Abstract
Herein, we present reliable, robust, stable, and cost-effective solid-contact ion-selective electrodes (ISEs) for perchlorate determination. Single-walled carbon nanotubes (SWCNTs) were used as solid-contact material and indium (III) 5, 10, 15, 20-(tetraphenyl) porphyrin chloride (InIII-porph) as an ion carrier. The sensor exhibited an improved sensitivity towards ClO4− ions with anionic slope of −56.0 ± 1.1 (R2 = 0.9998) mV/decade over a linear range 1.07 × 10−6 – 1.0 × 10−2 M and detection limit of 1.8 × 10−7 M. The short-term potential stability and the double-layer capacitance were measured by chronopotentiometric and electrochemical impedance spectroscopy (EIS) measurements, respectively. The sensor is used for ClO4− determination in fireworks and propellant powders. The results fairly agree with data obtained by ion chromatography.
1. Introduction
Perchlorate ions can be found due to either natural processes or as a result of human activities. These ions are characterized by their high solubility, high salvation capacity, and high reduction potential in water. These properties make perchlorate ions both chemically stable and risky towards human health [1]. Exposure to perchlorate can affect the thyroid gland function. It interferes with the uptake of iodide and the production of thyroid hormone. Standards were set by official agencies such as medical, clinical, or environmental laboratories in order to face these health threats [2]. Perchlorate salts were integrated in industry as rocket solid propellants and military explosives. In addition, they have been used as initiators, detonators, and blasting agents. Many aerospace programs in addition to more than 40 different weapon systems are based on perchlorate. Salts of perchlorate are used in the manufacturing of fireworks, flares, and coin-cell batteries. Moreover, they can be used as an automobile airbag initiator, in pyrotechnic devices, finishing leather, and in electronic tubes [3].
Various analytical techniques have been used for perchlorate determination [4,5,6,7,8,9,10,11,12,13,14]. Among of these methods are titrimetry [6], gravimetry [7], dye extraction spectrophotometry [8,9], atomic absorption spectrometry (AAS) [10,11], ion chromatography (IC) [12,13], and mass spectrometry based on electrospray ionization [14]. The main disadvantages of these analytical methods are the poor sensitivity and low selectivity [6,7], the high cost instrumentation [12,13,14], and the extensive sample pretreatment [8,9].
Potential-based sensors or the so-called “ion selective electrodes” (ISEs) have been extensively introduced in different analytical applications, such as in clinical analysis [15,16,17,18,19], environmental monitoring [20,21,22,23,24,25], pharmaceutical analysis [26,27,28,29,30,31], and quality control criteria [32]. This class of analytical devices is characterized by their low cost, high reliability and validity, and ease of operation.
Solid-contact ion-selective electrodes (SC-ISEs) as a different generation of ISEs are characterized by their suitable storage and servicing, ease of miniaturization, and high solidity [33]. The presence of the “blocked” interface between the electronic conductor and ion-selective membrane (ISM) is removed by the insertion of solid-contact materials, such as carbon nano-structures, conducting polymers or nano-noble metals. Signal noises and potential drifts, which can restrict the applications of ISEs are now removed [34]. In the literature, many ISEs have been reported for perchlorate assessment. Most of these electrodes are based on the use of perchlorate/metal chelates ion-association complexes [35,36,37,38,39], quaternary ammonium ions with long chains [40,41,42], and organic dyes [43,44,45]. These electrodes have poor sensitivity towards trace levels of ClO4− in presence of other many common anions, such as hydroxide, nitrate, thiocyanate, and iodide. Other reported perchlorate ISEs based on either neutral or charged carriers showed improved selectivity and sensitivity [45,46,47,48,49,50,51,52]. Other ISEs based on surfactant-modified zeolite Y (SMZ) nano-clusters have also been reported for perchlorate determination [53,54]. However, the development of robust and reliable ClO4− ISEs with good selectivity and high sensitivity is still a needed request for dealing with samples of small volumes.
In this study, we present a new robust, reliable, sensitive, and cost-effective solid-contact ISE for fast perchlorate determination. The sensor is based on indium-porphyrin ionophore in the sensing membrane and single-walled carbon nanotubes (SWCNTs) as solid-contact material. The structure of SWCNTs contributes to a high double layer capacitance because of their large specific surface area. It also reveals good electric conductivity in addition to its high hydrophobicity. The proposed sensor is used for the assay of ClO4− in fireworks and propellant samples.
2. Materials and Methods
2.1. Reagents
The ionophore indium (III) 5, 10, 15, 20-(tetraphenyl) porphyrin chloride (InIII-porph) was purchased from PorphyChem SAS (Dijon, France). Tetradodecylammonium tetrakis (4-chlorophenyl) borate (ETH 500), high molecular weight poly (vinyl chloride) (PVC), 2-nitrophenyl octyl ether (o-NPOE), tridodecylmethylammonium chloride (TDMAC), and tetrahydrofuran (THF) were purchased from Fluka AG (Buchs, Switzerland). Single-walled carbon nanotubes (SWCNTs) were purchased from XFnano Materials Tech Co., Ltd. (Nanjing, China).
