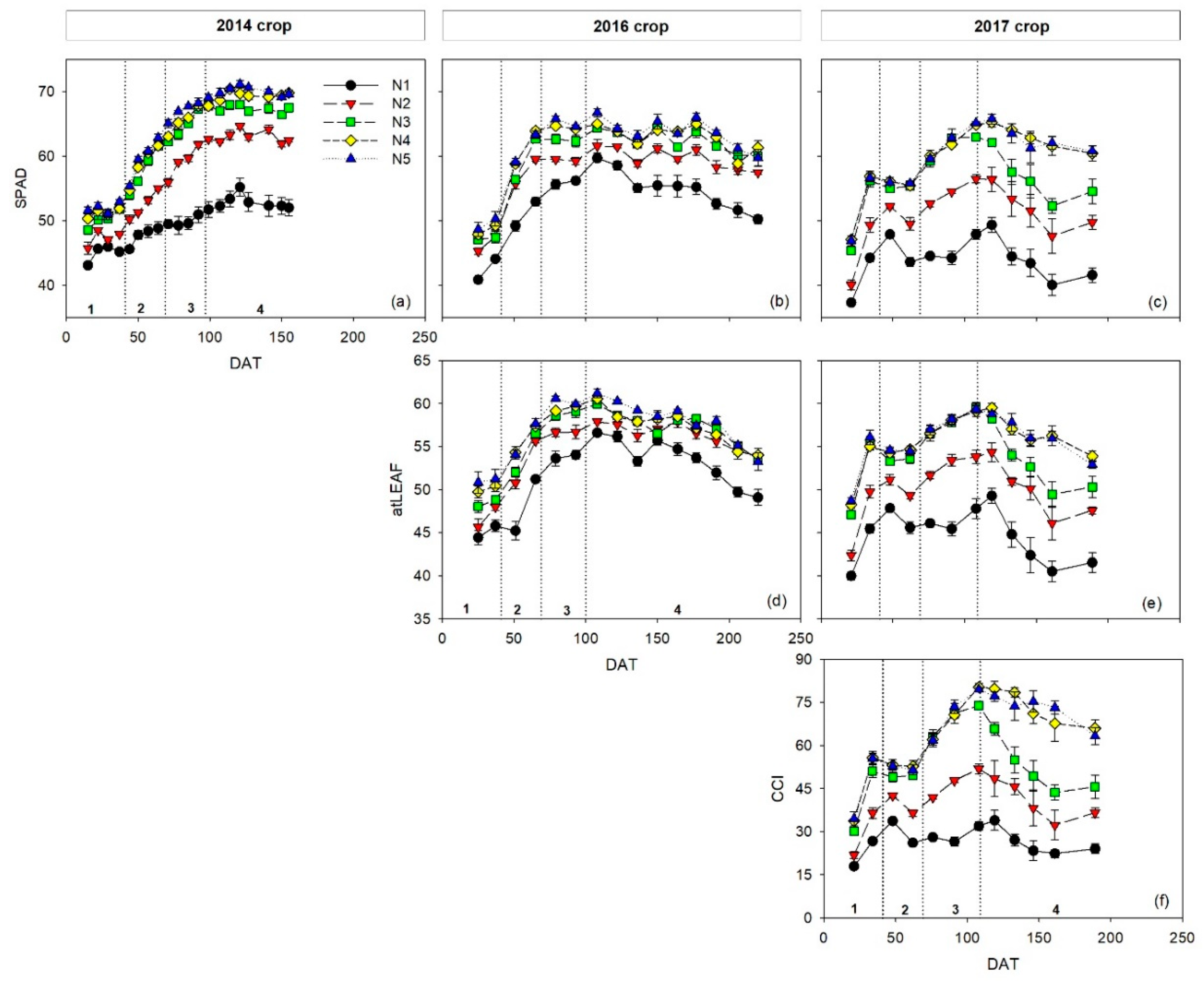
Chlorophyll meter measurements commenced on 27 (15 DAT), 18 (25 DAT), and 11 (21 DAT) August for the 2014, 2016, and 2017 crops, respectively. In the 2014 crop, measurements were made every seven days, and in the 2016 and 2017 crops every 14 days. In the three crops, measurements were made until the end of the crop. Three different leaf-clip chlorophyll meters were used, the SPAD-502 meter (Konica Minolta, Inc., Tokyo, Japan), the atLEAF+ meter (FT Green LLC, Wilmington, DE, USA), and the MC-100 Chlorophyll Concentration Meter (Apogee Instruments, Inc., Logan, UT, USA). The respective measurement values are SPAD units, atLEAF units, and chlorophyll content index (CCI). The SPAD-502 meter was used in each of the three crops (2014, 2016, and 2017). The atLEAF+ meter was used in the 2016 and 2017 crops. The MC-100 meter was used only in the 2017 crop. The areas measured in each measurement were 6 mm2 for the SPAD-502, 13 mm2 for the atLEAF+, and 63.6 mm2 for the MC-100.
