Single-Walled Carbon Nanotubes (SWCNTs) as Solid-Contact in All-Solid-State Perchlorate ISEs: Applications to Fireworks and Propellants Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus
2.3. Preparation Procedure of SC-ISEs
2.4. Sensors Calibration and ClO4− Determination
3. Results and Discussions
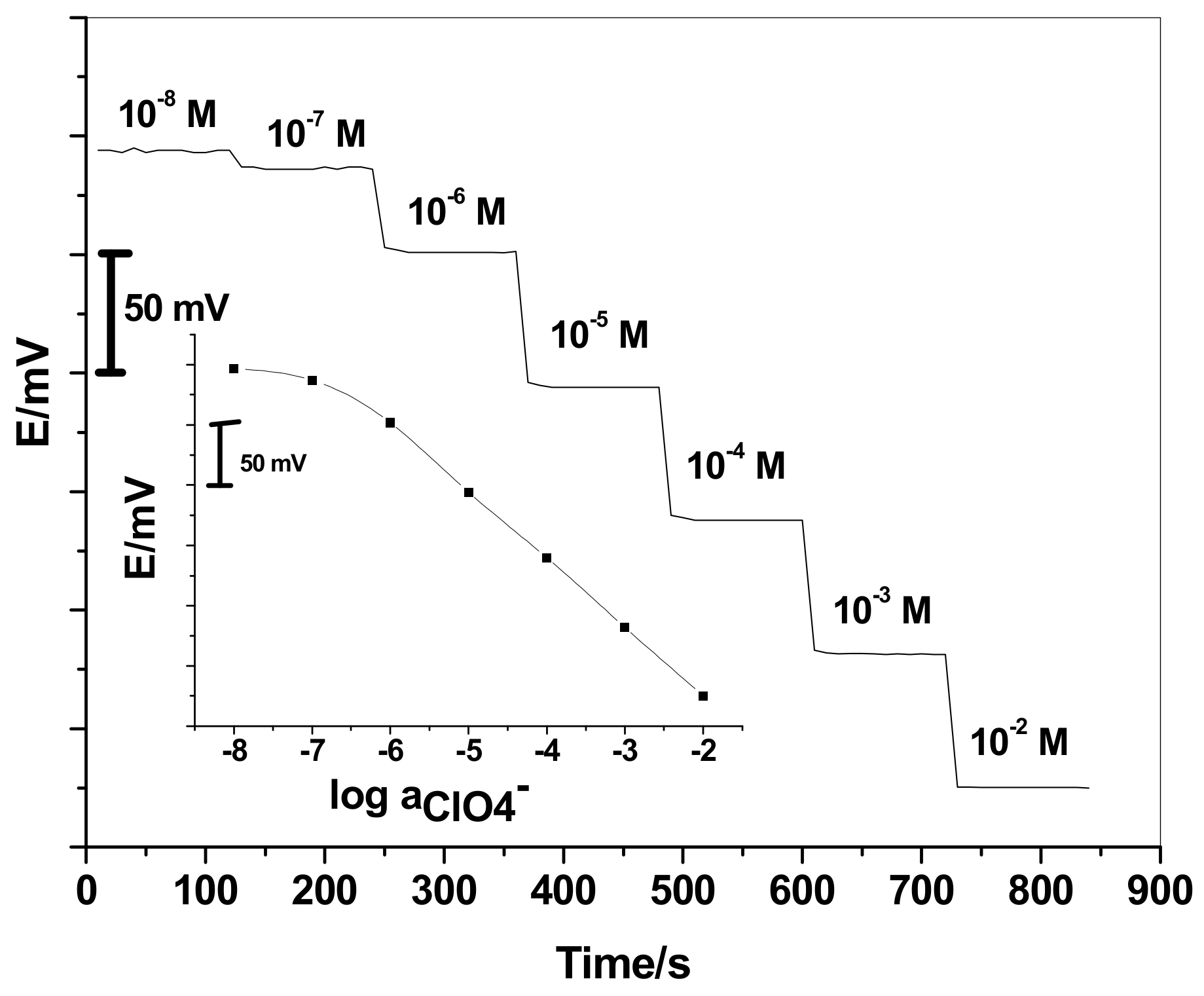
3.1. Performance Characteristics of All Solid-Contact Perchlorate ISEs
3.2. Interfering Ions Effect
3.3. Short-Term Potential Stability
3.4. Impedance Measurements
3.5. Determination of ClO4− in Commercial Fireworks Formulations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Team, P. Perchlorate: Overview of Issues, Status, and Remedial Options; Interstate Technology & Regulatory Council: Washinton, DC, USA, 2005. [Google Scholar]
- Urbansky, E.T. Perchlorate as an environmental contaminant. Environ. Sci. Pollut. Res. Int. 2002, 9, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Thorne, P.G. Field Screening Method for Perchlorate in Water and Soil; US Army Corps of Engineers, NTIS: Springfield, VA, USA, 2004.
