Point-of-Care Surface Plasmon Resonance Biosensor for Stroke Biomarkers NT-proBNP and S100β Using a Functionalized Gold Chip with Specific Antibody
Abstract
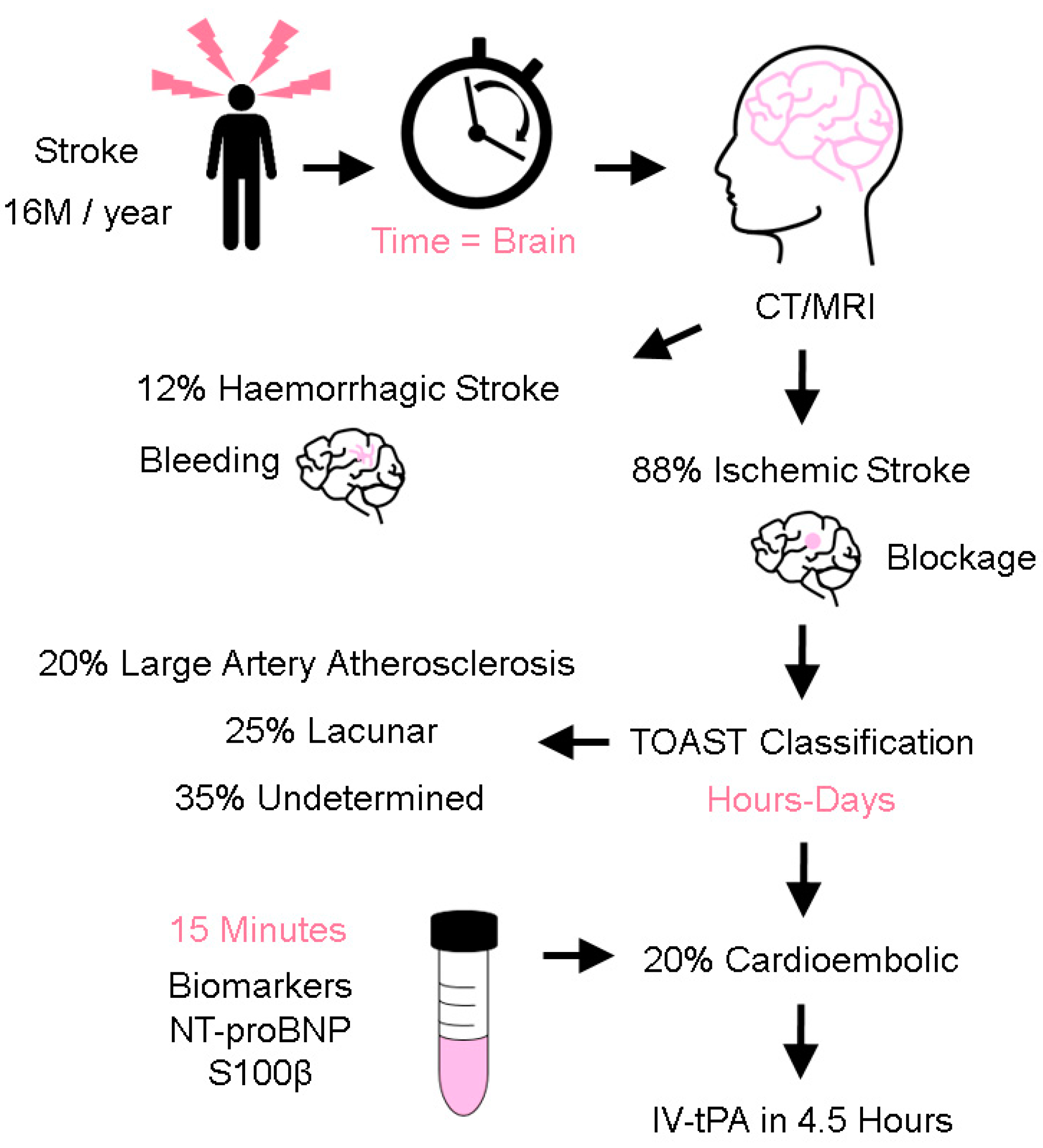
1. Introduction
2. Materials and Methods
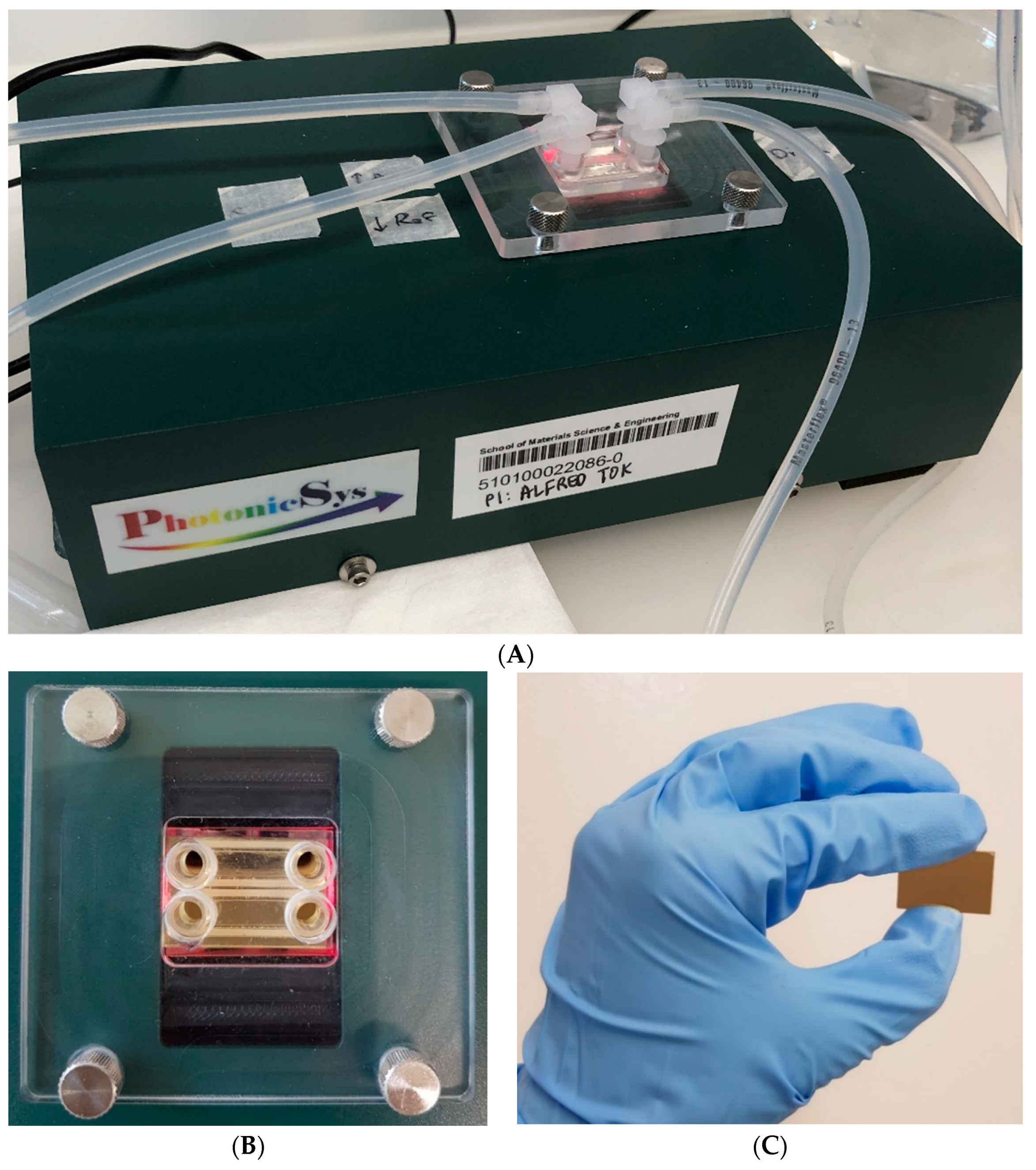
2.1. Equipment
2.2. Materials
2.3. Porcine Plasma
2.4. Device Calibration
2.5. Gold Chip Functionalization
2.6. Biomarker Measurement
2.7. Data Analysis
3. Results and Discussion
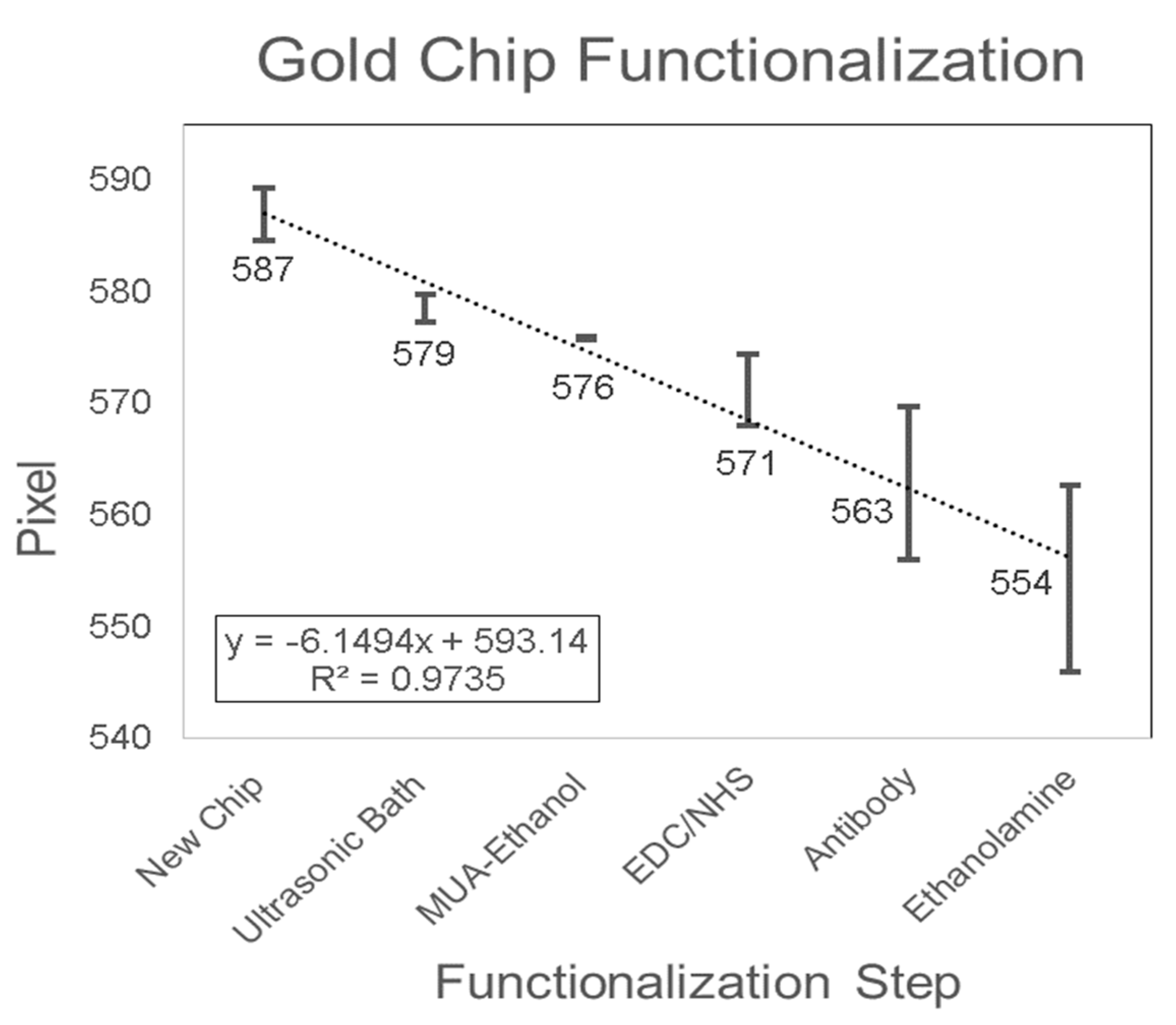
3.1. Gold Chip Functionalization
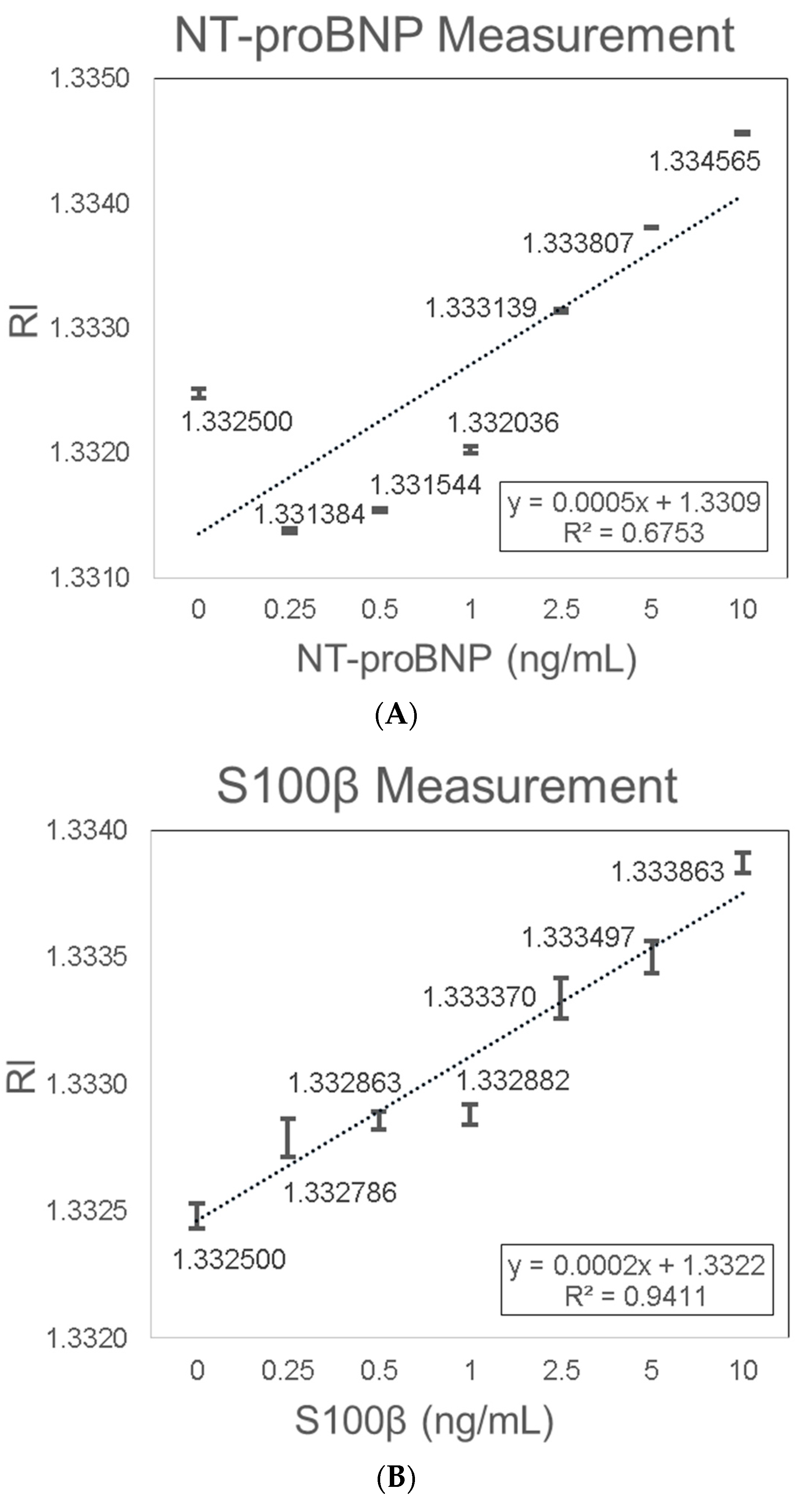
3.2. Biomarkers Measurement
3.3. Validation in Spiked Porcine Plasma Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harpaz, D.; Eltzov, E.; Seet, R.C.S.; Marks, R.S.; Tok, A.I.Y. Point-of-Care-Testing in Acute Stroke Management: An Unmet Need Ripe for Technological Harvest. Biosensors 2017, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Horgan, N.F.; O’Regan, M.; Cunningham, C.J.; Finn, A.M. Recovery after stroke: A 1-year profile. Disabil. Rehabil. 2009, 31, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, A. Human and economic burden of stroke. Age Ageing 2009, 38, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.P.; del Zoppo, G.; Alberts, M.J.; Bhatt, D.L.; Brass, L.; Furlan, A.; Grubb, R.L.; Higashida, R.T.; Jauch, E.C.; Kidwell, C.; et al. Guidelines for the Early Management of Adults with Ischemic Stroke: A Guideline From the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 2007, 38, 1655–1711. [Google Scholar] [PubMed]
- Hemmen, T.M.; Meyer, B.C.; McClean, T.L.; Lyden, P.D. Identification of nonischemic stroke mimics among 411 code strokes at the University of California, San Diego, Stroke Center. J. Stroke Cerebrovasc. Dis. 2008, 17, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L. Time is brain—Quantified. Stroke 2006, 37, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Landau, W.M.; Nassief, A. Editorial comment—Time to burn the TOAST. Stroke 2005, 36, 902–904. [Google Scholar] [CrossRef]
