The Effects of Filter’s Class, Cutoff Frequencies, and Independent Component Analysis on the Amplitude of Somatosensory Evoked Potentials Recorded from Healthy Volunteers
Abstract
1. Introduction
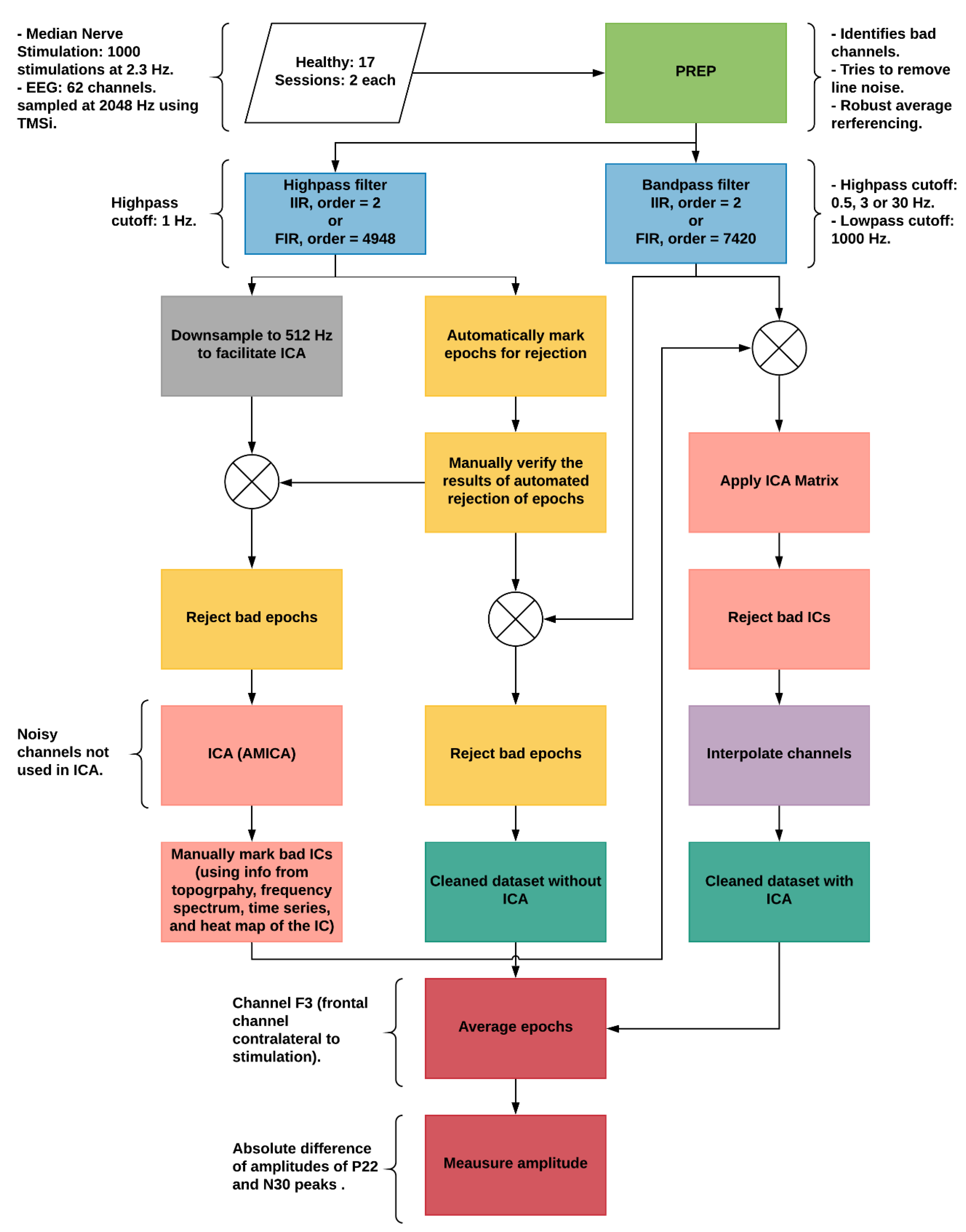
2. Methods
2.1. Experimental Protocol
2.2. Subjects
2.3. Median Nerve Stimulation
2.4. EEG
2.4.1. Artifact Identification
2.4.2. ICA
2.4.3. Cleaned Datasets
2.5. N30 Amplitude
2.6. Statistics
3. Results
3.1. Number of Artifacts
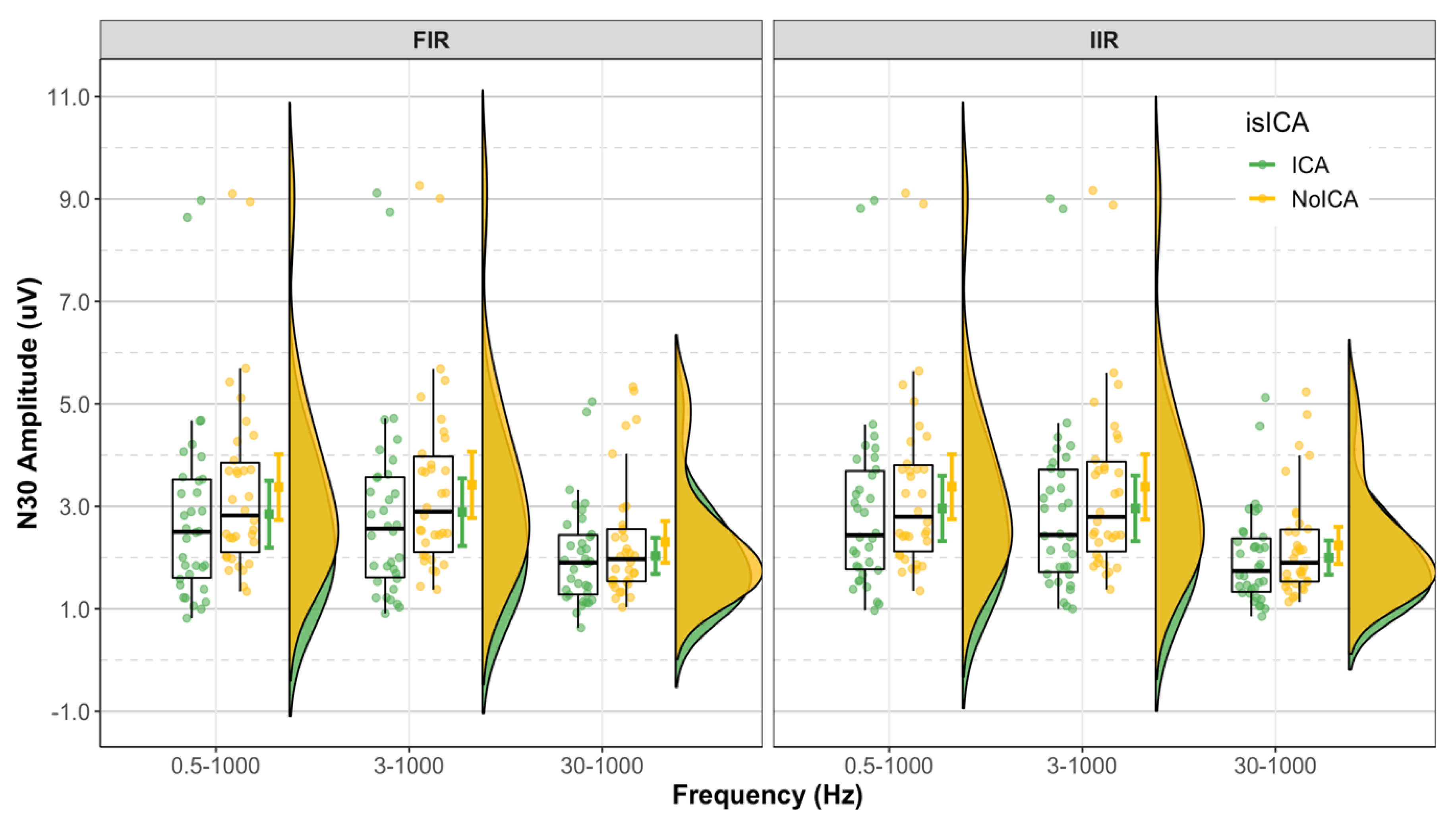