Aqueous solutions of the reagents and test solutions were prepared with de-ionized bi-distilled water. A stock solution of 0.1 M ClO4− was prepared by dissolving in NaClO4 and then diluted to working standard solutions with de-ionized bi-distilled water prior to measurements.
2.2. Apparatus
“All potentiometric measurements were carried out at 20–21 °C using an Orion-SA 720 pH/meter (MA, USA) in the galvanic cell: Ag/AgCl/(3 M KCl)/0.1 M LiOAc/sample solution//ISE membrane/SWCNTs/glassy carbon electrode (GCE). Selectivity coefficients for the proposed sensor towards ClO4− over different common anions were evaluated and calculated by the modified separate solution method (MSSM) [55]. The modified Debye–Hückel equation was employed for calculation of all activity coefficients of the tested ions [56]”.
“Ion chromatography measurements of perchlorate samples were conducted for comparison using a Thermo IC-1100 system equipped with GP50 gradient pump and ED40 electrochemical conductivity cell detector. A Dionex Ion Pac AS-16 separation column (2 × 250 mm2), AS16 guard column (2 × 50 mm2), 5 × 10−2 M NaOH eluent, 0.5 mL flow rate, and 500 μL perchlorate injection volumes were used”.
“Chronopotentiometry and electrochemical impedance spectroscopy (EIS) measurements were carried out using an Autolab Model 2000 potentiostat/galvanostat (Metrohom Instruments, Herisau, Switzerland). A three-electrode configuration cell containing silver/silver chloride (3 M KCl) reference electrode and an auxiliary electrode made from platinum wire was employed. The impedance spectra were measured and recorded at open-circuit potential in 0.01 M NaClO4 solution with excitation amplitude of 10 mV and a frequency range of 100 kHz–0.1 Hz”.
2.3. Preparation Procedure of SC-ISEs
“The ion-sensing membrane (ISM) is prepared as mentioned previously [57], by dissolving 360 mg of the membrane components in 2.5 mL of THF: (InIII-porph) (1 wt %), TDMAC (1 wt %), o-NPOE (49 wt %) and PVC (49.0 wt %). Using sonication, degassing for the membrane cocktail is done for 10 min. The solid-contact ISEs were fabricated as follows: (1) Glassy carbon electrode (GCE) was firstly polished with 0.3 µm γ-Al2O3 slurries, rinsed with water, sonicated for 10 min in ethanol and then, dried with ethanol. The resulting GCE was placed into a piece of matched PVC tubing at its distal end. (2) Mixture of 20 mg of ETH 500 and 2 mg of SWCNTs were spread onto the electrode surface, heated by an infrared lamp for 10 s till complete melting of the ETH 500. The mixture is then left to cool forming a uniform composite layer that is strongly adhered to the surface of GCE. (3) One-hundred microliters of the membrane cocktail was drop-cast onto the transducer layer and left to dry for 2 h. The GC/ClO4−-ISEs were prepared by the previously mentioned steps without using SWCNTs. The ClO4−-ISEs were firstly conditioned in 10−3M ClO4− for 1 day and then in 10−8 M ClO4− for another day”.
2.4. Sensors Calibration and ClO4− Determination
One-milliliter aliquots of 1.0 × 1−1–1.0 × 10−8 M ClO4− solutions were transferred to 25 mL beakers containing 9.0 mL of 50 mM phosphate buffer solution of pH 5.5. The GC/ETH500/SWCNTs/ClO4−-ISEs is inserted into the solution in conjunction with a double junction Ag/AgCl reference electrode. The EMF readings were recorded and plotted as a function of logaClO4−. The obtained calibration graph was used for all subsequent measurements of unknown ClO4− concentrations.
For successful assessment of perchlorate using the presented method, GC/ETH500/SWCNTs/ClO4−-ISEs were applied for perchlorate assessment in commercial firework samples. Two firework shell samples were homogenized using an agate mortar and left to dry under vacuum for one hour at room temperature. An accurate amount of the powder (0.5–1.0 gm) was transferred to a 50 mL beaker and was dissolved in 50 mL of de-ionized bi-distilled water. The solution is then carefully heated at 60 °C on a water-bath for 5 min. After that, it was left to cool, filtered, and completed to 100 mL with de-ionized bi-distilled water. As mentioned above, the amount of perchlorate was potentiometrically measured.
For comparison, determination of ClO4− using ion chromatography (IC) was carried out. Typically 10 mL of the above final test solution was further diluted to 100 mL. Before the analysis, ~5 mL of the test solution was filtered and 100 μL aliquots were injected into the chromatographic column.