- Goncharuk, V.V.; Zui, O.V.; Kushchevskaya, N.F. Methods of determining perchlorates. J. Water Chem. Technol. 2009, 31, 186–194. [Google Scholar] [CrossRef]
- Urbansky, E.T. Quantitation of perchlorate ion: Practices and advances applied to the analysis of common matrices. Crit. Rev. Anal. Chem. 2000, 30, 311–343. [Google Scholar] [CrossRef]
- Baczuk, R.J.; Bolleter, W.T. Conductometric titration of perchlorate with tetraphenylarsonium chloride. Anal. Chem. 1967, 39, 93–95. [Google Scholar] [CrossRef]
- Vogel, A.I. Text Book of Quantitative Inorganic Analysis, 4th ed.; Longman: London, UK, 1978. [Google Scholar]
- Weiss, J.A.; Stanbury, J.B. Spectrophotometric determination of microamounts of perchlorate in biological fluids. Anal. Chem. 1972, 44, 619–620. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.T.; Chimpalee, N.; Harrott, M. Flow-injection extraction-spectrophotometric determination of perchlorate with brilliant green. Anal. Chim. Acta 1989, 217, 177–181. [Google Scholar] [CrossRef]
- Gallego, M.; Valcarcel, M. Indirect atomic absorption spectrometric determination of perchlorate by liquid-liquid extraction in a flow-injection system. Anal. Chim. Acta 1985, 169, 161–169. [Google Scholar] [CrossRef]
- Chattaraj, S.; De, K.; Das, A.K. Indirect determination of perchlorate by atomic absorption spectrometry. Mikrochim. Acta 1992, 106, 183–190. [Google Scholar] [CrossRef]
- Narayanan, L.; Buttler, G.W.; Yu, K.O.; Mattie, D.R.; Fisher, J.W. Sensitive high-performance liquid chromatography method for the determination of low levels of perchlorate in biological samples. J. Chromatogr. B 2003, 788, 393–399. [Google Scholar] [CrossRef]
- Lamb, J.D.; Simpson, D.; Jensen, B.D.; Gardner, J.S.; Peterson, Q.P. Determination of perchlorate in drinking water by ion chromatography using macrocycle-based concentration and separation methods. J. Chromatogr. A 2006, 1118, 100–105. [Google Scholar] [CrossRef]
- Urbansky, E.T.; Magnuson, M.L.; Freeman, D.; Jelks, C. Quantitation of perchlorate ion by electrospray ionization mass spectrometry (ESI-MS) using stable association complexes with organic cations and bases to enhance selectivity. J. Anal. At. Spectrom. 1999, 14, 1861–1866. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Elnemma, E.M.; Mohamed, A.H.K. Novel Biomedical Sensors for Flow Injection Potentiometric Determination of Creatinine in Human Serum. Electroanalysis 2005, 17, 2246–2253. [Google Scholar] [CrossRef]
- Kamel, A.H. Conventional and miniaturized planar chip sensors for potentiometric assay of uric acid in biological fluids using flow injection analysis. J. Pharm. Biomed. Anal. 2007, 45, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.M.; Sayour, H.E.M.; Kamel, A.H. A simple potentiometric method for determination of acid and alkaline phosphatase enzymes in biological fluids and dairy products using a nitrophenylphosphate plastic membrane sensor. Anal. Chim. Acta 2009, 640, 75–81. [Google Scholar] [CrossRef]
