An LC Passive Wireless Gas Sensor Based on PANI/CNT Composite
Abstract
:1. Introduction
2. The Principle of Sensor
2.1. LC Resonant Circuit
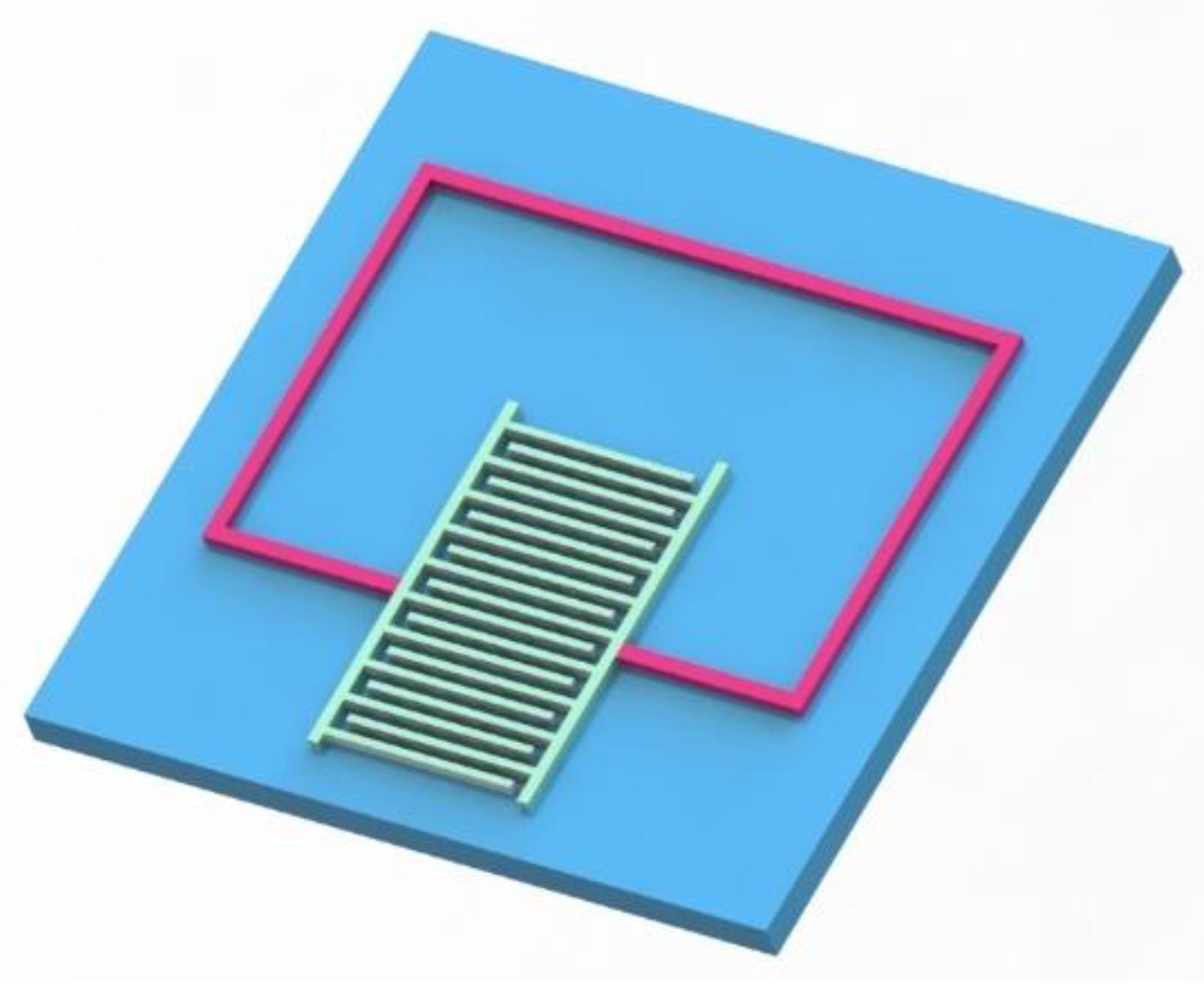
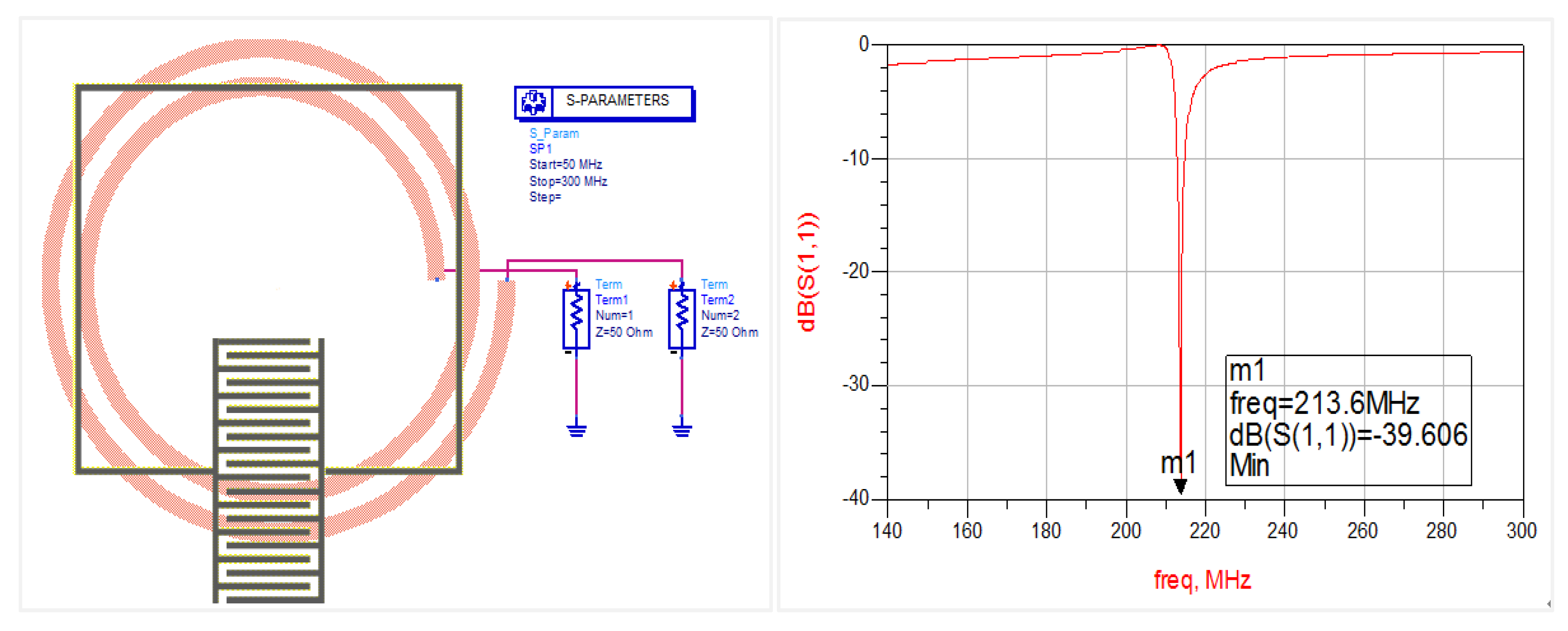
2.2. Gas Sensitive Infrastructure Simulation