3. Results and Discussions
3.1. Performance Characteristics of All Solid-Contact Perchlorate ISEs
The electrochemical performance of the SC/ISEs was evaluated according to the IUPAC recommendations [58]. Validation of the presented assay method was also done. After a period of three months, the performance characteristics of the proposed SC/ISEs are given in Table 1. As shown in Figure 1, the GC/ETH500/SWCNTs/ClO4−-ISEs reveals excellent response performance over a linear range between 1.0 × 10−2 and 1.0 × 10−6 M with a Nernstian response of −56.0 ± 1.1 mV/decade (n = 6, R2 = 0.9998) and detection limit of 1.8 × 10−7 M.

Table 1.
Performance characteristics of GC/ETH500/SWCNTs/ClO4−-ISE.
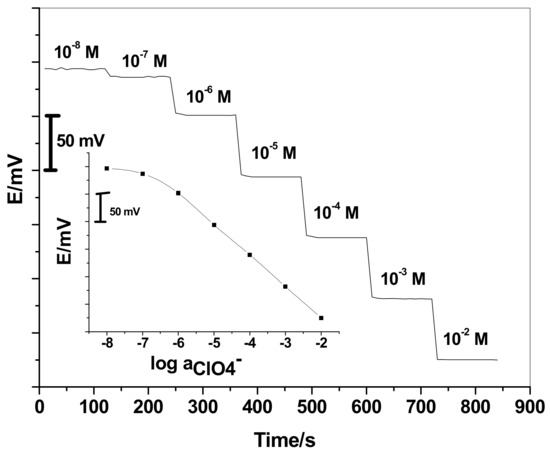
Figure 1.
Potentiometric response of perchlorate based sensor (GC/ETH500/SWCNTs/ClO4−-ISEs)
The transduction mechanism of using SWCNTs is linked to the formation of an electrical double layer at the interface between the ISM and SWCNTs [59]. This interface acts as an asymmetric capacitor confirming that the adsorption of a lipophilic TDMA+ cation in ISM onto the SWCNTs can contribute to the electrical double layer formation [60]. The mechanism of ion-to-electron transduction is schematically shown in Figure 2. At the interface between ISM and SWCNTs solid-contact, the large surface area of the later can provide more sites for TDMA+ adsorption and then it can facilitate the conversion of the ionic signal to an electrical signal [60].
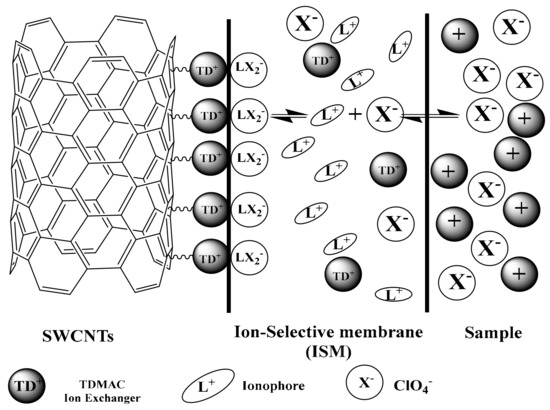
Figure 2.
Schematic illustration of the transduction mechanism.
Indium (III)-porphyrin ionophore interacts with ClO4− causing an increase of the coordination number of InIII central atom from 3 to 5 or 6. Binding of perchlorate and other anions with indium porphyrin are expected because the electron density on the central InIII atom varies by the extent of donation from the equatorial ligands. As reported before [61], InIII-porphyrin can bind with perchlorate forming mono-and di-perchlorate anion at its axial position without further complexation with other anions. It appears that at the interface between the ISM and sample, ClO4− ion binds selectively with central InIII in porphyrin ligand.
Using Equation (1), student’s (t) value was calculated from data obtained by repeated measurements (n = 6) of 5 μg/mL internal quality control (IQC) ClO4− sample. The texp was 0.912 at 95% confidence interval and compared with the theoretical value (t = 2.015). This indicates that the null hypothesis was held.
where μ is the IQC sample concentration, x is the found experimental average concentration, n is the number of replicates (n = 6) and σs is the standard deviation. All validation characteristics, such as accuracy, precision, within-day repeatability, between-days reproducibility and relative standard deviation were presented in Table 1. Precision (relative standard deviation (RSD) or the coefficient of variance (CV) of the method was checked by using six replicate measurements of 10 µg/mL of a quality control ClO4− sample. The precision and accuracy of the used procedure were calculated using the following equations:
where x, µ, and S are the average of the measured perchlorate concentration, the reference standard perchlorate concentration, and standard deviation, respectively. The relative standard deviation was calculated and found to be 1.6. The dynamic response time of the solid-contact electrode revealed a fast response time of <10 s. Elimination of the inner filling solution prefers the short time response of the solid-contact ISEs as previously reported [59,60].
texp = [(μ − x)√n]/σs
Accuracy, % = (x/µ) × 100
Precision (RSD), % = (S/x) × 100
Effect of pH on the potential response of GC/ETH500/SWCNTs/ClO4−-ISEs was tested. The potential-pH relations revealed no potential variation by more than that ± 1 mV within the pH range of 4.5–7.5. At pH < 3, hydronium ion (H3O+) along with the formation of H2ClO4+ ions were perhaps extracted in the membrane phase and then compete with perchlorate ion for the cationic site in the membrane. At pH > 8, severe interference from OH− ions were probably compete with ClO4− for InIII-porphyrin chelate ion. This is in a good approval with that reported by other workers in which the potential response of some anion-ISEs based on metalloporphyrin is affected by the change of pH within the range of 3–8 [61,62]. From all of the above, 50 mM phosphate buffer background of pH 5.5 was chosen for all subsequent measurements.