- Llombart, V.; Antolin-Fontes, A.; Bustamante, A.; Giralt, D.; Rost, N.S.; Furie, K.; Shibazaki, K.; Biteker, M.; Castillo, J.; Rodriguez-Yanez, M.; et al. B-type natriuretic peptides help in cardioembolic stroke diagnosis: Pooled data meta-analysis. Stroke 2015, 46, 1187–1195. [Google Scholar] [CrossRef]
- Kidwell, C.S.; Chalela, J.A.; Saver, J.L.; Starkman, S.; Hill, M.D.; Demchuk, A.M.; Butman, J.A.; Patronas, N.; Alger, J.R.; Latour, L.L.; et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA 2004, 292, 1823–1830. [Google Scholar] [CrossRef]
- Amarenco, P.; Bogousslavsky, J.; Caplan, L.R.; Donnan, G.A.; Hennerici, M.G. Classification of stroke subtypes. Cerebrovasc. Dis. 2009, 27, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Seet, R.C.S.; Friedman, P.A.; Rabinstein, A.A. Prolonged Rhythm Monitoring for the Detection of Occult Paroxysmal Atrial Fibrillation in Ischemic Stroke of Unknown Cause. Circulation 2011, 124, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Ng, G.J.L.; Quek, A.M.L.; Cheung, C.; Arumugam, T.V.; Seet, R.C.S. Stroke biomarkers in clinical practice: A critical appraisal. Neurochem. Int. 2017, 107, 11–22. [Google Scholar] [CrossRef]
- Strimbu, K.; Tavel, J.A. What are biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, A.; Lopez-Cancio, E.; Pich, S.; Penalba, A.; Giralt, D.; Garcia-Berrocoso, T.; Ferrer-Costa, C.; Gasull, T.; Hernandez-Perez, M.; Millan, M.; et al. Blood Biomarkers for the Early Diagnosis of Stroke: The Stroke-Chip Study. Stroke 2017, 48, 2419–2425. [Google Scholar] [CrossRef]
- Kawase, S.; Kowa, H.; Suto, Y.; Fukuda, H.; Kusumi, M.; Nakayasu, H.; Nakashima, K. Plasma Brain Natriuretic Peptide is a Marker of Prognostic Functional Outcome in Non-Cardioembolic Infarction. J. Stroke Cerebrovasc. Dis. 2015, 24, 2285–2290. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, M.; He, M.; Zeng, H.; Tan, F.; Li, K.; Chen, S.; Han, Q.; Wang, Q. Validation of the use of B-type natriuretic peptide point-of-care test platform in preliminary recognition of cardioembolic stroke patients in the ED. Am. J. Emerg. Med. 2015, 33, 521–526. [Google Scholar] [CrossRef]