3. Experiment Section
3.1. Preparation of PANI/CNT Composite
3.2. Characterization of Materials
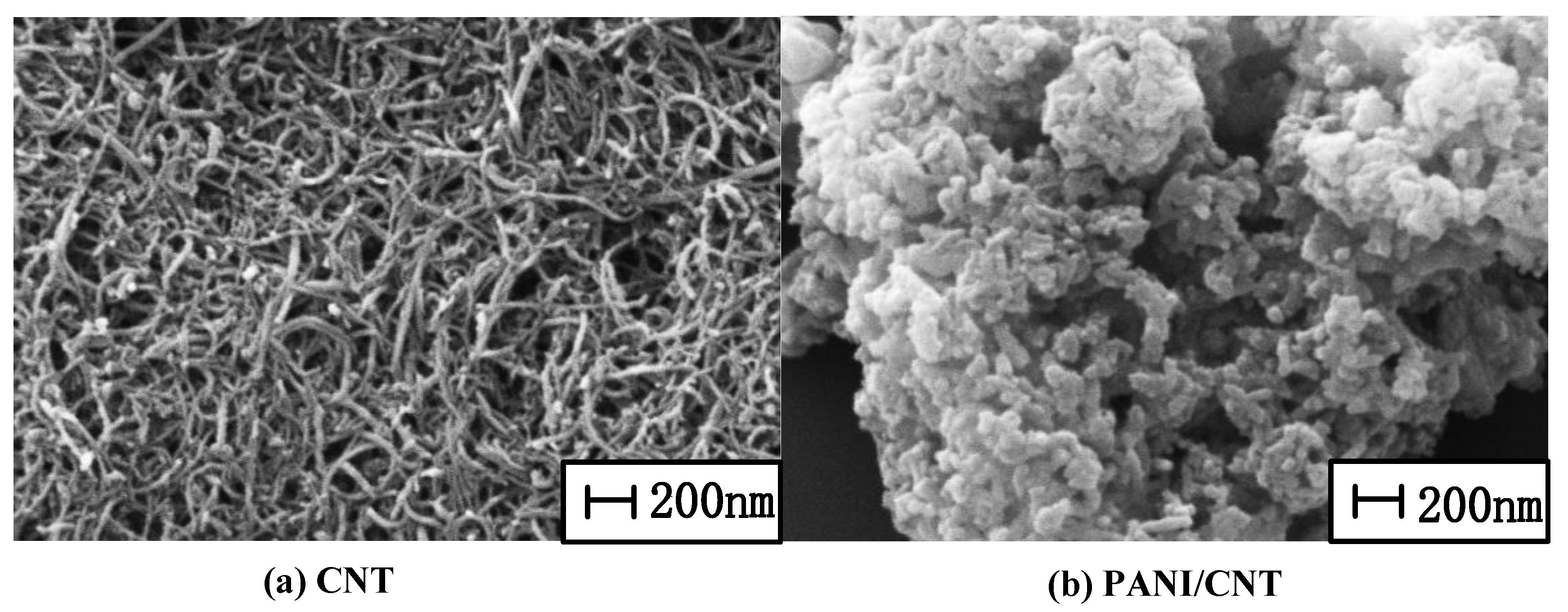