3.2. Interfering Ions Effect
Selectivity of GC/ETH500/SWCNTs/ClO4−-ISEs over many common anions was potentiometrically evaluated by measuring the selectivity coefficients using the modified separate solutions method (MSSM) [55]. This method is used to remove the effect of the inseparable limit in sensitivity on the potential response of the ISE toward the distinguished ions. The recorded results are presented in Table 2. As can be seen from these results, the selectivity coefficient values of GC/ETH500/SWCNTs/ClO4−-ISEs are in a good agreement with those obtained by the liquid-contact ISE based on the same used ionophore [45]. With the exclusion of SCN− ions, high concentration levels of other anions commonly present, have no effect on the potentiometric response of the sensors in presence of perchlorate ions. The order of selectivity was: ClO4− > SCN− > I− > Cl− > NO2− > Br− > NO3− > CN− > N3− > S2O32− > CH3COO− > S2− > SO42− > PO43−. Two possible mechanisms for the interaction of ClO4− anion with InIII-porphrin. According to neutral-carrier mechanism, ClO4− is extracted from the aqueous medium into the membrane containing the neutral indium mono-perchlorate complex as a 6th ligand for central InIII atom. This produces an octahedral negatively charged indium di-perchlorate complex. According to the mechanism of charged-carriers, interaction of perchlorate anion with indium-porphyrin charged molecule forms the neutral indium mono-perchlorate molecule and then the phase boundary potential is created.

Table 2.
Selectivity values (log Kpot ClO4−,j) for perchlorate solid-contact sensors.
3.3. Short-Term Potential Stability
Chronopotentiometry using current-reversed technique was used for short-term potential stability evaluation for the proposed sensors. As shown in Figure 3, the typical chronopotentiograms for the GC/ETH500/SWCNTs/ClO4−-ISEs and GC/ClO4−-ISEs were recorded in 1.0 × 10−4 M ClO4− solution. According to the equation ΔE/Δt = I/C proposed by Bobacka [63], the potential drift (ΔE/Δt) is correlated with the implemented current (I = 10−9A) and the electrode low-frequency capacitance (C). Therefore, ISEs have a large capacitance (C) reveal low drift in the potential. The potential drift of the GC/ETH500/SWCNTs/ClO4−-ISEs was found to be 2.61 ± 0.7 µV/s, while GC/ClO4−-ISE revealed a potential drift 123 ± 2.4 µV/s. The evaluated low-frequency capacitances for the GC/ETH500/SWCNTs/ClO4−-ISEs and GC/ClO4−-ISE were found to be 383.2 ± 0.7 µF and 8.1 ± 0.3 µF, respectively. These results indicate that the introduction of ETH500/SWCNTs between the ISM and electronic conductor substrate can effectively enhance the potential stability of all-solid-state ClO4−-ISEs via increasing the low-frequency capacitance on the interface between the solid-contact material and ISM.
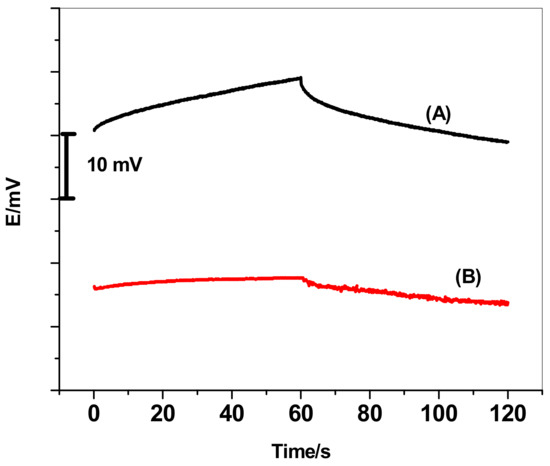
Figure 3.
Chronopotentiograms (applied current: ± 1 nA for 60 s) for all-solid-state perchlorate ISE: (A) GC/ClO4−-ISE; (B) GC/ETH500/SWCNTs/ClO4−-ISEs.