- Chaudhuri, J.R.; Sharma, V.K.; Mridula, K.R.; Balaraju, B.; Bandaru, V.C. Association of plasma brain natriuretic peptide levels in acute ischemic stroke subtypes and outcome. J. Stroke Cerebrovasc. Dis. 2015, 24, 485–491. [Google Scholar] [CrossRef]
- Cojocaru, I.M.; Cojocaru, M.; Sapira, V.; Ionescu, A.; Barlan, S.; Tacu, N. Could pro-BNP, uric acid, bilirubin, albumin and transferrin be used in making the distinction between stroke subtypes? Rom. J. Intern. Med. 2013, 51, 188–195. [Google Scholar] [CrossRef]
- Kara, K.; Gronewold, J.; Neumann, T.; Mahabadi, A.A.; Weimar, C.; Lehmann, N.; Berger, K.; Kalsch, H.I.; Bauer, M.; Broecker-Preuss, M.; et al. B-type natriuretic peptide predicts stroke of presumable cardioembolic origin in addition to coronary artery calcification. Eur. J. Neurol. 2014, 21, 914–921. [Google Scholar] [CrossRef]
- Maruyama, K.; Shiga, T.; Iijima, M.; Moriya, S.; Mizuno, S.; Toi, S.; Arai, K.; Ashihara, K.; Abe, K.; Uchiyama, S. Brain natriuretic peptide in acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2014, 23, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Shibazaki, K.; Kimura, K.; Aoki, J.; Kobayashi, K.; Fujii, S.; Okada, Y. Brain natriuretic peptide as a predictor of cardioembolism in acute ischemic stroke patients: Brain natriuretic peptide stroke prospective study. Eur. Neurol. 2013, 69, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Balion, C.; McKelvie, R.; Don-Wauchope, A.C.; Santaguida, P.L.; Oremus, M.; Keshavarz, H.; Hill, S.A.; Booth, R.A.; Ali, U.; Brown, J.A.; et al. B-type natriuretic peptide-guided therapy: A systematic review. Heart Fail. Rev. 2014, 19, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Clerico, A.; Franzini, M.; Masotti, S.; Prontera, C.; Passino, C. State of the art of immunoassay methods for B-type natriuretic peptides: An update. Crit. Rev. Clin. Lab. Sci. 2015, 52, 56–69. [Google Scholar] [CrossRef]