3.2.1. SEM Characterization
3.2.2. Electrical Characterization
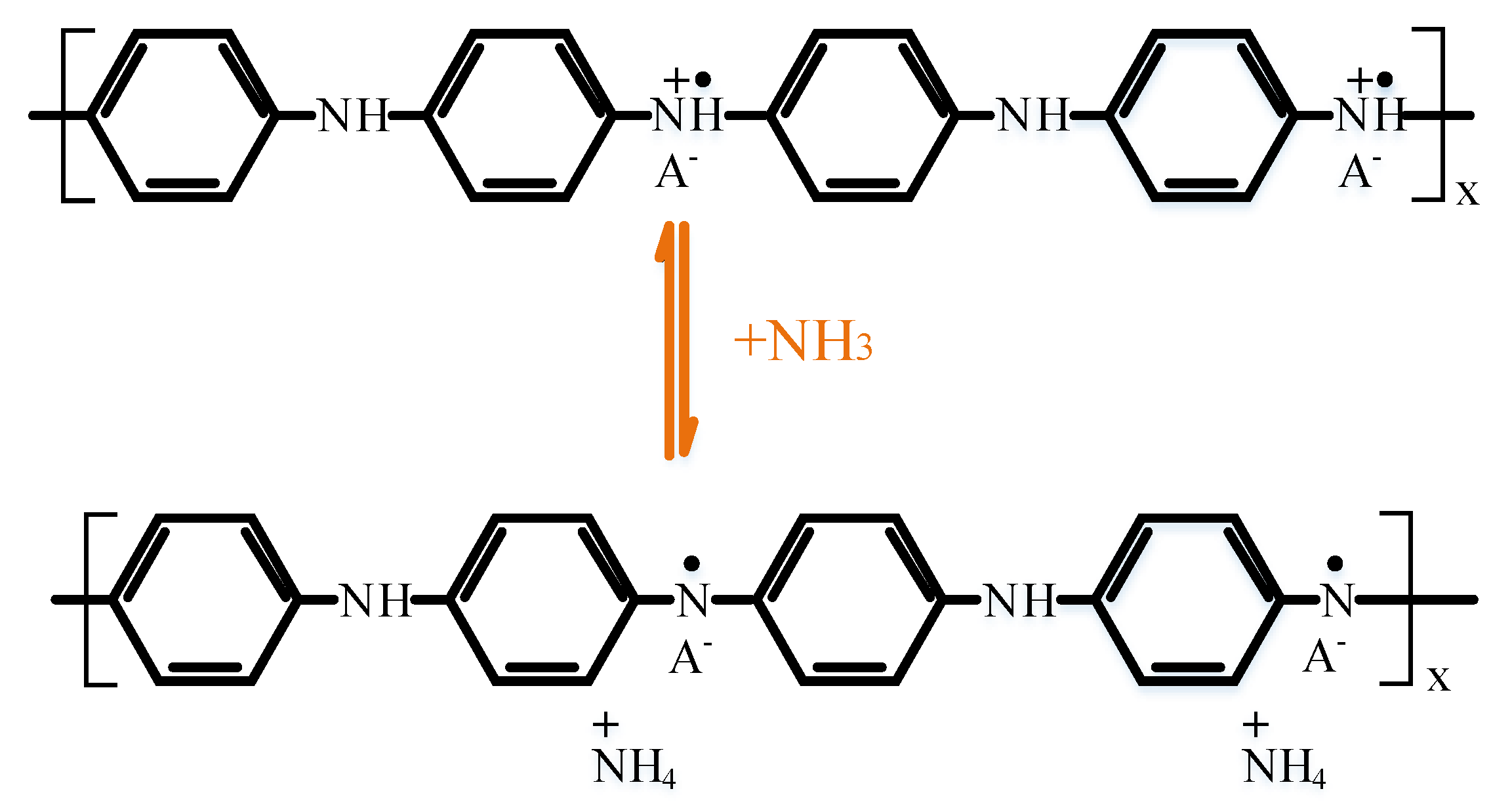