3.4. Impedance Measurements
The impedance spectra of GC/ETH500/SWCNTs/ClO4−-ISEs and GC/ClO4−-ISEs were tested in 1.0 × 10−4 M ClO4− solution to evaluate both high-frequency and charge-transfer resistances. In addition, double layer capacitances were also evaluated. As indicated in Figure 4, each ISE reveals a high-frequency semicircle, which represents the bulk resistance (Rb) and geometric capacitance of the ISM. In the high-frequency pat, the resistance values for GC/ETH500/SWCNTs/ClO4−-ISEs and GC/ClO4−-ISEs were 0.34 ± 0.02 and 0.33 ± 0.04 MΩ, respectively. In addition, in the low-frequency part, the GC/ClO4−-ISEs reveals a larger semicircle than the one obtained in GC/ETH500/SWCNTs/ClO4−-ISEs. The low-frequency capacitance (CL) for GC/ETH500/SWCNTs/ClO4−-ISEs and GC/ClO4−-ISEs was CL = 27.6 ± 0.7 and 6.5 ± 1.2 µF, respectively. This indicates the existence of a high double layer capacitance (CL) and low charge transfer resistance at the interface between the sensing membrane and GC electrode.
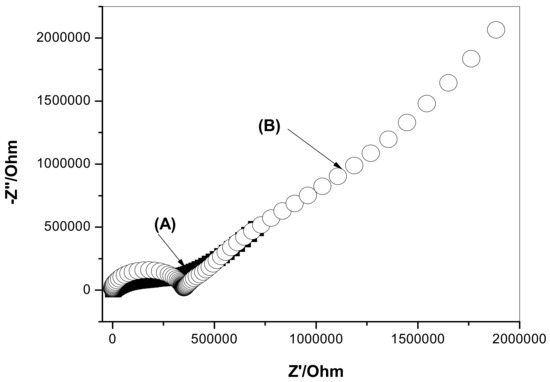
Figure 4.
Impedance spectra for the proposed (A) GC/ETH500/SWCNTs/ClO4−-ISEs and (B) GC/ClO4−-ISEs.
3.5. Determination of ClO4− in Commercial Fireworks Formulations
To test the validity of the proposed sensors, ClO4− ions were determined in some commercial fireworks. About more than 50% of the constituents of these commercial fireworks are additives, so the response of GC/ETH500/SWCNTs/ClO4−-ISEs towards these additives was investigated. No noticeable interferences were found by the presence of 1000-fold excess of reducing agents such as sulfur and charcoal, binders such as dextrin and lactose, linseed oil as color brighten and aluminum flakes as regulators. As shown in Table 3, F-test showed no significant difference at 95% confidence level between means and variances of the proposed potentiometric technique and the standard ion chromatography for comparison. Determination of ClO4− in some pure propellant powders of purity >99% was also carried out using the proposed perchlorate ISE. A shown in Table 4, the results obtained by the proposed sensor is in a close agreement and good reliability with this obtained by the ion chromatography method.

Table 3.
Potentiometric assessment of ClO4− in some commercial firework samples.

Table 4.
Potentiometric assessment of perchlorate in some propellants.
4. Conclusions
Simple and robust solid-contact ISE has been proposed for perchlorate determination. The fabrication of the sensor is based on the combination of using SWCNTs and the good adhesion ability revealed by ETH 500. As compared to GC/ClO4−-ISEs (CWEs), the proposed GC/ETH500/SWCNTs/ClO4−-ISEs revealed a significant enhancement in their potential stability. Moreover, the sensors introduced enhanced sensing characteristics including a broad linear range, fast response time, long-life span, and long-term stability. The sensors were used for the assessment of ClO4− content in some fireworks and propellant powders. Validation of the method is carried out and the data obtained by the proposed method were compared with those obtained by the standard ion chromatographic method. The sensors revealed enhanced features over many of those previously reported in terms of robustness, ease of fabrication, selectivity, and accuracy. The sensors can be introduced in a flow system for continuous monitoring. Sample pretreatment is not required for perchlorate analysis using these proposed sensors.
Author Contributions
The listed authors contributed to this work as described in the following: A.G.E., A.H.K., and S.S.M.H. gave the concepts of the work, interpreted the results, the experimental part and prepared the manuscript, A.H.K. and S.S.M.H. cooperated in the preparation of the manuscript and A.H.K., A.E.-G.E.A. and S.S.M.H. performed the revision before submission. A.E.-G.E.A. and M.A.A.-O. revealed the financial support for the work. All authors read and approved the final manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding through the Vice Deanship of Scientific Research Chairs.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Team, P. Perchlorate: Overview of Issues, Status, and Remedial Options; Interstate Technology & Regulatory Council: Washinton, DC, USA, 2005. [Google Scholar]
- Urbansky, E.T. Perchlorate as an environmental contaminant. Environ. Sci. Pollut. Res. Int. 2002, 9, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Thorne, P.G. Field Screening Method for Perchlorate in Water and Soil; US Army Corps of Engineers, NTIS: Springfield, VA, USA, 2004.