- Hajsadeghi, S.; Kashani Amin, L.; Bakhshandeh, H.; Rohani, M.; Azizian, A.R.; Jafarian Kerman, S.R. The diagnostic value of N-terminal pro-brain natriuretic peptide in differentiating cardioembolic ischemic stroke. J. Stroke Cerebrovasc. Dis. 2013, 22, 554–560. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, Z.; He, M.; Yang, L.; Xu, W. Experiences and the use of BNP POCT platform on suspected stroke patients by a Chinese emergency department. Ann. Indian Acad. Neurol. 2014, 17, 243–244. [Google Scholar]
- Shibazaki, K.; Kimura, K.; Iguchi, Y.; Okada, Y.; Inoue, T. Plasma brain natriuretic peptide can be a biological marker to distinguish cardioembolic stroke from other stroke types in acute ischemic stroke. Intern. Med. 2009, 48, 259–264. [Google Scholar] [CrossRef]
- Yang, H.L.; Lin, Y.P.; Long, Y.; Ma, Q.L.; Zhou, C. Predicting cardioembolic stroke with the B-type natriuretic peptide test: A systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 2014, 23, 1882–1889. [Google Scholar] [CrossRef]
- Fonseca, A.C.; Matias, J.S.; Pinho e Melo, T.; Falcao, F.; Canhao, P.; Ferro, J.M. N-terminal probrain natriuretic peptide as a biomarker of cardioembolic stroke. Int. J. Stroke 2011, 6, 398–403. [Google Scholar] [CrossRef]
- Herrmann, M.; Ehrenreich, H. Brain derived proteins as markers of acute stroke: Their relation to pathophysiology, outcome prediction and neuroprotective drug monitoring. Restor. Neurol. Neurosci. 2003, 21, 177–190. [Google Scholar]
- Herrmann, M.; Vos, P.; Wunderlich, M.T.; de Bruijn, C.H.; Lamers, K.J. Release of glial tissue-specific proteins after acute stroke: A comparative analysis of serum concentrations of protein S-100B and glial fibrillary acidic protein. Stroke 2000, 31, 2670–2677. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, M.T.; Ebert, A.D.; Kratz, T.; Goertler, M.; Jost, S.; Herrmann, M. Early neurobehavioral outcome after stroke is related to release of neurobiochemical markers of brain damage. Stroke 1999, 30, 1190–1195. [Google Scholar] [CrossRef]
- Cata, J.P.; Abdelmalak, B.; Farag, E. Neurological biomarkers in the perioperative period. Br. J. Anaesth. 2011, 107, 844–858. [Google Scholar] [CrossRef]