3.3. Gas Sensitivity Mechanism
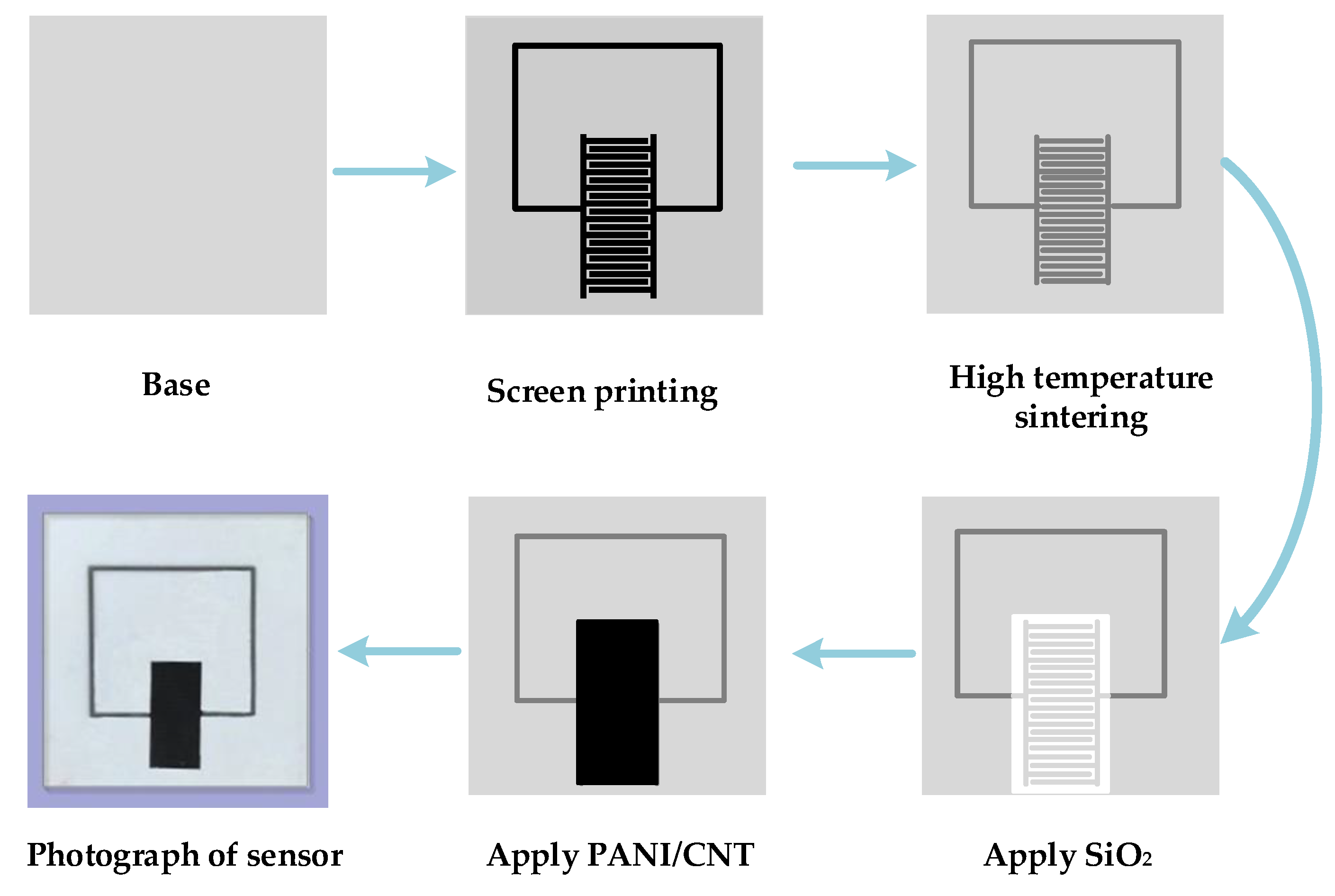
3.4. Preparation of LC Wireless Passive Gas Sensor