- Goncharuk, V.V.; Zui, O.V.; Kushchevskaya, N.F. Methods of determining perchlorates. J. Water Chem. Technol. 2009, 31, 186–194. [Google Scholar] [CrossRef]
- Urbansky, E.T. Quantitation of perchlorate ion: Practices and advances applied to the analysis of common matrices. Crit. Rev. Anal. Chem. 2000, 30, 311–343. [Google Scholar] [CrossRef]
- Baczuk, R.J.; Bolleter, W.T. Conductometric titration of perchlorate with tetraphenylarsonium chloride. Anal. Chem. 1967, 39, 93–95. [Google Scholar] [CrossRef]
- Vogel, A.I. Text Book of Quantitative Inorganic Analysis, 4th ed.; Longman: London, UK, 1978. [Google Scholar]
- Weiss, J.A.; Stanbury, J.B. Spectrophotometric determination of microamounts of perchlorate in biological fluids. Anal. Chem. 1972, 44, 619–620. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.T.; Chimpalee, N.; Harrott, M. Flow-injection extraction-spectrophotometric determination of perchlorate with brilliant green. Anal. Chim. Acta 1989, 217, 177–181. [Google Scholar] [CrossRef]
- Gallego, M.; Valcarcel, M. Indirect atomic absorption spectrometric determination of perchlorate by liquid-liquid extraction in a flow-injection system. Anal. Chim. Acta 1985, 169, 161–169. [Google Scholar] [CrossRef]
- Chattaraj, S.; De, K.; Das, A.K. Indirect determination of perchlorate by atomic absorption spectrometry. Mikrochim. Acta 1992, 106, 183–190. [Google Scholar] [CrossRef]
- Narayanan, L.; Buttler, G.W.; Yu, K.O.; Mattie, D.R.; Fisher, J.W. Sensitive high-performance liquid chromatography method for the determination of low levels of perchlorate in biological samples. J. Chromatogr. B 2003, 788, 393–399. [Google Scholar] [CrossRef]
- Lamb, J.D.; Simpson, D.; Jensen, B.D.; Gardner, J.S.; Peterson, Q.P. Determination of perchlorate in drinking water by ion chromatography using macrocycle-based concentration and separation methods. J. Chromatogr. A 2006, 1118, 100–105. [Google Scholar] [CrossRef]
- Urbansky, E.T.; Magnuson, M.L.; Freeman, D.; Jelks, C. Quantitation of perchlorate ion by electrospray ionization mass spectrometry (ESI-MS) using stable association complexes with organic cations and bases to enhance selectivity. J. Anal. At. Spectrom. 1999, 14, 1861–1866. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Elnemma, E.M.; Mohamed, A.H.K. Novel Biomedical Sensors for Flow Injection Potentiometric Determination of Creatinine in Human Serum. Electroanalysis 2005, 17, 2246–2253. [Google Scholar] [CrossRef]
- Kamel, A.H. Conventional and miniaturized planar chip sensors for potentiometric assay of uric acid in biological fluids using flow injection analysis. J. Pharm. Biomed. Anal. 2007, 45, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.M.; Sayour, H.E.M.; Kamel, A.H. A simple potentiometric method for determination of acid and alkaline phosphatase enzymes in biological fluids and dairy products using a nitrophenylphosphate plastic membrane sensor. Anal. Chim. Acta 2009, 640, 75–81. [Google Scholar] [CrossRef]
- Yan, R.; Qiu, S.; Tong, L.; Qian, Y. Review of progresses on clinical applications of ion selective electrodes for electrolytic ion tests: From conventional ISEs to graphene-based SEs (Review). Chem. Spec. Bioavail. 2016, 28, 72–77. [Google Scholar] [CrossRef]
- Dimeski, G.; Badrick, T.; John, A.S. Ion Selective Electrodes (ISEs) and interferences-A review (Review). Clin. Chim. Acta 2010, 411, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.M.; Marzouk, S.A.M.; Mohamed, A.H.K.; Badawy, N.M. Novel dicyanoargentate polymeric membrane sensors for selective determination of cyanide ions. Electroanalysis 2004, 16, 298–303. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Mahmoud, W.H.; Mohamed, A.H.K.; Kelany, A.E. Mercury(II) Ion-Selective Polymeric Membrane Sensors for Analysis of Mercury in Hazardous Wastes. Anal. Sci. 2006, 22, 877–881. [Google Scholar] [CrossRef]
- Kamel, A.H.; Galal, H.R.; Awaad, N.S. Cost-effective and handmade paper-based potentiometric sensing platform for piperidine determination. Anal. Methods 2018, 10, 5406–5415. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Badr, I.H.A.; Kamel, A.H.; Mohamed, M.S. A Novel Poly (Vinyl Chloride) Matrix Membrane Sensor for Batch and Flow-injection Determination of Thiocyanate, Cyanide and Some Metal Ions. Anal. Sci. 2009, 25, 911–917. [Google Scholar] [CrossRef]