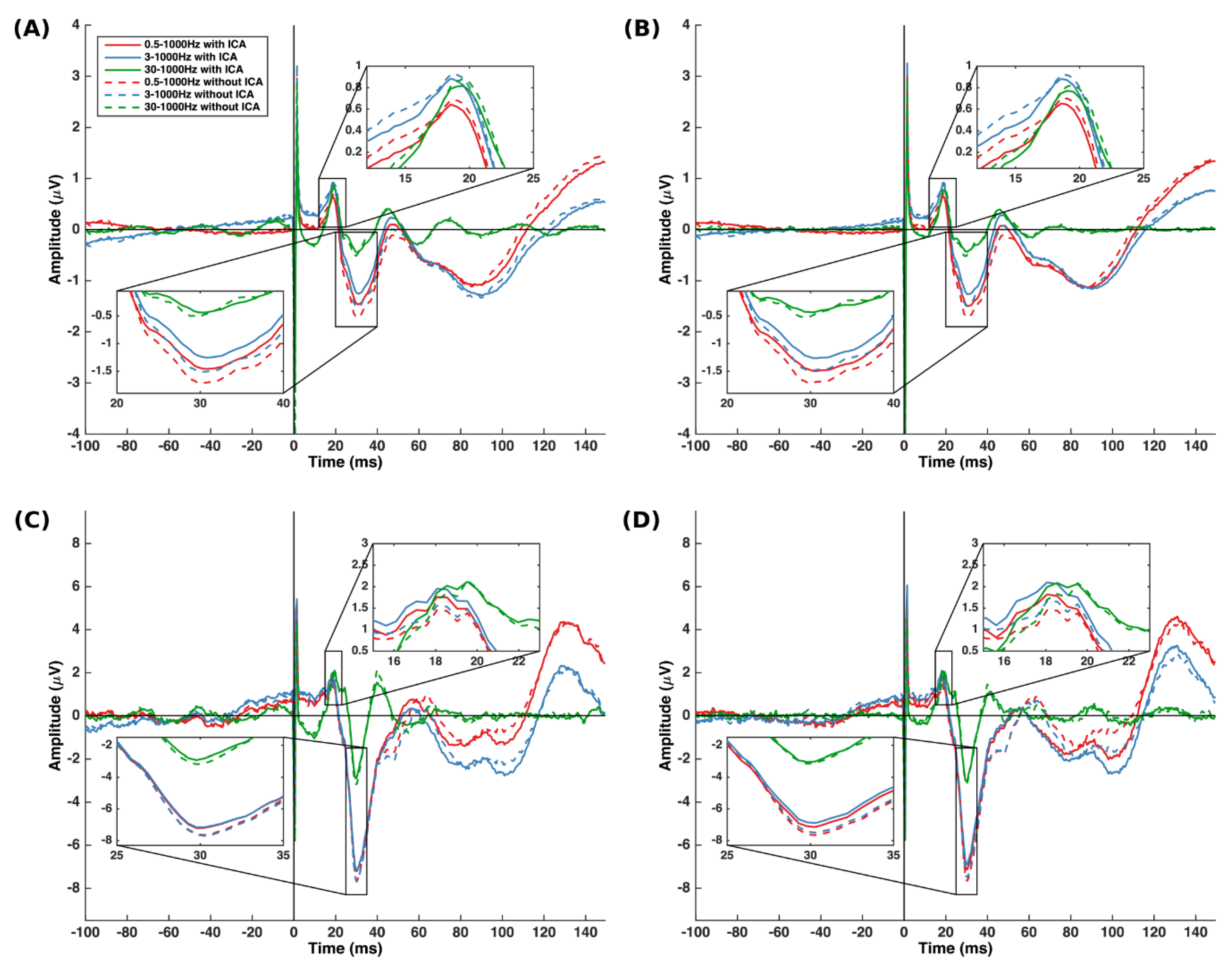
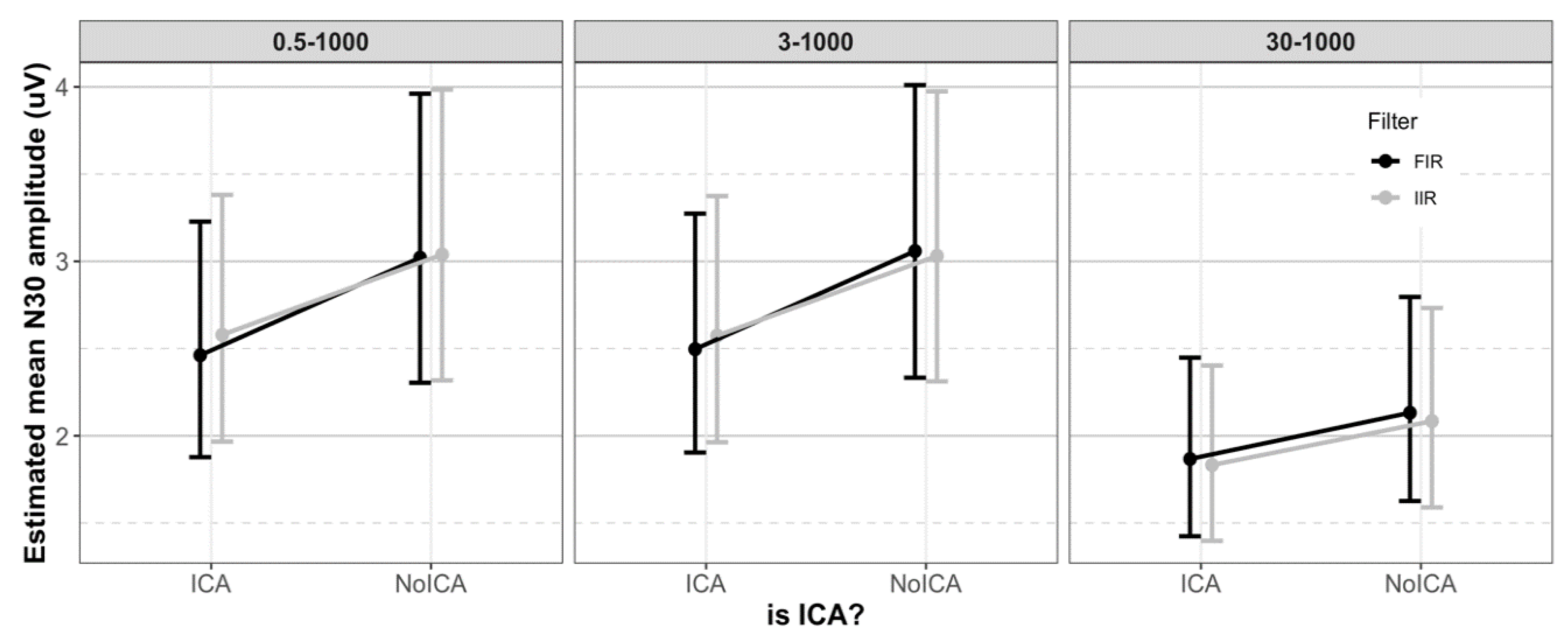
3.2. N30 Amplitude
3.2.1. Effect of Filter Class
3.2.2. Effect of Cutoff Frequency
3.2.3. Effect of ICA
4. Discussion
4.1. Selection of Preprocessing Parameters
4.2. Number of Artifacts
4.3. Filter Class
4.4. Frequency Band
4.5. ICA
4.6. Limitations
4.7. Toolboxes
4.8. Recommendations
- In the preprocessing section of the methodology, filter class (FIR, IIR, high-pass, low-pass, band-pass), order, slope/transition bandwidth, cutoff frequencies should be reported.
- Filtering should always be performed on continuous data in double precision.
- Use FIR, as it is more stable and less likely to introduce phase distortions. Additionally, we found it easier to design and understand the FIR response giving the cutoff frequencies and transition bandwidth in Hz, instead of roll-off in octaves or decades.
- Use 0.5 Hz (or lower) as the cutoff frequency for the high-pass filter to remove DC and slow drifts but keeps the rest of the EEG spectra.