- Yokobori, S.; Hosein, K.; Burks, S.; Sharma, I.; Gajavelli, S.; Bullock, R. Biomarkers for the clinical differential diagnosis in traumatic brain injury—A systematic review. CNS Neurosci. Ther. 2013, 19, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Kapural, M.; Krizanac-Bengez, L.; Barnett, G.; Perl, J.; Masaryk, T.; Apollo, D.; Rasmussen, P.; Mayberg, M.R.; Janigro, D. Serum S-100beta as a possible marker of blood-brain barrier disruption. Brain Res. 2002, 940, 102–104. [Google Scholar] [CrossRef]
- Gazzolo, D.; Abella, R.; Frigiola, A.; Giamberti, A.; Tina, G.; Nigro, F.; Florio, P.; Colivicchi, M.; Temporini, F.; Ricotti, A.; et al. Neuromarkers and unconventional biological fluids. J. Matern. Fetal Neonatal Med. 2010, 23, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.J.; Yandle, T.G.; Nicholls, M.G.; Richards, A.M.; Espiner, E.A. The amino-terminal portion of pro-brain natriuretic peptide (Pro-BNP) circulates in human plasma. Biochem. Biophys. Res. Commun. 1995, 214, 1175–1183. [Google Scholar] [CrossRef]
- Atisha, D.; Bhalla, M.A.; Morrison, L.K.; Felicio, L.; Clopton, P.; Gardetto, N.; Kazanegra, R.; Chiu, A.; Maisel, A.S. A prospective study in search of an optimal B-natriuretic peptide level to screen patients for cardiac dysfunction. Am. Heart J. 2004, 148, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Sudoh, T.; Kangawa, K.; Minamino, N.; Matsuo, H. A new natriuretic peptide in porcine brain. Nature 1988, 332, 78–81. [Google Scholar] [CrossRef]
- Ziskoven, D.F.W.; Holthausen, U.; Menz, G.; Addicks, K.; Rippegater GIn Kaufmann, W.; Wambach, G. Calcium Calmodulin Antagonists Influences the release of Cardiodilatin/ANP from Atrial Cardiocytes. In Handbook Endocrinology of the Heart; Springer: Berlin, Germany, 1989. [Google Scholar]
- Weber, M.; Hamm, C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart 2006, 92, 843–849. [Google Scholar] [CrossRef]