- Cuartero, M.; Crespo, G.A. All-solid-state potentiometric sensors: A new wave for in situ aquatic research (Review). Curr. Opin. Electrochem. 2018, 10, 98–106. [Google Scholar] [CrossRef]
- Sak-Bosnar, M.; Madunić-Čačić, D.; Grabarić, Z.; Grabarić, B. Potentiometric Determination of Anionic and Nonionic Surfactants in Surface Waters and Wastewaters. Handb. Environ. Chem. 2015, 31, 157–176. [Google Scholar]
- Moreira, F.T.C.; Guerreiro, J.R.L.; Azevedo, V.L.; Kamel, A.H.; Sales, M.G.F. New biomimetic sensors for the determination of tetracycline in biological samples: Batch and flow mode operations. Anal. Methods 2010, 2, 2039–2045. [Google Scholar] [CrossRef][Green Version]
- Hassan, S.S.M.; Kamel, A.H.; Abd El-Naby, H. New Potentiometric Sensors Based on Selective Recognition Sites for Determination of Ephedrine in Some Pharmaceuticals and Biological Fluids. Talanta 2013, 103, 330–336. [Google Scholar] [CrossRef] [PubMed]
- El-Naby, E.H.; Kamel, A.H. Potential transducers based man-tailored biomimetic sensors for selective recognition of dextromethorphan as an antitussive drug. Mater. Sci. Eng. C 2015, 54, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Lenik, J. Cyclodextrins based electrochemical sensors for biomedical and pharmaceutical analysis-review. Curr. Med. Chem. 2017, 24, 2359–2391. [Google Scholar] [CrossRef] [PubMed]
- Lenik, J. Application of PVC in Constructions of Ion Selective Electrodes for Pharmaceutical Analysis. In Handbook of Polymers for Pharmaceutical Technologies; Processing and Applications; Thakur, V.K., Thakur, M.K., Eds.; Wiley Scrivener Publishing: Hoboken, NJ, USA, 2015; Volume 2, pp. 195–227. [Google Scholar]
- de Souza Gil, E.; de Melo, G.R. Electrochemical biosensors in pharmaceutical analysis. Braz. J. Pharm. Sci. 2010, 6, 376–391. [Google Scholar]
- Lindner, E.; Gyurcsanyi, R.E. Quality control criteria for solid-contact, solvent polymeric membrane ion-selective electrodes. J. Solid State Electrochem. 2009, 13, 51–68. [Google Scholar] [CrossRef]
- Hu, J.; Stein, A.; Bühlmann, P. Rational design of all-solid-state ion-selective electrodes and reference electrodes. Trends Anal. Chem. 2016, 76, 102–114. [Google Scholar] [CrossRef]
- Ashmawy, N.H.; Almehizia, A.A.; Youssef, T.A.; Amr, A.E.; Al-Omar, M.A.; Kamel, A.H. Novel Carbon/PEDOT/PSS-Based Screen-Printed Biosensors for Acetylcholine Neurotransmitter and Acetylcholinesterase Detection in Human Serum. Molecules 2019, 24, 1539. [Google Scholar] [CrossRef]
- Ishibashi, N.; Kohara, H. Perchlorate ion-selective electrodes with the liquid membranes of the o-phenanthroline chelate or its related compounds. Anal. Lett. 1971, 4, 785–792. [Google Scholar] [CrossRef]
- Rohm, T.J.; Guilbault, G.G. New methods for the preparation of perchlorate ion-selective electrodes. Anal. Chem. 1974, 46, 590–592. [Google Scholar] [CrossRef]
- Wilson, A.C.; Pool, K.H. An improved ion-selective electrode for perchlorate. Talanta 1976, 23, 387–388. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Elsaied, M.M. A new liquid-membrane electrode for selective determination of perchlorate. Talanta 1986, 33, 679–684. [Google Scholar] [CrossRef]
- Jain, K.; Jahan, M.; Tyagi, V. Construction and assessment of some perchlorate-selective liquid membrane electrodes. Anal. Chim. Acta 1990, 231, 69–75. [Google Scholar] [CrossRef]
- Coetzee, C.J.; Freiser, H. Liquid-liquid membrane electrodes based on ion-association extraction systems. Anal. Chem. 1969, 41, 1128–1130. [Google Scholar] [CrossRef]
- Back, S. Selectivity studies on anion-selective membrane electrodes. Anal. Chem. 1972, 44, 1696–1698. [Google Scholar] [CrossRef]
- Fogg, A.G.; Pathan, A.S.; Burns, D.T. A liquid-state perchlorate ion-selective electrode based on brilliant green perchlorate. Anal. Chim. Acta 1974, 73, 220–223. [Google Scholar] [CrossRef]
- Kataoka, M.; Kambara, T. A liquid membrane type perchlorate ion-selective electrode. J. Electroanal. Chem. 1976, 73, 279–284. [Google Scholar] [CrossRef]
- Sanchez-Pedreno, C.; Ortuno, J.A.; Hernandez, J. Perchlorate-selective polymeric membrane electrode based on a gold (I) complex: Application to water and urine analysis. Anal. Chim. Acta 2000, 415, 159–164. [Google Scholar] [CrossRef]