Measurements were made on one leaf of each of the 16 marked plants in each replicate plot. The value for each replicate plot was the mean of the 16 individual measurements. They were made at the same time of day (8:00 to 10:00 solar time), before irrigation/fertigation. Measurements were made on each plant on the most recently fully expanded and well-lit leaf, on the distal part of the adaxial side of the leaf, midway between the margin and the mid-rib of the leaf. Measurement was made by clipping the sensor onto the leaf. Leaves with physical damage or with condensed water were not measured, alternative plants being selected.
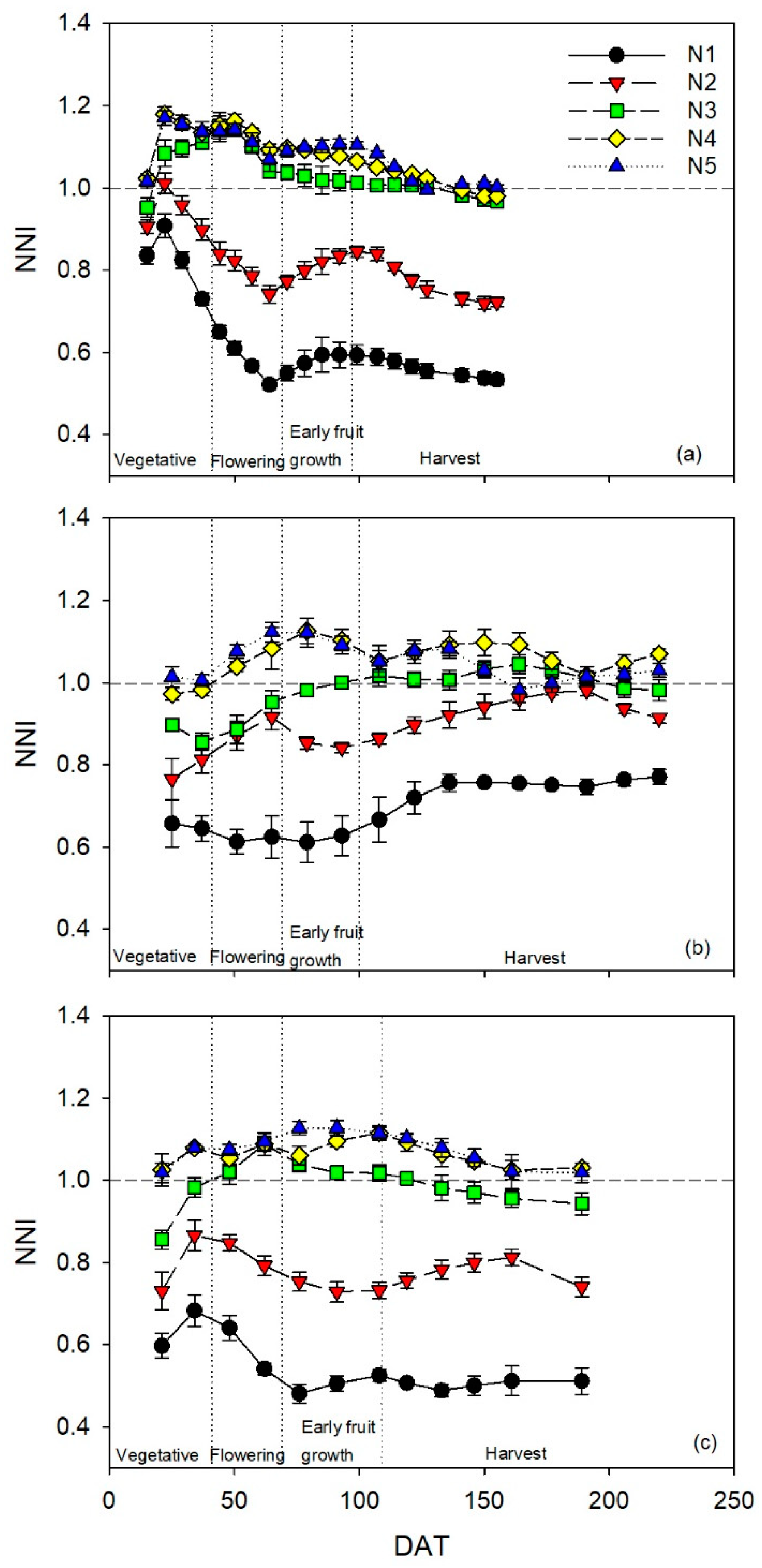
2.4. Determination of Crop Nitrogen Nutrition Index
The critical N curve derived for greenhouse-grown sweet pepper, Nc = 4.488·DMP−0.196 (A. Rodríguez and R.B. Thompson, University of Almeria, personal communication), where DMP is dry matter production, was used to calculate the nitrogen nutrition index (NNI) as a measure of crop N status.
The
NNI was calculated as:
where
Nact is the measured N content of the crop and
Nc is the critical N content obtained from the critical N curve for each treatment for each biomass sampling date.
NNI values for each day of chlorophyll meter measurement were calculated by interpolating DMP and crop N content values between the two biomass samplings on either side of the measurement date. Above-ground dry matter production during the crop was measured by periodic biomass sampling (approximately every 14 days) by removing two complete plants in each replicate plot. All fresh material of each biomass component (stem, leaf, and fruit) was weighed, and the dry matter contents determined by oven-drying representative sub-samples at 65 °C until a constant weight was reached. Fruit production and pruning was determined throughout the crop, in eight selected plants in each replicate plot. Representative samples of leaves, stems, and fruit from each biomass sampling, from each replicate plot, were ground sequentially in knife and