Size of Heparin-Imprinted Nanoparticles Reflects the Matched Interactions with the Target Molecule
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Template Immobilization on Glass Beads
2.3. Synthesis of MIP-NPs
2.4. Evaluation of the Binding Ability of MIP-NPs with Glycosaminoglycans of Quartz Crystal Microbalance (QCM)
3. Results
3.1. Sensitivity of the Size of MIP-NPs to the Template or Analogue
3.2. Sensitivity of the QCM Sensor Coated with the Template or Analogue to the MIP-NPs
3.3. Sensitivity of the Zeta Potential of the MIP-NPs to the Template or the Analogue
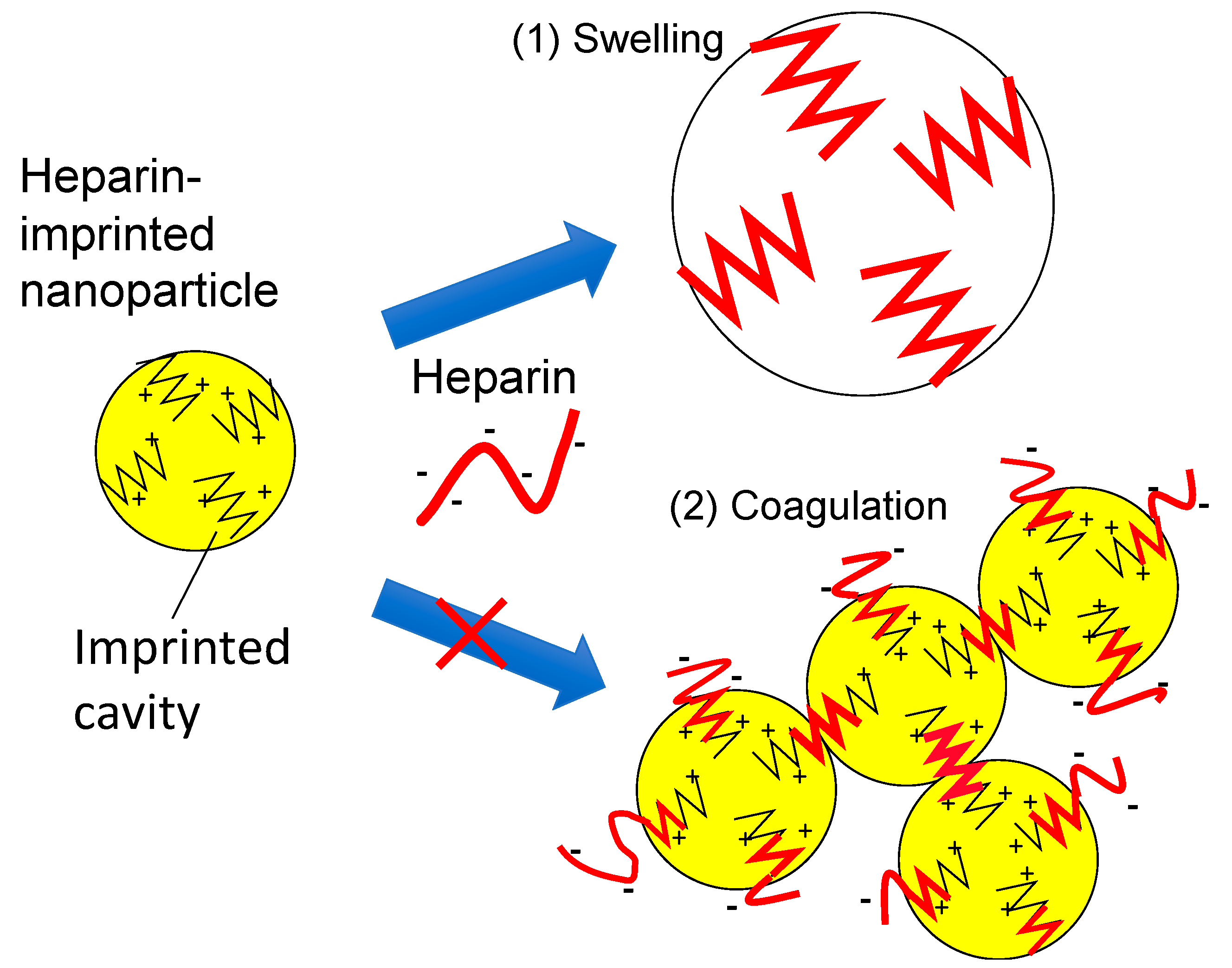
4. Discussion
- (a)
- The UFH-imprinted MIP-NP also binds with CSC as indicated by QCM. Thus, CSC would also have crosslinked particles based on the coagulation assumption. Therefore, the hypothesis is inconsistent with the result that the radius of the particle was insensitive to CSC.