- Yan, R.; Qiu, S.; Tong, L.; Qian, Y. Review of progresses on clinical applications of ion selective electrodes for electrolytic ion tests: From conventional ISEs to graphene-based SEs (Review). Chem. Spec. Bioavail. 2016, 28, 72–77. [Google Scholar] [CrossRef]
- Dimeski, G.; Badrick, T.; John, A.S. Ion Selective Electrodes (ISEs) and interferences-A review (Review). Clin. Chim. Acta 2010, 411, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.M.; Marzouk, S.A.M.; Mohamed, A.H.K.; Badawy, N.M. Novel dicyanoargentate polymeric membrane sensors for selective determination of cyanide ions. Electroanalysis 2004, 16, 298–303. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Mahmoud, W.H.; Mohamed, A.H.K.; Kelany, A.E. Mercury(II) Ion-Selective Polymeric Membrane Sensors for Analysis of Mercury in Hazardous Wastes. Anal. Sci. 2006, 22, 877–881. [Google Scholar] [CrossRef]
- Kamel, A.H.; Galal, H.R.; Awaad, N.S. Cost-effective and handmade paper-based potentiometric sensing platform for piperidine determination. Anal. Methods 2018, 10, 5406–5415. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Badr, I.H.A.; Kamel, A.H.; Mohamed, M.S. A Novel Poly (Vinyl Chloride) Matrix Membrane Sensor for Batch and Flow-injection Determination of Thiocyanate, Cyanide and Some Metal Ions. Anal. Sci. 2009, 25, 911–917. [Google Scholar] [CrossRef]
- Cuartero, M.; Crespo, G.A. All-solid-state potentiometric sensors: A new wave for in situ aquatic research (Review). Curr. Opin. Electrochem. 2018, 10, 98–106. [Google Scholar] [CrossRef]
- Sak-Bosnar, M.; Madunić-Čačić, D.; Grabarić, Z.; Grabarić, B. Potentiometric Determination of Anionic and Nonionic Surfactants in Surface Waters and Wastewaters. Handb. Environ. Chem. 2015, 31, 157–176. [Google Scholar]
- Moreira, F.T.C.; Guerreiro, J.R.L.; Azevedo, V.L.; Kamel, A.H.; Sales, M.G.F. New biomimetic sensors for the determination of tetracycline in biological samples: Batch and flow mode operations. Anal. Methods 2010, 2, 2039–2045. [Google Scholar] [CrossRef][Green Version]
- Hassan, S.S.M.; Kamel, A.H.; Abd El-Naby, H. New Potentiometric Sensors Based on Selective Recognition Sites for Determination of Ephedrine in Some Pharmaceuticals and Biological Fluids. Talanta 2013, 103, 330–336. [Google Scholar] [CrossRef] [PubMed]
- El-Naby, E.H.; Kamel, A.H. Potential transducers based man-tailored biomimetic sensors for selective recognition of dextromethorphan as an antitussive drug. Mater. Sci. Eng. C 2015, 54, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Lenik, J. Cyclodextrins based electrochemical sensors for biomedical and pharmaceutical analysis-review. Curr. Med. Chem. 2017, 24, 2359–2391. [Google Scholar] [CrossRef] [PubMed]
- Lenik, J. Application of PVC in Constructions of Ion Selective Electrodes for Pharmaceutical Analysis. In Handbook of Polymers for Pharmaceutical Technologies; Processing and Applications; Thakur, V.K., Thakur, M.K., Eds.; Wiley Scrivener Publishing: Hoboken, NJ, USA, 2015; Volume 2, pp. 195–227. [Google Scholar]
- de Souza Gil, E.; de Melo, G.R. Electrochemical biosensors in pharmaceutical analysis. Braz. J. Pharm. Sci. 2010, 6, 376–391. [Google Scholar]