ball mills. The total N content (%N) of each sample was determined using a Dumas-type elemental analyzer system (model Rapid N, Elementar, Analysensysteme GmbH, Hanau, Germany). The mass of N in each relevant component was calculated from the %N and the corresponding mass of dry matter. Total crop N uptake in each replicate plot, at each biomass sampling, was the sum of N in all relevant components. Crop N content (%N) for each biomass sampling was calculated, for each replicate plot, as crop N uptake divided by DMP.
2.5. Data Analysis
To account for differences in planting dates that occur in vegetable crops, and to facilitate the use and interpretation of chlorophyll meters in practical farming, measurements and analyses were based on phenological stage rather than on days after transplanting. Because of the frequent measurements with chlorophyll meters during the pepper cycle, there were several dates of measurements within each phenological stage. To integrate the various dates of measurement to provide a unique value for each phenological stage, integrated values of each chlorophyll meter measurement (
SPADi,
atLEAFi, and
CCIi) and for the crop nitrogen nutrition index (
NNIi), were calculated for each phenological stage. These integrated values were calculated as:
where
D was the total number of days of each phenological stage,
V was the value measured at each measurement date, and ds was the interval between two successive measurement dates (values of each measurement date were pondered by the time elapsed between two consecutive measurements). Four major phenological stages were considered: (i) vegetative, (ii) flowering, (iii) early fruit growth, and (iv) harvest. The vegetative stage was from transplanting to the beginning of flowering. The flowering stage was from the beginning of flowering until fruit set. Early fruit growth was from fruit set until fruit maturation. The harvest stage commenced with the first fruit harvest and ended when the crop finished, this was the longest of the four phenological stages.