- (b)
- Figure 2 shows that the size increase of UFH-imprinted MIP-NPs by UFH was saturated. If the increase resulted from coagulation, it would not be saturated.
- (c)
- The long-chain UFH is more advantageous than short-chain LMWH for crosslinking the MIP nanoparticles. However, the size of the UFH-imprinted nanoparticles increased in the presence of LMWH, whereas the size of LMWH-MIP-NPs was insensitive to UFH.
- (d)
- If bound UFH existed on the surface of the UFH-MIP-NPs, the zeta potential would have shifted negatively. However, the result was the opposite. It is likely that UFH or CSC was absorbed in the bulk of the particles, preventing the glycosaminoglycans from crosslinking the nanoparticles.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, S.; Ge, Y.; Piletsky, S.A.; Lunec, J. Molecularly Imprinted Sensors, Overview and Application; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Lin, T.-Y.; Hu, C.-H.; Chou, T.-C. Determination of albumin concentration by MIP-QCM sensor. Biosens. Bioelectron. 2004, 20, 75–81. [Google Scholar] [CrossRef]
- Çiçek, Ç.; Yılmaz, F.; Özgür, E.; Yavuz, H.; Denizli, A. Molecularly imprinted quartz crystal microbalance sensor (qcm) for bilirubin detection. Chemosensors 2016, 4, 21. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Tian, X.-G.; Cai, L.-G.; Xu, Z.-L.; Lei, H.-T.; Wang, H.; Sun, Y.-M. Molecularly imprinted polymer based surface plasmon resonance sensors for detection of Sudan dyes. Anal. Methods 2014, 6, 3751–3757. [Google Scholar] [CrossRef]
- Cennamom, N.; D’Agostin, G.; Pesavento, M.; Zen, L. High selectivity and sensitivity sensor based on MIP and SPR in tapered plastic optical fibers for the detection of l-nicotine. Sens. Actuators B 2014, 191, 529–536. [Google Scholar] [CrossRef]
- Perdomo, Y.; Arancibia, V.; García-Beltrán, O.; Nagles, E. Adsorptive Stripping voltammetric determination of amaranth and tartrazine in drinks and gelatins using a screen-printed carbon electrode. Sensors 2017, 17, 2665. [Google Scholar] [CrossRef]
- Prasad, B.B.; Jauhari, D.; Tiwari, M.P. A dual-template imprinted polymer-modified carbon ceramic electrode for ultra trace simultaneous analysis of ascorbic acid and dopamine. Biosens. Bioelectron. 2013, 50, 19–27. [Google Scholar] [CrossRef]
- Elshafey, R.; Radi, A.-E. Electrochemical impedance sensor for herbicide alachlor based on imprinted polymer receptor. J. Electroanal. Chem. 2018, 813, 171–177. [Google Scholar] [CrossRef]
- Smolinska-Kempisty, K.; Ahmad, O.S.; Guerreiro, A.; Karim, K.; Piletska, E.; Piletsky, S. New potentiometric sensor based on molecularly imprinted nanoparticles for cocaine detection. Biosens. Bioelectron. 2017, 96, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.-C.; Chang, P.-H.; Lin, C.-C.; Hong, C.-L. A disposable microfluidic biochip with on-chip molecularly imprinted biosensors for optical detection of anesthetic propofol. Biosens. Bioelectron. 2010, 25, 2058–2064. [Google Scholar] [CrossRef]
- Yoshimi, Y.; Ohdaira, R.; Iiyama, C.; Sakai, K. “Gate effect” of thin layer of molecularly-imprinted poly (methacrylic acid-co-ethyleneglycol dimethacrylate). Sens. Actuators B 2001, 73, 49–53. [Google Scholar] [CrossRef]
- Sekine, S.; Watanabe, Y.; Yoshimi, Y.; Hattori, K.; Sakai, K. Influence of solvents on chiral discriminative gate effect of molecularly imprinted poly (ethylene glycol dimethacrylate-co-methacrylic acid). Sens. Actuators B Chem. 2007, 127, 512–517. [Google Scholar] [CrossRef]
- Yoshimi, Y.; Ishii, N. Improved gate effect enantioselectivity of phenylalanine-imprinted polymers in water by blending crosslinkers. Anal. Chim. Acta 2015, 862, 77–85. [Google Scholar] [CrossRef]