- Use ICA to remove non-brain/noisy components from the EEG, as this may improve the statistical power by keeping a higher number of trials in the data and maintaining the brain activity for the analysis.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Study | Electrodes 1 | SEP Components | Filter Class | Filter Order | Filter Roll Off/Transition Bandwidth | Filter Cutoff Frequency (Hz) |
|---|---|---|---|---|---|---|
| (Di Lorenzo et al., 2016) [32] | Skin | N9, N13, N20, P25, N33 | − | − | − | 0–450 |
| (Puta et al., 2016) [33] | Skin | N9, N20 | − | − | −12 dB/octave | 3–750 |
| (Haavik-Taylor and Murphy, 2007) [34] | Skin | N9, N11, N13, P14-N18 complex, N20 (P14-N20, N20-P27 complexes), N30 (P22-N30 complex) | − | − | −6 dB/octave | 3–1000 |
| (Fedele et al., 2017) [35] | ECoG | N20, HFO | Butterworth | 2 | −12 dB/octave | N20 (30–1000), HFO (400–1000) |
| (Roser et al., 2016) [36] | ECoG | N20, P40 | − | − | − | 10–1000 |
| (Baars and von Klitzing, 2017) [37] | Skin | N20 | − | − | − | 3–800 |
| (Ares et al., 2018) [38] | Scalp needle and Skin | − | − | − | − | Scalp needle (3–300), Skin (30–1000) |
| (Burnos et al., 2016) [39] | Skin and ECoG | N20, HFO | Butterworth | 2 | −12 dB/octave | N20 (30–1000), HFO (500–1000) |
| (Sakuma et al., 2004) [40] | Skin | N9, N13, N20, P25, HFO | − | − | − | HFO (400–800), rest (0.3–3000) |
| (Bailey et al., 2016) [41] | Skin | N20-P25 complex | − | − | − | 2–2500 |
| (Maegaki et al., 2000) [42] | ECoG | N20, P20, P25, N30 | − | − | − | 30–2000 |
| (Endisch et al., 2016) [43] | Skin | N20, N20-P25 complex, HFO | HFO (FIR) | − | − | HFO (450–750), rest (5–1500) |
| (Murakami et al., 2008) [44] | Skin | P14–N20, N20–P25, P25–N33, baseline-N13, N13onset-N13peak complexes | − | − | − | 0.3–3000 |
| (Andrew et al., 2015) [45] | Skin | N9, N13, N18 (P14–N18 complex), N20 (P14–N20 complex), N24 (P22–N24 complex), N30 (P22–N30 complex) | − | − | − | 0.2–1000 |
| (Mideksa et al., 2012) [46] | Skin | N20 | − | − | − | 0.01–200 |
| (Han et al., 2014) [47] | Skin | N20, P37 | − | − | − | 20–3000 |
| (Boostani et al., 2016) [48] | Skin | N9, N11, N13, N20 | − | − | − | 5–2000 |
| (Lelic et al., 2016) [15] | Skin | N30 | − | − | − | 1–1000 |
| (Haavik et al., 2017) [16] | Skin | N9, N11, N13, P14, N18, N20, N30 | − | − | −6 dB/octave | 3–1000 |
| (Taylor and Murphy, 2010) [18] | Skin | N9, N11, N13, P14, N18, N20, N30 | − | − | −6 dB/octave | 3–1000 |
| (Tinazzi et al., 2000) [49] | Skin | N9, N13, P14, N20, P27, N30 | − | − | −6 dB/octave | 5–1500 |
| (Haavik Taylor and Murphy, 2007) [17] | Skin | N9, N11, N13, P14, N18, N20, N30 | − | − | −6 dB/octave | 3–1000 |
| (Adhikari et al., 2016) [50] | Skin | N20, P24, P40, N48 | − | − | − | 10–250 |
| (Balzamo et al., 2004) [51] | ECoG | N20-P30, P20-N30 | − | − | − | 1–1000 |
| (Hoshiyama and Kakigi, 2000) [52] | Skin | N20, P25, N33, P20, N30 | − | − | − | 1–500 |
| (Kaňovský et al., 2003) [53] | ECoG | N30 | − | − | − | 10–1000 |
| (Tinazzi et al., 2000) [54] | Skin | N13, P14, N20, P27, N30 | − | − | −6 dB/octave | 5–1500 |
| (Taylor and Murphy, 2010) [55] | Skin | N9, N11 N13, P14, N18, N20 N30 | − | − | − | 3–1000 |
| (Van Rijn et al., 2009) [56] | Skin | N9, N14, N20, N35 | − | − | − | 30–1000 |
| (Costa et al., 2007) [57] | Scalp needle | − | − | − | − | 30–1000 |
| (Jin et al., 2014) [58] | Scalp needle | N20, P25 | − | − | − | 30–1000 |
References
- Toleikis, J.R. Intraoperative Monitoring Using Somatosensory Evoked Potentials. J. Clin. Monit. Comput. 2005, 19, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Nash, C.L.; Lorig, R.A.; Schatzinger, L.A.; Brown, R.H. Spinal cord monitoring during operative treatment of the spine. Clin. Orthop. Relat. Res. 1977, 126, 100–105. [Google Scholar] [CrossRef]
- Passmore, S.R.; Murphy, B.; Lee, T.D. The origin, and application of somatosensory evoked potentials as a neurophysiological technique to investigate neuroplasticity. J. Can. Chiropr. Assoc. 2014, 58, 170–183. [Google Scholar] [PubMed]
- Smith, S. Digital Signal Processing; Elsevier: Amsterdam, The Netherlands, 2003; ISBN 9780750674447. [Google Scholar]
- Widmann, A.; Schröger, E.; Maess, B. Digital filter design for electrophysiological data-a practical approach. J. Neurosci. Methods 2015, 250, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Acunzo, D.J.; MacKenzie, G.; van Rossum, M.C.W. Systematic biases in early ERP and ERF components as a result of high-pass filtering. J. Neurosci. Methods 2012, 209, 212–218. [Google Scholar] [CrossRef] [PubMed]
- VanRullen, R. Four common conceptual fallacies in mapping the time course of recognition. Front. Psychol. 2011, 2, 365. [Google Scholar] [CrossRef] [PubMed]
- American Clinical Neurophysiological Society. Guideline 11B: Recommended Standards for Intraoperative Monitoring of Somatosensory Evoked Potentials. 2009. Available online: http://www.acns.org/pdf/guidelines/Guideline-11B.pdf (accessed on 13 July 2018).