To evaluate the sensitivity of
SPADi,
atLEAFi, and
CCIi, to estimate crop
NNIi, regression analyses were performed for each phenological stage. Four types of regression equations (linear, quadratic, power, and exponential) were considered, and the best equation was selected using the Akaike information criterion [
31], which represents the best compromise between the goodness of fit and the complexity of a model. These regression analyses were performed for each phenological stage in each crop, and for each crop in its entirety. Additionally, where there was more than one crop in which a particular chlorophyll meter was used (SPAD, atLEAF+), these regression analyses were conducted on: (a) pooled data for each phenological stage from the different crops, and (b) composite whole crop data from the different crops. The CurveExpert Professional® 2.2.0 software (Daniel G. Hyams) was used for these regression analyses.
Sufficiency values of chlorophyll meter measurements for maximum crop growth were derived for each phenological stage from the relationship between integrated chlorophyll meter measurements and
NNIi. The approach of Padilla et al. [
4] was used, in which the Akaike Information Criterion (AIC) best-fit equations that related chlorophyll meter measurements to
NNI were solved for
NNI = 1, which is the value of
NNI that represents the optimal N nutrition for maximum growth. Sufficiency values of chlorophyll meter measurements were calculated for: (a) each phenological stage for each crop considered separately, (b) each whole crop, (c) each phenological stage for multiple crops, and (d) the whole crop using data from multiple crops.
4. Discussion
Integrated measurements of the three chlorophyll meters (SPAD-502, atLEAF+, and MC-100) were very strongly related to integrated
NNI for: (a) each of the four phenological stages (vegetative, flowering, early fruit growth, and harvest) of each pepper crop, (b) each crop considered in its entirety, (c) individual phenological stage, using composite data for all crops in which measurements were made, and (d) single values for the entirety of the crop, for the crops in which measurements were made. These results demonstrate that the three chlorophyll meters provided good estimations of the crop N status of sweet pepper. This is in agreement with studies that reported strong relationships between chlorophyll meter measurements and crop N status, in various horticultural [
4,
17,
32] and cereal crops [
33,
34,
35].
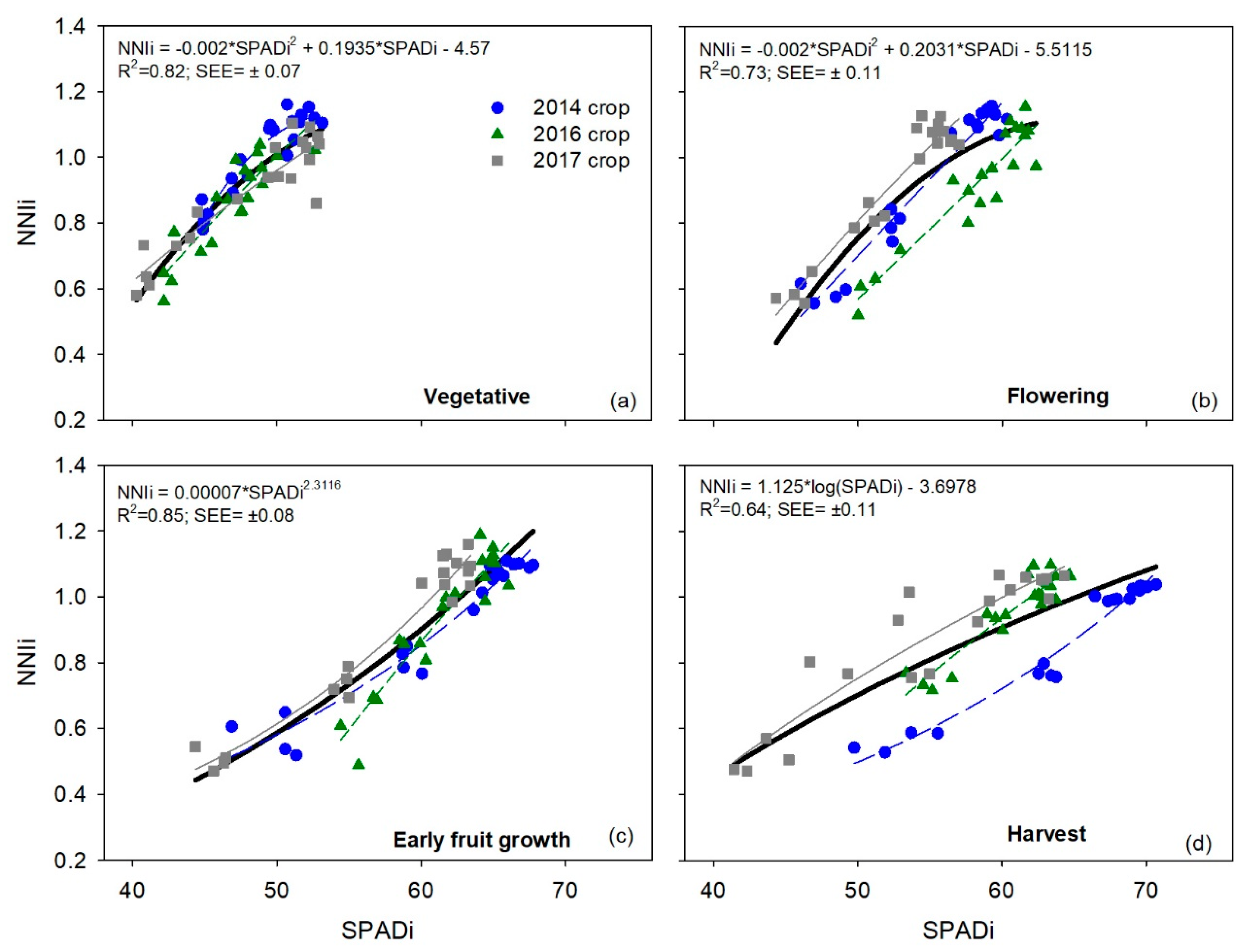
Considering the four individual phenological stages, the strongest relationships between the three integrated chlorophyll meter measurements and