- Yoshimi, Y.; Sato, K.; Ohshima, M.; Piletska, E. Application of the ‘gate effect’ of a molecularly imprinted polymer grafted on an electrode for the real-time sensing of heparin in blood. Analyst 2013, 138, 5121–5128. [Google Scholar] [CrossRef] [PubMed]
- Yoshimi, Y.; Inaba, R.; Ogawa, T.; Yoshino, W.; Inoue, M.; Kuwana, K. Stabilized sensing of heparin in whole blood using the ‘gate effect’ of heparin-imprinted polymer grafted onto an electrode. Mol. Impr. 2016, 4, 13–20. [Google Scholar] [CrossRef]
- Yoshimi, Y.; Yagisawa, Y.; Yamaguchi, R.; Seki, M. Blood heparin sensor made from a paste electrode of graphite particles grafted with molecularly imprinted polymer. Sens. Actuators B Chem. 2018, 259, 455–462. [Google Scholar] [CrossRef]
- Poma, A.; Guerreiro, A.; Whitcombe, M.J.; Piletska, E.V.; Turner, A.P.F.; Piletsky, S.A. Solid-phase synthesis of molecularly imprinted polymer nanoparticles with a reusable template—“Plastic Antibodies”. Adv. Funct. Mater. 2013, 23, 2821–2827. [Google Scholar] [CrossRef]
- Barns, G.T.; Gentle, I.R. Interfacial Science, 2nd ed.; Oxford University Press: Oxford, UK, 2011; 253p. [Google Scholar]
- Piletsky, S.; Panasyk, T.; Piletskaya, E.; Nicholls, I.; Ulbricht, M. Receptor and transport properties of imprinted polymer membranes—A review. J. Membr. Sci. 1999, 157, 263–278. [Google Scholar] [CrossRef]
- Ohira, K.; Orihara, K.; Hikichi, A.; Arita, T.; Muguruma, H.; Yoshimi, Y. Heparin molecularly imprinted polymer thin film on gold electrode by plasma-induced graft polymerization for label-free biosensor. J. Pharm. Biomed. Anal. 2018, 151, 324–330. [Google Scholar]
- Piletsky, S.A.; Mijangos, I.; Guerreiro, A.; Piletska, E.V.; Chianella, I.; Karim, K.; Turner, A.P.F. Polymer Cookery: Influence of polymerization time and different initiation conditions on performance of molecularly imprinted polymers. Macromolecules 2005, 38, 1410–1414. [Google Scholar] [CrossRef]
- Israelachivili, J.N. Intermolecular and Surface Forces, 3rd ed.; Academic Press: Amsterdam, The Netherlands, 2011; Chapter 8. [Google Scholar]
- Watanabe, M.; Akahoshi, T.; Tabata, Y.; Nakayama, D. Molecular specific swelling change of hydrogels in accordance with the concentration of guest molecules. J. Am. Chem. Soc. 1998, 120, 5577–5578. [Google Scholar] [CrossRef]
- Sellergren, B.; Hall, A.J. Fundamental aspects of imprinted network polymers. In Molecularly Imprinted Polymers; Elsevier: Amsterdam, The Netherlands, 2012; pp. 22–57. [Google Scholar]
- Yoshimi, Y.; Arai, R.; Nakayama, S. Influence of the solvent on nature of gate effect in molecularly imprinted membrane. Anal. Chim. Acta 2010, 682, 110–116. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshimi, Y.; Oino, D.; Ohira, H.; Muguruma, H.; Moczko, E.; Piletsky, S.A. Size of Heparin-Imprinted Nanoparticles Reflects the Matched Interactions with the Target Molecule. Sensors 2019, 19, 2415. https://doi.org/10.3390/s19102415
Yoshimi Y, Oino D, Ohira H, Muguruma H, Moczko E, Piletsky SA. Size of Heparin-Imprinted Nanoparticles Reflects the Matched Interactions with the Target Molecule. Sensors. 2019; 19(10):2415. https://doi.org/10.3390/s19102415
Chicago/Turabian StyleYoshimi, Yasuo, Daichi Oino, Hirofumi Ohira, Hitoshi Muguruma, Ewa Moczko, and Sergey A. Piletsky. 2019. "Size of Heparin-Imprinted Nanoparticles Reflects the Matched Interactions with the Target Molecule" Sensors 19, no. 10: 2415. https://doi.org/10.3390/s19102415
APA StyleYoshimi, Y., Oino, D., Ohira, H., Muguruma, H., Moczko, E., & Piletsky, S. A. (2019). Size of Heparin-Imprinted Nanoparticles Reflects the Matched Interactions with the Target Molecule. Sensors, 19(10), 2415. https://doi.org/10.3390/s19102415