- American Clinical Neurophysiology Society. Guideline 9D: Guidelines on short-latency somatosensory evoked potentials. J. Clin. Neurophysiol. 2006, 23, 168–179. [Google Scholar] [CrossRef]
- American Electroencephalographic Society. Guideline Eleven: Guidelines for Intraoperative Monitoring of Sensory Evoked Potentials. J. Clin. Neurophysiol. 1994, 11, 77–87. [Google Scholar] [CrossRef]
- Cruccu, G.; Aminoff, M.J.; Curio, G.; Guerit, J.M.; Kakigi, R.; Mauguiere, F.; Rossini, P.M.; Treede, R.D.; Garcia-Larrea, L. Recommendations for the clinical use of somatosensory-evoked potentials. Clin. Neurophysiol. 2008, 119, 1705–1719. [Google Scholar] [CrossRef]
- Luck, S.J. An Introduction to The Event-Related Potential Technique; MIT Press: Cambridge, MA, USA, 2014; ISBN 9780262525855. [Google Scholar]
- Widmann, A.; Schröger, E. Filter effects and filter artifacts in the analysis of electrophysiological data. Front. Psychol. 2012, 3, 233. [Google Scholar] [CrossRef]
- Lelic, D.; Valeriani, M.; Fischer, I.W.D.; Dahan, A.; Drewes, A.M. Venlafaxine and oxycodone have different effects on spinal and supraspinal activity in man: a somatosensory evoked potential study. Br. J. Clin. Pharmacol. 2017, 83, 764–776. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lelic, D.; Niazi, I.K.; Holt, K.; Jochumsen, M.; Dremstrup, K.; Yielder, P.; Murphy, B.; Drewes, A.M.; Haavik, H. Manipulation of Dysfunctional Spinal Joints Affects Sensorimotor Integration in the Prefrontal Cortex: A Brain Source Localization Study. Neural Plast. 2016, 2016, 3704964. [Google Scholar] [CrossRef] [PubMed]
- Haavik, H.; Niazi, I.K.; Holt, K.; Murphy, B. Effects of 12 Weeks of Chiropractic Care on Central Integration of Dual Somatosensory Input in Chronic Pain Patients: A Preliminary Study. J. Manip. Physiol. Ther. 2017, 40, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Haavik, T.H.; Murphy, B.A. Altered cortical integration of dual somatosensory input following the cessation of a 20 min period of repetitive muscle activity. Exp. Brain Res. 2007, 178, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.H.; Murphy, B. Altered Central Integration of Dual Somatosensory Input After Cervical Spine Manipulation. J. Manip. Physiol. Ther. 2010, 33, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Calderon, J.; Luck, S.J. ERPLAB: An open-source toolbox for the analysis of event-related potentials. Front. Hum. Neurosci. 2014, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Bigdely-Shamlo, N.; Mullen, T.; Kothe, C.; Su, K.-M.; Robbins, K.A. The PREP pipeline: Standardized preprocessing for large-scale EEG analysis. Front. Neuroinform. 2015, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Onton, J. High-frequency broadband modulation of electroencephalographic spectra. Front. Hum. Neurosci. 2009, 3, 61. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Palmer, J.; Onton, J.; Oostenveld, R.; Makeig, S. Independent EEG sources are dipolar. PLoS ONE 2012, 7, e30135. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.P.; Makeig, S.; Humphries, C.; Lee, T.W.; Mckeown, M.J.; Iragui, V.; Sejnowski, T.J. Removing electroencephalographic artifacts by blind source separation. Psychophysiology 2000, 37, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Chaumon, M.; Bishop, D.V.M.; Busch, N.A. A practical guide to the selection of independent components of the electroencephalogram for artifact correction. J. Neurosci. Methods 2015, 250, 47–63. [Google Scholar] [CrossRef] [PubMed]
- R Core Team R. A Language and Environment for Statistical Computing; The R Development Core Team: Vienna, Austria, 2018. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R. Emmeans: Estimated Marginal Means, aka Least-Squares Means. 2018. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 28 December 2018).
- Allen, M.; Poggiali, D.; Whitaker, K.; Marshall, T.R.; Kievit, R. Raincloud plots: A multi-platform tool for robust data visualization. Peer J. Prepr. 2018, 6, e27137v1. [Google Scholar] [CrossRef] [PubMed]