NNIi were obtained in the flowering and early fruit growth stages, which occurred in the middle of the crops, for individual crops, and for when data was combined from multiple crops. Similarly, the strongest relationship between chlorophyll meter measurements and leaf N concentration occurred in the middle of the growing season in potatoes [
3]. In the present study, there were also strong relationships at the beginning (in the vegetative stage) and at the end of the crop (in the harvest stage), but with slightly lower R
2 values than in the flowering and early fruit growth stages. The strong relationship in the vegetative stage of sweet pepper, in this study, contrasts with the results for cucumber in a previous study, where there was a weak relationship between SPAD measurements and
NNI in the vegetative stage [
17], which was attributed to limited differentiation of the N treatments at the beginning of that crop [
17]. In the current study, the high R
2 values between the integrated chlorophyll meters measurements and
NNIi in each of the four phenological stages, regardless of year and chlorophyll meter, demonstrated the robust ability of chlorophyll meter measurements to be used as indicators of the crop N status of sweet pepper.
For the three chlorophyll meters, there was no evidence of saturation when relating measurements to
NNIi in any of the four phenological stages and in the different crops. Regression analysis showed that
SPADi,
atLEAFi, and
CCIi values increased when
NNIi values exceeded the optimal value for the crop growth of one. Saturation of SPAD-502 and atLEAF+ measurements at high chlorophyll contents, which are associated with high crop N contents has been often reported [
11,
33,
36]. However, saturation of chlorophyll meter measurements does not always occur at higher crop N contents [
3,
4], as it depends on whether leaf chlorophyll contents are sufficiently high to cause saturation [
11]. None of the three chlorophyll meters evaluated were able to differentiate between the N4 and N5 treatments. This was not due to a saturation response of the chlorophyll meters, but rather was due to the similar crop N status of these two treatments, as indicated by the very similar
NNIi values. The
NNIi values of treatments N4 and N5 were not significantly different for any of the three crops.
Regarding the calculation of sufficiency values of the SPAD-502 meter, there were only small differences in sufficiency values for each phenological stage between the three different crops. Similarly, there were only small differences between sufficiency values for individual crops and the corresponding sufficiency value for the combined crop data set, for a given phenological stage. These data indicate that the sufficiency values determined for each phenological stage were very consistent between the three different years, and that SPAD sufficiency values obtained with the combined dataset are representative of the three crops.