- Donato, R. Intracellular and extracellular roles of S100 proteins. Microsc. Res. Tech. 2003, 60, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Kaca-Orynska, M.; Tomasiuk, R.; Friedman, A. Neuron-specific enolase and S 100B protein as predictors of outcome in ischaemic stroke. Neurol. Neurochir. Pol. 2010, 44, 459–463. [Google Scholar] [CrossRef]
- Selcuk, O.; Yayla, V.; Cabalar, M.; Guzel, V.; Uysal, S.; Gedikbasi, A. The Relationship of Serum S100B Levels with Infarction Size and Clinical Outcome in Acute Ischemic Stroke Patients. Noro Psikiyatr. Ars. 2014, 51, 395. [Google Scholar] [CrossRef] [PubMed]
- Glushakova, O.; Glushakov, A.; Miller, E.; Valadka, A.; Hayes, R. Biomarkers for acute diagnosis and management of stroke in neurointensive care units. Brain Circ. 2016, 2, 28–47. [Google Scholar] [CrossRef] [PubMed]
- Donato, R. S100: A multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int. J. Biochem. Cell Boil. 2001, 33, 637–668. [Google Scholar] [CrossRef]
- Mariani, S.; Minunni, M. Surface plasmon resonance applications in clinical analysis. Anal. Bioanal. Chem. 2014, 406, 2303–2323. [Google Scholar] [CrossRef] [PubMed]
- Pitarke, J.M.; Silkin, V.M.; Chulkov, E.V.; Echenique, P.M. Theory of surface plasmons and surface-plasmon polaritons. Rep. Prog. Phys. 2006, 70. [Google Scholar] [CrossRef]
- Wijaya, E.; Lenaerts, C.; Maricot, S.; Hastanin, J.; Habraken, S.; Vilcot, J.-P.; Boukherroub, R.; Szunerits, S. Surface plasmon resonance-based biosensors: From the development of different SPR structures to novel surface functionalization strategies. Curr. Opin. Solid State Mater. Sci. 2011, 15, 208–224. [Google Scholar] [CrossRef]
- Abdulhalim, I. Coupling configurations between extended surface electromagnetic waves and localized surface plasmons for ultrahigh field enhancement. Nanophotonics 2018, 7, 1891–1916. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Abdulhalim, I. Self-referenced sensor utilizing extra-ordinary optical transmission from metal nanoslits array. Opt. Lett. 2015, 40, 2425–2428. [Google Scholar] [CrossRef]
- Photomicsys Technology. 2019. Available online: https://www.photonicsys.com/technology (accessed on 4 March 2019).