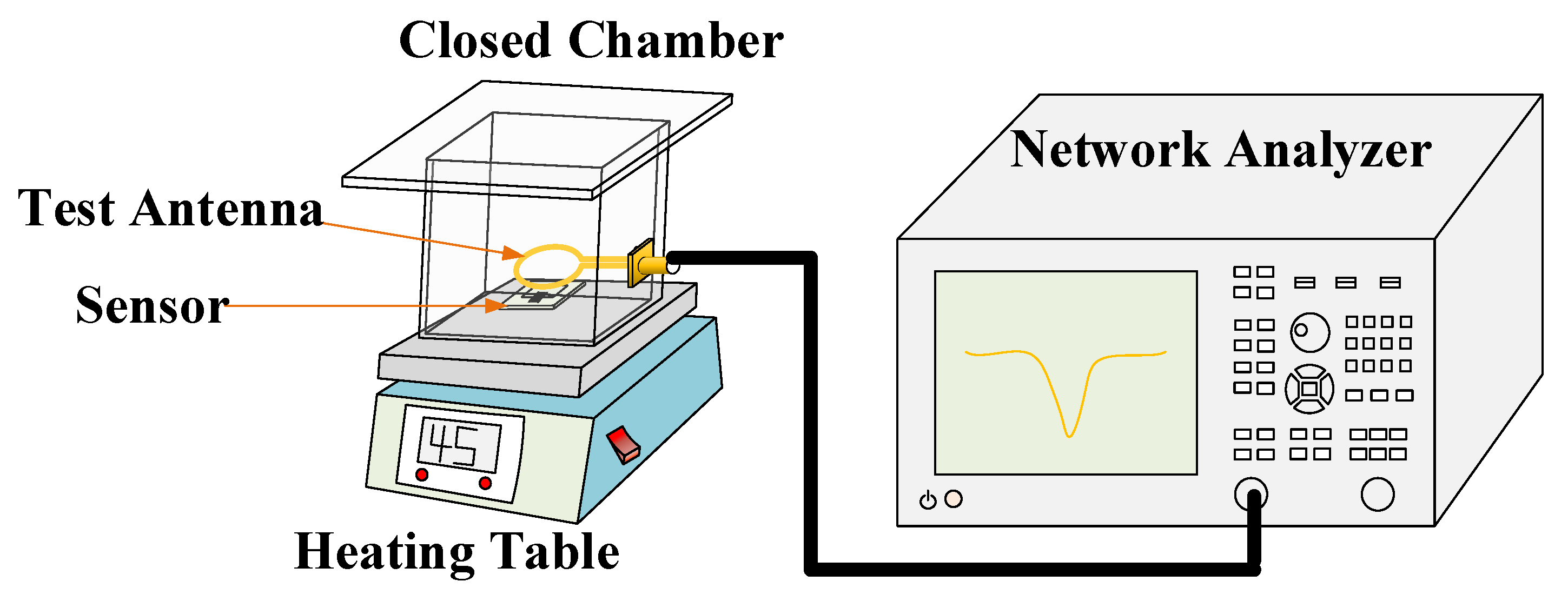
3.5. Sensitivity Test of Gas Sensor
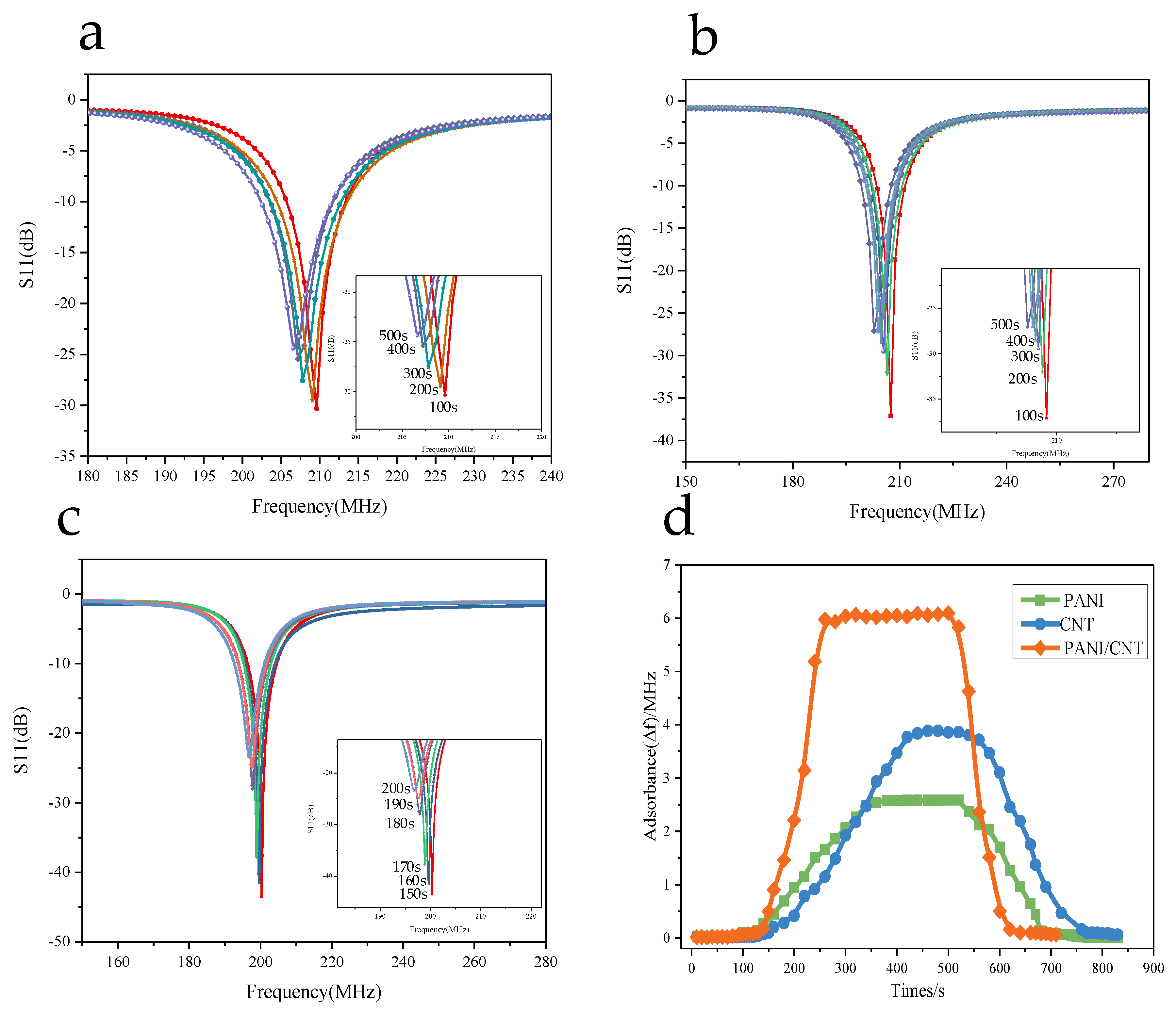
3.5.1. The Relationship between