- Cohen, X.M. Analyzing Neural Time Series Data: Theory and practice; MIT Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 2011, 1. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Coppola, G.; Bracaglia, M.; Di Lenola, D.; Evangelista, M.; Sirianni, G.; Rossi, P.; Di Lorenzo, G.; Serrao, M.; Parisi, V.; et al. Cortical functional correlates of responsiveness to short-lasting preventive intervention with ketogenic diet in migraine: A multimodal evoked potentials study. J. Headache Pain 2016, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Puta, C.; Franz, M.; Blume, K.R.; Gabriel, H.H.W.; Miltner, W.H.R.; Weiss, T. Are There Abnormalities in Peripheral and Central Components of Somatosensory Evoked Potentials in Non-Specific Chronic Low Back Pain? Front. Hum. Neurosci. 2016, 10, 521. [Google Scholar] [CrossRef]
- Haavik-Taylor, H.; Murphy, B. Cervical spine manipulation alters sensorimotor integration: A somatosensory evoked potential study. Clin. Neurophysiol. 2007, 118, 391–402. [Google Scholar] [CrossRef]
- Fedele, T.; Schönenberger, C.; Curio, G.; Serra, C.; Krayenbühl, N.; Sarnthein, J. Intraoperative subdural low-noise EEG recording of the high frequency oscillation in the somatosensory evoked potential. Clin. Neurophysiol. 2017, 128, 1851–1857. [Google Scholar] [CrossRef]
- Roser, F.; Ebner, F.H.; Liebsch, M.; Tatagiba, M.S.; Naros, G. The role of intraoperative neuromonitoring in adults with Chiari I malformation. Clin. Neurol. Neurosurg. 2016, 150, 27–32. [Google Scholar] [CrossRef]
- Baars, J.H.; von Klitzing, J.-P. Easily applicable SEP-monitoring of the N20 wave in the intensive care unit. Neurophys. Clin. Clin. Neurophysiol. 2017, 47, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Ares, W.J.; Grandhi, R.M.; Panczykowski, D.M.; Weiner, G.M.; Thirumala, P.; Habeych, M.E.; Crammond, D.J.; Horowitz, M.B.; Jankowitz, B.T.; Jadhav, A.; et al. Diagnostic accuracy of somatosensory evoked potential monitoring in evaluating neurological complications during endovascular aneurysm treatment. Op. Neurosurg. 2018, 14, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Burnos, S.; Fedele, T.; Schmid, O.; Krayenbühl, N.; Sarnthein, J. Detectability of the somatosensory evoked high frequency oscillation (HFO) co-recorded by scalp EEG and ECoG under propofol. NeuroImage Clin. 2016, 10, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, K.; Takeshima, T.; Ishizaki, K.; Nakashima, K. Somatosensory evoked high-frequency oscillations in migraine patients. Clin. Neurophysiol. 2004, 115, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.Z.; Asmussen, M.J.; Nelson, A.J. Short-latency afferent inhibition determined by the sensory afferent volley. J. Neurophysiol. 2016, 116, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Maegaki, Y.; Najm, I.; Terada, K.; Morris, H.H.; Bingaman, W.E.; Kohaya, N.; Takenobu, A.; Kadonaga, Y.; Lüders, H.O. Somatosensory evoked high-frequency oscillations recorded directly from the human cerebral cortex. Clin. Neurophysiol. 2000, 111, 1916–1926. [Google Scholar] [CrossRef]
- Endisch, C.; Waterstraat, G.; Storm, C.; Ploner, C.J.; Curio, G.; Leithner, C. Cortical somatosensory evoked high-frequency (600 Hz) oscillations predict absence of severe hypoxic encephalopathy after resuscitation. Clin. Neurophysiol. 2016, 127, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Sakuma, K.; Nakashima, K. Somatosensory evoked potentials and high-frequency oscillations in athletes. Clin. Neurophysiol. 2008, 119, 2862–2869. [Google Scholar] [CrossRef] [PubMed]
- Andrew, D.; Haavik, H.; Dancey, E.; Yielder, P.; Murphy, B. Somatosensory evoked potentials show plastic changes following a novel motor training task with the thumb. Clin. Neurophysiol. 2015, 126, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Mideksa, K.G.; Hellriegel, H.; Hoogenboom, N.; Krause, H.; Schnitzler, A.; Deuschl, G.; Raethjen, J.; Heute, U.; Muthuraman, M. Source analysis of median nerve stimulated somatosensory evoked potentials and fields using simultaneously measured EEG and MEG signals. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 4903–4906. [Google Scholar]