- Abdulhalim, I.; Zourob, M.; Lakhtakia, A. Surface Plasmon Resonance for Biosensing: A Mini-Review. Electromagnetics 2008, 28, 214–242. [Google Scholar] [CrossRef]
- Manuel, M.; Vidal, B.; López, R.; Alegret, S.; Alonso-Chamarro, J.; Garces, I.; Mateo, J. Determination of probable alcohol yield in musts by means of an SPR optical sensor. Sens. Actuators B Chem. 1993, 11, 455–459. [Google Scholar] [CrossRef]
- Liedberg, B.; Lundström, I.; Stenberg, E. Principles of biosensing with an extended coupling matrix and surface plasmon resonance. Sens. Actuators B Chem. 1993, 11, 63–72. [Google Scholar] [CrossRef]
- Watad, I.; Abdulhalim, I. Phase-shifted polarimetric surface plasmon resonance sensor using a liquid crystal retarder and a diverging beam. Opt. Lett. 2019, 44, 1607–1610. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Surface Plasmon Resonance Based Sensors. In Springer Series on Chemical Sensors and Biosensors; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Lecaruyer, P.; Canva, M.; Rolland, J. Metallic film optimization in a surface plasmon resonance biosensor by the extended Rouard method. Appl. Opt. 2007, 46, 2361–2369. [Google Scholar] [CrossRef] [PubMed]
- Szunerits, S.; Castel, X.; Boukherroub, R. Surface Plasmon Resonance Investigation of Silver and Gold Films Coated with Thin Indium Tin Oxide Layers: Influence on Stability and Sensitivity. J. Phys. Chem. C 2008, 112, 15813–15817. [Google Scholar] [CrossRef]
- Ulman, A. Formation and Structure of Self-Assembled Monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef]
- Touahir, L.; Niedziól̷ka-Jönsson, J.; Galopin, E.; Boukherroub, R.; Gouget-Laemmel, A.C.; Solomon, I.; Petukhov, M.; Chazalviel, J.-N.; Ozanam, F.; Szunerits, S. Surface Plasmon Resonance on Gold and Silver Films Coated with Thin Layers of Amorphous Silicon—Carbon Alloys. Langmuir 2010, 26, 6058–6065. [Google Scholar] [CrossRef]
- Johnsson, B.; Löfås, S.; Lindquist, G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Anal. Biochem. 1991, 198, 268–277. [Google Scholar] [CrossRef]
- Chen, Y.; Ming, H. Review of surface plasmon resonance and localized surface plasmon resonance sensor. Photonic Sens. 2012, 2, 37–49. [Google Scholar] [CrossRef]
- Fujiwara, K.; Watarai, H.; Itoh, H.; Nakahama, E.; Ogawa, N. Measurement of antibody binding to protein immobilized on gold nanoparticles by localized surface plasmon spectroscopy. Anal. Bioanal. Chem. 2006, 386, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, Y.; Jauw, J.; Linman, M.J.; Cheng, Q. Highly Sensitive Detection of Protein Toxins by Surface Plasmon Resonance with Biotinylation-Based Inline Atom Transfer Radical Polymerization Amplification. Anal. Chem. 2010, 82, 3679–3685. [Google Scholar] [CrossRef] [PubMed]
- Bassoa, C.R.; Tozato, C.C.; Junior, J.P.A.; Pedrosaa, V.A. A fast and highly sensitive method for the detection of canine distemper virus using gold nanoparticles. Anal. Methods 2015, 7, 2264–2267. [Google Scholar] [CrossRef]
- Karabchevsky, A.; Tsapovsky, L.; Marks, R.S.; Abdulhalim, I. Study of Immobilization Procedure on Silver Nanolayers and Detection of Estrone with Diverged Beam Surface Plasmon Resonance (SPR) Imaging. Biosensors 2013, 3, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Holland, W.R.; Hall, D.G. Surface-plasmon dispersion relation: Shifts induced by the interaction with localized plasma resonances. Phys. Rev. B 1983, 27, 7765–7768. [Google Scholar] [CrossRef]