- Lindner, E.; Gyurcsanyi, R.E. Quality control criteria for solid-contact, solvent polymeric membrane ion-selective electrodes. J. Solid State Electrochem. 2009, 13, 51–68. [Google Scholar] [CrossRef]
- Hu, J.; Stein, A.; Bühlmann, P. Rational design of all-solid-state ion-selective electrodes and reference electrodes. Trends Anal. Chem. 2016, 76, 102–114. [Google Scholar] [CrossRef]
- Ashmawy, N.H.; Almehizia, A.A.; Youssef, T.A.; Amr, A.E.; Al-Omar, M.A.; Kamel, A.H. Novel Carbon/PEDOT/PSS-Based Screen-Printed Biosensors for Acetylcholine Neurotransmitter and Acetylcholinesterase Detection in Human Serum. Molecules 2019, 24, 1539. [Google Scholar] [CrossRef]
- Ishibashi, N.; Kohara, H. Perchlorate ion-selective electrodes with the liquid membranes of the o-phenanthroline chelate or its related compounds. Anal. Lett. 1971, 4, 785–792. [Google Scholar] [CrossRef]
- Rohm, T.J.; Guilbault, G.G. New methods for the preparation of perchlorate ion-selective electrodes. Anal. Chem. 1974, 46, 590–592. [Google Scholar] [CrossRef]
- Wilson, A.C.; Pool, K.H. An improved ion-selective electrode for perchlorate. Talanta 1976, 23, 387–388. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Elsaied, M.M. A new liquid-membrane electrode for selective determination of perchlorate. Talanta 1986, 33, 679–684. [Google Scholar] [CrossRef]
- Jain, K.; Jahan, M.; Tyagi, V. Construction and assessment of some perchlorate-selective liquid membrane electrodes. Anal. Chim. Acta 1990, 231, 69–75. [Google Scholar] [CrossRef]
- Coetzee, C.J.; Freiser, H. Liquid-liquid membrane electrodes based on ion-association extraction systems. Anal. Chem. 1969, 41, 1128–1130. [Google Scholar] [CrossRef]
- Back, S. Selectivity studies on anion-selective membrane electrodes. Anal. Chem. 1972, 44, 1696–1698. [Google Scholar] [CrossRef]
- Fogg, A.G.; Pathan, A.S.; Burns, D.T. A liquid-state perchlorate ion-selective electrode based on brilliant green perchlorate. Anal. Chim. Acta 1974, 73, 220–223. [Google Scholar] [CrossRef]
- Kataoka, M.; Kambara, T. A liquid membrane type perchlorate ion-selective electrode. J. Electroanal. Chem. 1976, 73, 279–284. [Google Scholar] [CrossRef]
- Sanchez-Pedreno, C.; Ortuno, J.A.; Hernandez, J. Perchlorate-selective polymeric membrane electrode based on a gold (I) complex: Application to water and urine analysis. Anal. Chim. Acta 2000, 415, 159–164. [Google Scholar] [CrossRef]
- Almeer, S.H.M.A.; Zogby, I.A.; Hassan, S.S.M. Novel miniaturized sensors for potentiometric batch and flow-injection analysis (FIA) of perchlorate in fireworks and propellants. Talanta 2014, 129, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Gholamian, F.; Sheikh-Mohseni, M.A.; Salavati-Niasari, M. Highly selective determination of perchlorate by a novel potentiometric sensor based on a synthesized complex of copper. Mater. Sci. Eng. C 2011, 31, 1688–1691. [Google Scholar] [CrossRef]