The relative constancy of SPAD sufficiency values over time can be assessed by comparing the sufficiency values of the different phenological stages, using the combined data of the three crops. The relative difference between the sufficiency values of the vegetative and flowering stages was 12.2%, between flowering and early fruit growth was 9.7%, and between the early fruit growth and harvest stages was 3.8%. The differences between the sufficiency values for successive stages diminished as the crop grew. This was attributed to the temporal dynamics of chlorophyll meter values (
Figure 2) because SPAD measurements increased in the early part of the crops and reached relatively stable values midway through the crops. There was a large difference in SPAD sufficiency values between the harvest stage (last phenological stage of the crop) and the vegetative stage (first phenological stage of the crop) of 15.5 SPAD units, the relative difference being 23.4%. This large difference during the crop suggests that a single SPAD sufficiency value cannot be used for a whole sweet pepper crop. In contrast, single SPAD sufficiency values for a whole crop have been proposed for cucumber [
17,
22] and grapevine [
37].
The SPAD sufficiency values derived for sweet pepper, in the present study, are generally higher than reported for other horticultural crops; the highest sufficiency value obtained in the present work was 64.0 ± 1.3 SPAD units. In indeterminate tomato, the average sufficiency value for the complete crop cycle was 54.2 SPAD units [
21]. In cucumber, sufficiency values have been recommended for the whole crop of 45.2 SPAD units [
17] and 44.9 SPAD units [
22]. In potato, a whole crop sufficiency value of 38.2 SPAD units was recommended [
38]. The appreciably higher sufficiency values for the SPAD-502 meter for sweet pepper in the present work can be explained by the very high leaf chlorophyll content of sweet pepper [
11]. In a study with 22 common crop species, sweet pepper had the highest leaf chlorophyll concentration, which was double that of maize [
39].
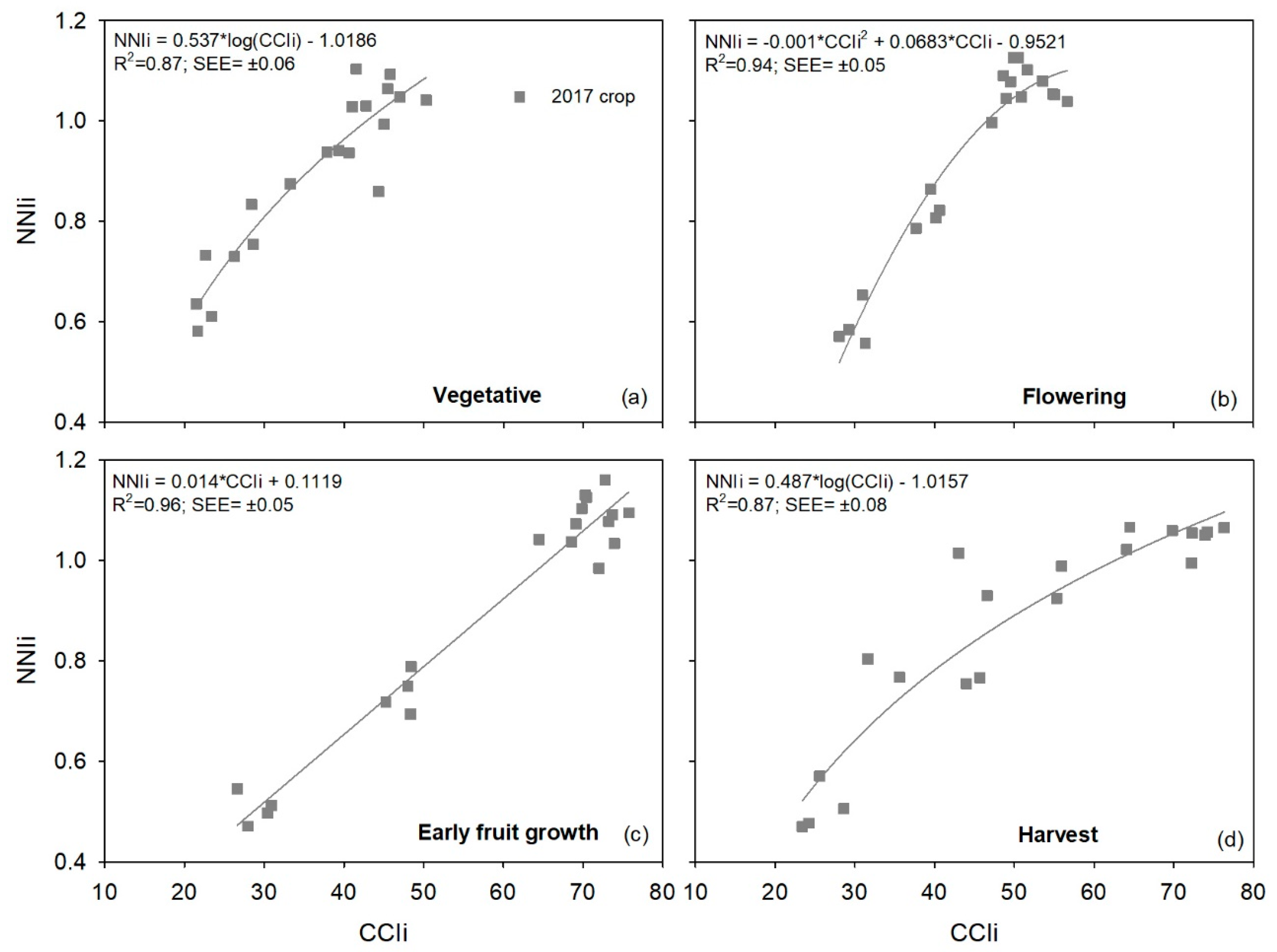
The performance of the atLEAF+ and MC-100 meters was similar to the SPAD-502 meter in terms of sufficiency values. With both the atLEAF+ and MC-100 meters, the lowest sufficiency values were in the vegetative stage, and the highest in the early fruit growth and harvest stages. As with the SPAD, these temporal variations were associated with the dynamics of chlorophyll meter measurements throughout the crop, which initially increased and then were relatively constant in the second half of the crop. For the atLEAF+ meter, there were only small differences in the sufficiency value, for each of the four phenological stages, between the 2016 and 2017 crops. This indicates that the atLEAF sufficiency values determined were consistent between the two crops. It also demonstrates that the sufficiency values