- Kausaite, A.; van Dijk, M.; Castrop, J.; Ramanaviciene, A.; Baltrus, J.P.; Acaite, J.; Ramanavicius, A. Surface plasmon resonance label-free monitoring of antibody antigen interactions in real time. Biochem. Mol. Biol. Educ. 2007, 35, 57–63. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Chik, C.E.N.C.E.; Mahdi, M.A. Development of an optical sensor based on surface plasmon resonance phenomenon for diagnosis of dengue virus E-protein. Sens. Bio Sens. Res. 2018, 20, 16–21. [Google Scholar] [CrossRef]
- Luo, B.; Wu, S.; Zhang, Z.; Zou, W.; Shi, S.; Zhao, M.; Zhong, N.; Liu, Y.; Zou, X.; Wang, L.; et al. Human heart failure biomarker immunosensor based on excessively tilted fiber gratings. Biomed. Opt. Express 2016, 8, 57–67. [Google Scholar] [CrossRef]
- Teramura, Y.; Arima, Y.; Iwata, H. Surface plasmon resonance-based highly sensitive immunosensing for brain natriuretic peptide using nanobeads for signal amplification. Anal. Chem. 2006, 357, 208–215. [Google Scholar] [CrossRef]
- Jang, H.R.; Wark, A.W.; Baek, S.H.; Chung, B.H.; Lee, H.J. Ultrasensitive and Ultrawide Range Detection of a Cardiac Biomarker on a Surface Plasmon Resonance Platform. Anal. Chem. 2014, 86, 814–819. [Google Scholar] [CrossRef]
- Kurita, R.; Yokota, Y.; Sato, Y.; Mizutani, F.; Niwa, O. On-chip enzyme immunoassay of a cardiac marker using a microfluidic device combined with a portable surface plasmon resonance system. Anal. Chem. 2006, 78, 5525–5531. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.; Hutschenreiter, S.; Hultschig, C.; Zeilinger, C.; Zimmermann, B.; Kleinjung, F.; Schuchhardt, J.; Eickhoff, H.; Herberg, F.W. Differential binding studies applying functional protein microarrays and surface plasmon resonance. Proteomics 2006, 6, 5132–5139. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, E. Measuring Binding of S100 Proteins to RAGE by Surface Plasmon Resonance. In Calcium-Binding Proteins and RAGE: From Structural Basics to Clinical Applications; Heizmann, C.W., Ed.; Humana Press: Totowa, NJ, USA, 2013; pp. 201–213. [Google Scholar]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harpaz, D.; Koh, B.; Marks, R.S.; Seet, R.C.S.; Abdulhalim, I.; Tok, A.I.Y. Point-of-Care Surface Plasmon Resonance Biosensor for Stroke Biomarkers NT-proBNP and S100β Using a Functionalized Gold Chip with Specific Antibody. Sensors 2019, 19, 2533. https://doi.org/10.3390/s19112533
Harpaz D, Koh B, Marks RS, Seet RCS, Abdulhalim I, Tok AIY. Point-of-Care Surface Plasmon Resonance Biosensor for Stroke Biomarkers NT-proBNP and S100β Using a Functionalized Gold Chip with Specific Antibody. Sensors. 2019; 19(11):2533. https://doi.org/10.3390/s19112533
Chicago/Turabian StyleHarpaz, Dorin, Brescia Koh, Robert S. Marks, Raymond C.S. Seet, Ibrahim Abdulhalim, and Alfred I.Y. Tok. 2019. "Point-of-Care Surface Plasmon Resonance Biosensor for Stroke Biomarkers NT-proBNP and S100β Using a Functionalized Gold Chip with Specific Antibody" Sensors 19, no. 11: 2533. https://doi.org/10.3390/s19112533
APA StyleHarpaz, D., Koh, B., Marks, R. S., Seet, R. C. S., Abdulhalim, I., & Tok, A. I. Y. (2019). Point-of-Care Surface Plasmon Resonance Biosensor for Stroke Biomarkers NT-proBNP and S100β Using a Functionalized Gold Chip with Specific Antibody. Sensors, 19(11), 2533. https://doi.org/10.3390/s19112533