- Rezaei, B.; Meghdadi, S.; Bagherpour, S. Perchlorate-selective polymeric membrane electrode based on bis (dibenzoylmethanato) cobalt (II) complex as a neutral carrier. J. Hazard. Mater. 2009, 161, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Segui, M.J.; Lizondo-Sabater, J.; Martinez-Manez, R.; Sancenon, F.; Soto, J.; Garcia-Breijo, E.; Gil, I. An ion-selective electrode for anion perchlorate in thick-film technology. Sensors 2006, 6, 480–491. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Yousefi, M.; Poursaberi, T.; Naji, L.; Salavati-Niasari, M.; Shamsipur, M. Highly Selective and Sensitive Perchlorate Sensors Based on Some Recently Synthesized Ni (II)-Hexaazacyclotetradecane Complexes. Electroanalysis 2003, 15, 1476–1480. [Google Scholar] [CrossRef]
- Shamsipur, M.; Soleymanpour, A.; Akhond, M.; Sharghi, H.; Hasaninejad, A.R. Perchlorate selective membrane electrodes based on a phosphorus (V)–tetraphenylporphyrin complex. Sens. Actuators B 2003, 89, 9–14. [Google Scholar] [CrossRef]
- Lizondo-Sabater, J.; Segui, M.J.; Lioris, J.M.; Martinez-Manez, R.; Pardo, T.; Sancenon, F.; Soto, J. New membrane perchlorate-selective electrodes containing polyazacycloalkanes as carriers. Sens. Actuators B 2004, 101, 20–27. [Google Scholar] [CrossRef]
- Zanjanchi, M.A.; Arvand, M.; Akbari, M.; Tabatabaeian, K.; Zaraei, G. Perchlorate-selective polymeric membrane electrode based on a cobaloxime as a suitable carrier. Sens. Actuators B 2006, 113, 304–309. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Badri, A. Application of surfactant modified zeolite membrane electrode towards potentiometric determination of perchlorate. J. Electroanal. Chem. 2011, 660, 71–79. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Badri, A. Perchloate selective membrane electrode based on surfactant-modified zeolite Y nanocluster. Anal. Bioanal. Electrochem. 2011, 3, 565–586. [Google Scholar]
- Bakker, E. Determination of improved selectivity coefficients of polymer membrane ion-selective electrodes by conditioning with a discriminated ion. J. Electrochem. Soc. 1996, 143, L83–L85. [Google Scholar] [CrossRef]
- Kamaata, S.; Bhale, A.; Fukunaga, Y.; Murata, H. Copper(II)-selective electrode using thiuram disulfide neutral carriers. Anal. Chem. 1988, 60, 2464–2467. [Google Scholar]
- Liang, R.; Yin, T.; Qin, W. A simple approach for fabricating solid-contact ion-selective electrodes using nanomaterials as transducers. Anal. Chim. Acta 2015, 1, 291–296. [Google Scholar] [CrossRef] [PubMed]
- IUPAC. Analytical Chemistry Division, Commission on Analytical Nomenclature. Pure Appl. Chem. 1994, 66, 2527–2536. [Google Scholar]
- Ping, J.F.; Wang, Y.X.; Ying, Y.B.; Wu, J. Application of electrochemically reduced graphene oxide on screen-printed ion-selective electrode. Anal. Chem. 2013, 84, 3473–3479. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Li, F.H.; Qin, W. An all-solid-state Cd2+-selective electrode with a low detection limit. Sens. Actuators B 2011, 155, 919–922. [Google Scholar] [CrossRef]
- Khorasani, J.H.; Amini, M.K.; Motaghi, H.; Tangestaninejad, S.; Moghadam, M. Manganese porphyrin derivatives as ionophores for thiocyanate-selective electrodes: The influence of porphyrin substituents and additives on the response properties. Sens. Actuators B 2002, 87, 448–456. [Google Scholar] [CrossRef]