calculated using the combined data set of the two crops are representative of both crops. For the MC-100 meter, this is one of the first studies to provide sufficiency values of CCI. With this chlorophyll meter in the current study, there was only one crop; so, it was not possible to assess the consistency of sufficiency values between crops. The relative differences in sufficiency values between each of the four phenological stages, for the atLEAF+ sensor and the MC-100 meter, were calculated to assess the consistency of sufficiency values for each chlorophyll meter over time. The atLEAF+ meter had the narrowest range in sufficiency values, with the difference between the early fruit growth stage (maximum sufficiency value) and the vegetative stage (minimum sufficiency value) being 11.2%. The largest range of sufficiency values was with the MC-100 meter, where the relative difference between the maximum sufficiency value (early fruit growth stage) and the minimum sufficiency value (vegetative stage) was 34.0%.
Following the evaluation and the derivation of sufficiency values, chlorophyll meters could be used to frequently assess the crop N status of fertigated pepper crops that frequently receive N by regular drip irrigation. In greenhouses in SE Spain, N and other nutrients are applied every one to four days in each irrigation. Sufficiency values are required for practical real time monitoring of crop N status, using chlorophyll meters. Frequent effective monitoring of crop N status will enable rapid correction of crop N status by adjusting mineral N fertilizer application when chlorophyll meter measurements deviate from sufficiency values [
4], thereby ensuring optimal N nutrition. This will also reduce excessive “insurance” N applications that are applied to avoid the risk of N deficiency. In crops grown with fertigation systems, where N is applied in every irrigation, adjustment in N fertilization can be made very soon after such deviations are detected [
5]. The results obtained in this study may be applied to sweet pepper crops grown in greenhouses; for sweet pepper crops grown outdoors, further research is required to validate these sufficiency values.
Overall, the results of this study show the potential of chlorophyll meters for monitoring crop N status and to assist with N fertilizer management of sweet pepper. The strong relationship between integrated chlorophyll meter measurements and NNIi for each phenological stage of each crop, when considered separately and as a combined dataset from different crops, demonstrated the consistency and robustness of chlorophyll meter measurements as indicators of crop N status. The sufficiency values calculated for chlorophyll meter measurements in each phenological stage and their consistency throughout crops showed the potential for the sufficiency values to be used in commercial farming to achieve improved N management of sweet pepper crops.