- Han, E.Y.; Jung, H.Y.; Kim, M.O. Absent median somatosensory evoked potential is a predictor of type I complex regional pain syndrome after stroke. Disabil. Rehabilit. 2014, 36, 1080–1084. [Google Scholar] [CrossRef]
- Boostani, R.; Poorzahed, A.; Ahmadi, Z.; Mellat, A. Median Nerve Somatosensory Evoked Potential in HTLV-I Associated Myelopathy. Electron. Phys. 2016, 8, 2361–2365. [Google Scholar] [CrossRef] [PubMed]
- Tinazzi, M.; Priori, A.; Bertolasi, L.; Frasson, E.; Mauguière, F.; Fiaschi, A. Abnormal central integration of a dual somatosensory input in dystonia. Evidence for sensory overflow. Brain 2000, 123, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, R.B.; Takeda, M.; Kolakshyapati, M.; Sakamoto, S.; Morishige, M.; Kiura, Y.; Okazaki, T.; Shinagawa, K.; Ichinose, N.; Yamaguchi, S.; et al. Somatosensory evoked potentials in carotid artery stenting: Effectiveness in ascertaining cerebral ischemic events. J. Clin. Neurosci. 2016, 30, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Balzamo, E.; Marquis, P.; Chauvel, P.; Régis, J. Short-latency components of evoked potentials to median nerve stimulation recorded by intracerebral electrodes in the human pre- and postcentral areas. Clin. Neurophysiol. 2004, 115, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Hoshiyama, M.; Kakigi, R. Vibratory stimulation of proximal muscles does not affect cortical components of somatosensory evoked potential following distal nerve stimulation. Clin. Neurophysiol. 2000, 111, 1607–1610. [Google Scholar] [CrossRef]
- Kaňovský, P.; Bareš, M.; Rektor, I. The selective gating of the N30 cortical component of the somatosensory evoked potentials of median nerve is different in the mesial and dorsolateral frontal cortex: Evidence from intracerebral recordings. Clin. Neurophysiol. 2003, 114, 981–991. [Google Scholar] [CrossRef]
- Tinazzi, M.; Fiaschi, A.; Rosso, T.; Faccioli, F.; Grosslercher, J.; Aglioti, S.M. Neuroplastic changes related to pain occur at multiple levels of the human somatosensory system: A somatosensory-evoked potentials study in patients with cervical radicular pain. J. Neurosci. 2000, 20, 9277–9283. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.H.; Murphy, B. The Effects of Spinal Manipulation on Central Integration of Dual Somatosensory Input Observed After Motor Training: A Crossover Study. J. Manip. Physiol. Ther. 2010, 33, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Van Rijn, M.A.; Van Hilten, J.J.; Van Dijk, J.G. Spatiotemporal integration of sensory stimuli in complex regional pain syndrome and dystonia. J. Neural Transm. 2009, 116, 559–565. [Google Scholar] [CrossRef]
- Costa, P.; Bruno, A.; Bonzanino, M.; Massaro, F.; Caruso, L.; Vincenzo, I.; Ciaramitaro, P.; Montalenti, E. Somatosensory- and motor-evoked potential monitoring during spine and spinal cord surgery. Spinal Cord 2007, 45, 86–91. [Google Scholar] [CrossRef]
- Jin, S.-H.; Chung, C.K.; Kim, J.E.; Choi, Y.D. A new measure for monitoring intraoperative somatosensory evoked potentials. J. Korean Neurosurg. Soc. 2014, 56, 455–462. [Google Scholar] [CrossRef] [PubMed]
| Filter | Frequency (Hz) | ICA | N30 Amplitude (μV) (Mean ± SD) |
|---|---|---|---|
| FIR | 0.5–1000 | Yes | 2.85 ± 1.87 |
| No | 3.38 ± 1.84 | ||
| 3–1000 | Yes | 2.89 ± 1.89 | |
| No | 3.42 ± 1.85 | ||
| 30–1000 | Yes | 2.03 ± 1.01 | |
| No | 2.31 ± 1.17 | ||
| IIR | 0.5–1000 | Yes | 2.96 ± 1.83 |
| No | 3.39 ± 1.81 | ||
| 3–1000 | Yes | 2.96 ± 1.84 | |
| No | 3.38 ± 1.82 | ||
| 30–1000 | Yes | 2.00 ± 0.96 | |
| No | 2.24 ± 1.04 |
| Model Coefficients | Estimate | Standard Error | t Value | p Value | |
|---|---|---|---|---|---|
| Intercept | 1.11 | 0.14 | 8.00 | <0.0001 | |
| Filter = IIR | 0.01 | 0.04 | 0.13 | 0.8930 | |
| ICA = Yes | −0.21 | 0.04 | −4.59 | <0.0001 | |
| Frequency = 3–1000 | 0.01 | 0.04 | 0.28 | 0.7800 | |
| Frequency = 30–1000 | −0.35 | 0.04 | −7.82 | <0.0001 | |
| Filter = IIR: ICA = Yes | 0.04 | 0.06 | 0.64 | 0.5210 | |
| Filter = IIR: Frequency = 3–1000 | 0.02 | 0.06 | −0.24 | 0.8120 | |
| Filter = IIR: Frequency = 30–1000 | 0.03 | 0.06 | −0.46 | 0.6480 | |
| ICA = Yes: Frequency = 3–1000 | 0.00 | 0.06 | 0.03 | 0.9800 | |
| ICA = Yes: Frequency = 30–1000 | 0.07 | 0.06 | 1.14 | 0.2540 | |