- Almeer, S.H.M.A.; Zogby, I.A.; Hassan, S.S.M. Novel miniaturized sensors for potentiometric batch and flow-injection analysis (FIA) of perchlorate in fireworks and propellants. Talanta 2014, 129, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Gholamian, F.; Sheikh-Mohseni, M.A.; Salavati-Niasari, M. Highly selective determination of perchlorate by a novel potentiometric sensor based on a synthesized complex of copper. Mater. Sci. Eng. C 2011, 31, 1688–1691. [Google Scholar] [CrossRef]
- Rezaei, B.; Meghdadi, S.; Bagherpour, S. Perchlorate-selective polymeric membrane electrode based on bis (dibenzoylmethanato) cobalt (II) complex as a neutral carrier. J. Hazard. Mater. 2009, 161, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Segui, M.J.; Lizondo-Sabater, J.; Martinez-Manez, R.; Sancenon, F.; Soto, J.; Garcia-Breijo, E.; Gil, I. An ion-selective electrode for anion perchlorate in thick-film technology. Sensors 2006, 6, 480–491. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Yousefi, M.; Poursaberi, T.; Naji, L.; Salavati-Niasari, M.; Shamsipur, M. Highly Selective and Sensitive Perchlorate Sensors Based on Some Recently Synthesized Ni (II)-Hexaazacyclotetradecane Complexes. Electroanalysis 2003, 15, 1476–1480. [Google Scholar] [CrossRef]
- Shamsipur, M.; Soleymanpour, A.; Akhond, M.; Sharghi, H.; Hasaninejad, A.R. Perchlorate selective membrane electrodes based on a phosphorus (V)–tetraphenylporphyrin complex. Sens. Actuators B 2003, 89, 9–14. [Google Scholar] [CrossRef]
- Lizondo-Sabater, J.; Segui, M.J.; Lioris, J.M.; Martinez-Manez, R.; Pardo, T.; Sancenon, F.; Soto, J. New membrane perchlorate-selective electrodes containing polyazacycloalkanes as carriers. Sens. Actuators B 2004, 101, 20–27. [Google Scholar] [CrossRef]
- Zanjanchi, M.A.; Arvand, M.; Akbari, M.; Tabatabaeian, K.; Zaraei, G. Perchlorate-selective polymeric membrane electrode based on a cobaloxime as a suitable carrier. Sens. Actuators B 2006, 113, 304–309. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Badri, A. Application of surfactant modified zeolite membrane electrode towards potentiometric determination of perchlorate. J. Electroanal. Chem. 2011, 660, 71–79. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Badri, A. Perchloate selective membrane electrode based on surfactant-modified zeolite Y nanocluster. Anal. Bioanal. Electrochem. 2011, 3, 565–586. [Google Scholar]
- Bakker, E. Determination of improved selectivity coefficients of polymer membrane ion-selective electrodes by conditioning with a discriminated ion. J. Electrochem. Soc. 1996, 143, L83–L85. [Google Scholar] [CrossRef]
- Kamaata, S.; Bhale, A.; Fukunaga, Y.; Murata, H. Copper(II)-selective electrode using thiuram disulfide neutral carriers. Anal. Chem. 1988, 60, 2464–2467. [Google Scholar]
- Liang, R.; Yin, T.; Qin, W. A simple approach for fabricating solid-contact ion-selective electrodes using nanomaterials as transducers. Anal. Chim. Acta 2015, 1, 291–296. [Google Scholar] [CrossRef] [PubMed]
- IUPAC. Analytical Chemistry Division, Commission on Analytical Nomenclature. Pure Appl. Chem. 1994, 66, 2527–2536. [Google Scholar]
- Ping, J.F.; Wang, Y.X.; Ying, Y.B.; Wu, J. Application of electrochemically reduced graphene oxide on screen-printed ion-selective electrode. Anal. Chem. 2013, 84, 3473–3479. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Li, F.H.; Qin, W. An all-solid-state Cd2+-selective electrode with a low detection limit. Sens. Actuators B 2011, 155, 919–922. [Google Scholar] [CrossRef]
- Khorasani, J.H.; Amini, M.K.; Motaghi, H.; Tangestaninejad, S.; Moghadam, M. Manganese porphyrin derivatives as ionophores for thiocyanate-selective electrodes: The influence of porphyrin substituents and additives on the response properties. Sens. Actuators B 2002, 87, 448–456. [Google Scholar] [CrossRef]
- Shamsipur, M.; Javanbakht, M.; Hassaninejad, A.R.; Sharghi, H.; Ganjali, M.R.; Mousavi, M.F. Highly selective PVC-membrane electrodes based on three derivatives of (tetraphenylporphyrinato) cobalt (III) acetate for determination of trace amounts of nitrite ion. Electroanalysis 2003, 15, 1251–1259. [Google Scholar] [CrossRef]
- Bobacka, J. Potential stability of all-solid-state ion-selective electrodes using conducting polymers as ion-to-electron transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).