- Shamsipur, M.; Javanbakht, M.; Hassaninejad, A.R.; Sharghi, H.; Ganjali, M.R.; Mousavi, M.F. Highly selective PVC-membrane electrodes based on three derivatives of (tetraphenylporphyrinato) cobalt (III) acetate for determination of trace amounts of nitrite ion. Electroanalysis 2003, 15, 1251–1259. [Google Scholar] [CrossRef]
- Bobacka, J. Potential stability of all-solid-state ion-selective electrodes using conducting polymers as ion-to-electron transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef]



| Parameter * | GC/ETH500/SWCNTs/ClO4−-ISE |
|---|---|
| Slope, (mV/decade) | −56.0 ± 1.1 |
| Correlation coefficient, (r2) | −0.9998 |
| Lower detection limit, (M) | 1.8 × 10−7 |
| Linear range, (M) | 1.07 × 10−6–1.0 × 10−2 |
| Working acidity range, (pH) | 4.5–7.5 |
| Response time, (s) | <10 |
| Life span, (week) | 8 |
| Precision, (%) | 1.6 |
| Accuracy, (%) | 98.5 |
| Standard deviation, (σmV) | 0.82 |
| Interfering Ion, j | GC/ETH500/SWCNTs/ClO4−-ISE * |
|---|---|
| SCN− | −0.9 ± 0.07 |
| I− | −2.9 ± 0.5 |
| Cl− | −3.3 ± 0.6 |
| NO2− | −3.7 ± 0.7 |
| Br− | −4.1 ± 0.4 |
| NO3− | −4.2 ± 0.6 |
| CN− | −4.5 ± 0.3 |
| N3− | −4.6 ± 0.7 |
| S2O32− | −5.6 ± 0.4 |
| CH3COO− | −6.1 ± 0.2 |
| S2− | −6.5 ± 0.7 |
| SO42− | −7.2 ± 0.3 |
| PO43− | −7.8 ± 0.6 |
| Fireworks | [ClO4−] (%) a | F-test b | |||
|---|---|---|---|---|---|
| Potentiometry | RSD, % | Ion Chromatography | RSD, % | ||
| Sample 1 | 35.3 ± 1.2 | 3.4 | 31.2 ± 0.9 | 2.8 | 2.341 |
| Sample 2 | 39.1 ± 1.7 | 4.3 | 35.7 ± 0.4 | 1.1 | 1.663 |
| Sample 3 | 46.3 ± 2.2 | 4.7 | 42.1 ± 1.5 | 3.5 | 1.851 |
| Compound | [ClO4] (%) * | RSD, % | |
|---|---|---|---|
| Calculated | Found | ||
| Urea perchlorate | 62.0 | 61.3 ± 0.7 | 1.1 |
| Hydrazine perchlorate | 75.1 | 73.6 ± 1.5 | 2.1 |
| Ethylenediamine perchlorate | 62.0 | 60.4 ± 1.1 | 1.8 |
| Ammonium perchlorate | 84.7 | 81.2 ± 0.6 | 0.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, S.S.M.; Galal Eldin, A.; Amr, A.E.-G.E.; Al-Omar, M.A.; Kamel, A.H. Single-Walled Carbon Nanotubes (SWCNTs) as Solid-Contact in All-Solid-State Perchlorate ISEs: Applications to Fireworks and Propellants Analysis. Sensors 2019, 19, 2697. https://doi.org/10.3390/s19122697
Hassan SSM, Galal Eldin A, Amr AE-GE, Al-Omar MA, Kamel AH. Single-Walled Carbon Nanotubes (SWCNTs) as Solid-Contact in All-Solid-State Perchlorate ISEs: Applications to Fireworks and Propellants Analysis. Sensors. 2019; 19(12):2697. https://doi.org/10.3390/s19122697
Chicago/Turabian StyleHassan, Saad S. M., Ahmed Galal Eldin, Abd El-Galil E. Amr, Mohamed A. Al-Omar, and Ayman H. Kamel. 2019. "Single-Walled Carbon Nanotubes (SWCNTs) as Solid-Contact in All-Solid-State Perchlorate ISEs: Applications to Fireworks and Propellants Analysis" Sensors 19, no. 12: 2697. https://doi.org/10.3390/s19122697
APA StyleHassan, S. S. M., Galal Eldin, A., Amr, A. E.-G. E., Al-Omar, M. A., & Kamel, A. H. (2019). Single-Walled Carbon Nanotubes (SWCNTs) as Solid-Contact in All-Solid-State Perchlorate ISEs: Applications to Fireworks and Propellants Analysis. Sensors, 19(12), 2697. https://doi.org/10.3390/s19122697