| Filter = IIR: ICA = Yes: Frequency = 3–1000 | −0.00 | 0.09 | −0.01 | 0.9910 | |
| Filter = IIR: ICA = Yes: Frequency = 30–1000 | −0.04 | 0.09 | −0.41 | 0.6830 | |
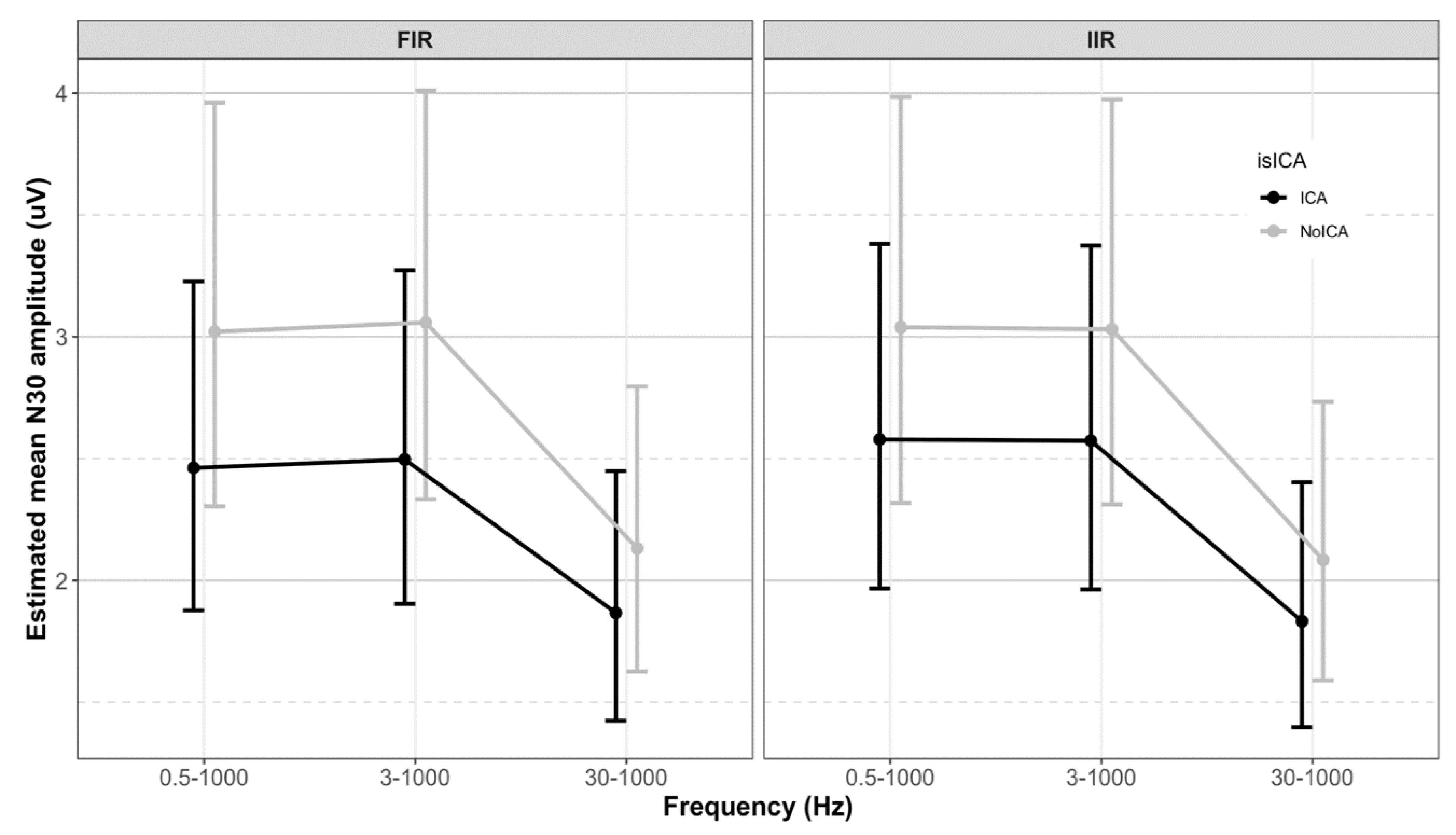
| Filter | Frequency (Hz) | ICA | N30 Amplitude (μV) | Standard Error (μV) | 95% CI LCL | 95% CI UCL |
|---|---|---|---|---|---|---|
| FIR | 0.5–1000 | Yes | 2.46 | 0.34 | 1.88 | 3.23 |
| No | 3.02 | 0.42 | 2.30 | 3.96 | ||
| 3–1000 | Yes | 2.50 | 0.35 | 1.90 | 3.27 | |
| No | 3.06 | 0.42 | 2.33 | 4.01 | ||
| 30–1000 | Yes | 1.87 | 0.26 | 1.42 | 2.45 | |
| No | 2.13 | 0.29 | 1.63 | 2.80 | ||
| IIR | 0.5–1000 | Yes | 2.58 | 0.36 | 1.97 | 3.38 |
| No | 3.04 | 0.42 | 2.32 | 3.98 | ||
| 3–1000 | Yes | 2.57 | 0.36 | 1.96 | 3.37 | |
| No | 3.03 | 0.42 | 2.31 | 3.97 | ||
| 30–1000 | Yes | 1.83 | 0.25 | 1.40 | 2.40 | |
| No | 2.08 | 0.29 | 1.59 | 2.73 |
| ICA | Frequency (Hz) | Ratio (FIR/IIR) | Standard Error (μV) | 95% CI LCL | 95% CI UCL | z Ratio | p Value |
|---|---|---|---|---|---|---|---|
| Yes | 0.5–1000 | 0.95 | 0.04 | 0.87 | 1.04 | −1.04 | 0.2972 |
| 3–1000 | 0.97 | 0.04 | 0.89 | 1.06 | −0.68 | 0.4943 | |
| 30–1000 | 1.02 | 0.05 | 0.93 | 1.11 | 0.42 | 0.6755 | |
| No | 0.5–1000 | 0.99 | 0.04 | 0.91 | 1.08 | −0.13 | 0.8932 |
| 3–1000 | 1.01 | 0.04 | 0.92 | 1.10 | 0.20 | 0.8396 | |
| 30–1000 | 1.02 | 0.05 | 0.94 | 1.12 | 0.51 | 0.6095 |
| Filter | ICA | Contrast | Ratio | Standard Error (μV) | 95% CI LCL | 95% CI UCL | z Ratio | p Value |
|---|---|---|---|---|---|---|---|---|
| FIR | Yes | 0.5–1000/3–1000 | 0.99 | 0.04 | 0.89 | 1.09 | −0.32 | 0.9467 |
| 0.5–1000/30–1000 | 1.32 | 0.06 | 1.19 | 1.46 | 6.20 | <0.0001 | ||
| 3–1000/30–1000 | 1.34 | 0.06 | 1.20 | 1.48 | 6.52 | <0.0001 | ||
| No | 0.5–1000/3–1000 | 0.99 | 0.04 | 0.89 | 1.10 | −0.28 | 0.9579 | |
| 0.5–1000/30–1000 | 1.42 | 0.06 | 1.28 | 1.57 | 7.82 | <0.0001 | ||
| 3–1000/30–1000 | 1.43 | 0.06 | 1.29 | 1.59 | 8.09 | <0.0001 | ||
| IIR | Yes | 0.5–1000/3–1000 | 1.00 | 0.04 | 0.90 | 1.11 | 0.04 | 0.9989 |
| 0.5–1000/30–1000 | 1.41 | 0.06 | 1.27 | 1.56 | 7.66 | <0.0001 | ||
| 3–1000/30–1000 | 1.40 | 0.06 | 1.27 | 1.56 | 7.62 | <0.0001 | ||
| No | 0.5–1000/3–1000 | 1.00 | 0.04 | 0.90 | 1.11 | 0.06 | 0.9982 | |
| 0.5–1000/30–1000 | 1.46 | 0.06 | 1.31 | 1.62 | 8.46 | <0.0001 | ||
| 3–1000/30–1000 | 1.45 | 0.06 | 1.31 | 1.61 | 8.41 | <0.0001 |
| Filter | Frequency (Hz) | Ratio (NoICA/ICA) | Standard Error (μV) | 95% CI LCL | 95% CI UCL | z Ratio | p Value |
|---|---|---|---|---|---|---|---|
| FIR | 0.5–1000 | 1.23 | 0.05 | 1.12 | 1.34 | 4.59 | <0.0001 |
| 3–1000 | 1.23 | 0.05 | 1.12 | 1.34 | 4.56 | <0.0001 | |
| 30–1000 | 1.14 | 0.05 | 1.05 | 1.25 | 2.98 | 0.0029 | |
| IIR | 0.5–1000 | 1.18 | 0.05 | 1.08 | 1.29 | 3.69 | 0.0002 |
| 3–1000 | 1.18 | 0.05 | 1.08 | 1.29 | 3.67 | 0.0002 | |
| 30–1000 | 1.14 | 0.05 | 1.04 | 1.24 | 2.89 | 0.0039 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navid, M.S.; Niazi, I.K.; Lelic, D.; Drewes, A.M.; Haavik, H. The Effects of Filter’s Class, Cutoff Frequencies, and Independent Component Analysis on the Amplitude of Somatosensory Evoked Potentials Recorded from Healthy Volunteers. Sensors 2019, 19, 2610. https://doi.org/10.3390/s19112610
Navid MS, Niazi IK, Lelic D, Drewes AM, Haavik H. The Effects of Filter’s Class, Cutoff Frequencies, and Independent Component Analysis on the Amplitude of Somatosensory Evoked Potentials Recorded from Healthy Volunteers. Sensors. 2019; 19(11):2610. https://doi.org/10.3390/s19112610
Chicago/Turabian StyleNavid, Muhammad Samran, Imran Khan Niazi, Dina Lelic, Asbjørn Mohr Drewes, and Heidi Haavik. 2019. "The Effects of Filter’s Class, Cutoff Frequencies, and Independent Component Analysis on the Amplitude of Somatosensory Evoked Potentials Recorded from Healthy Volunteers" Sensors 19, no. 11: 2610. https://doi.org/10.3390/s19112610
APA StyleNavid, M. S., Niazi, I. K., Lelic, D., Drewes, A. M., & Haavik, H. (2019). The Effects of Filter’s Class, Cutoff Frequencies, and Independent Component Analysis on the Amplitude of Somatosensory Evoked Potentials Recorded from Healthy Volunteers. Sensors, 19(11), 2610. https://doi.org/10.3390/s19112610