the Resonant Frequency Variation and the Operating Temperature
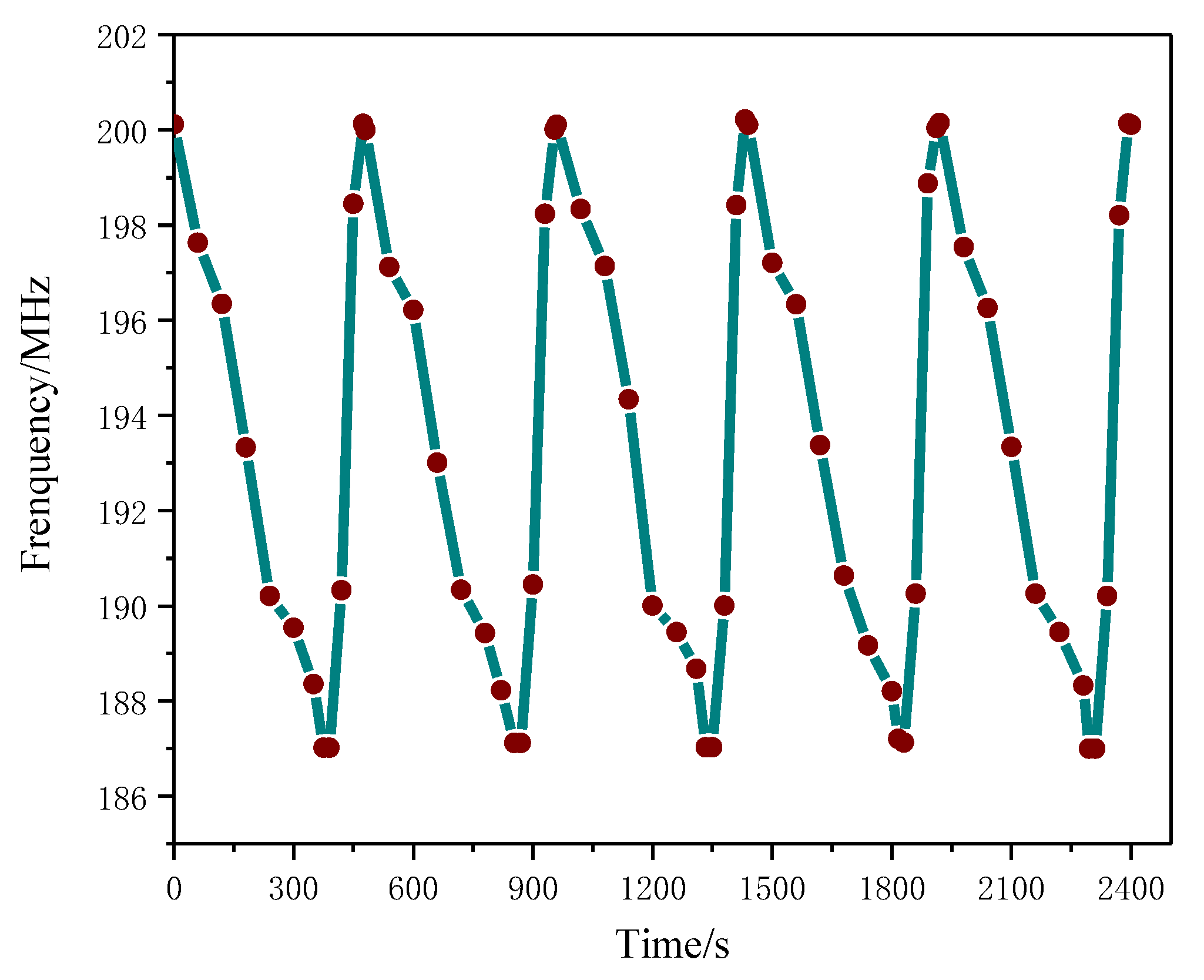
3.5.2. Repeatability Test
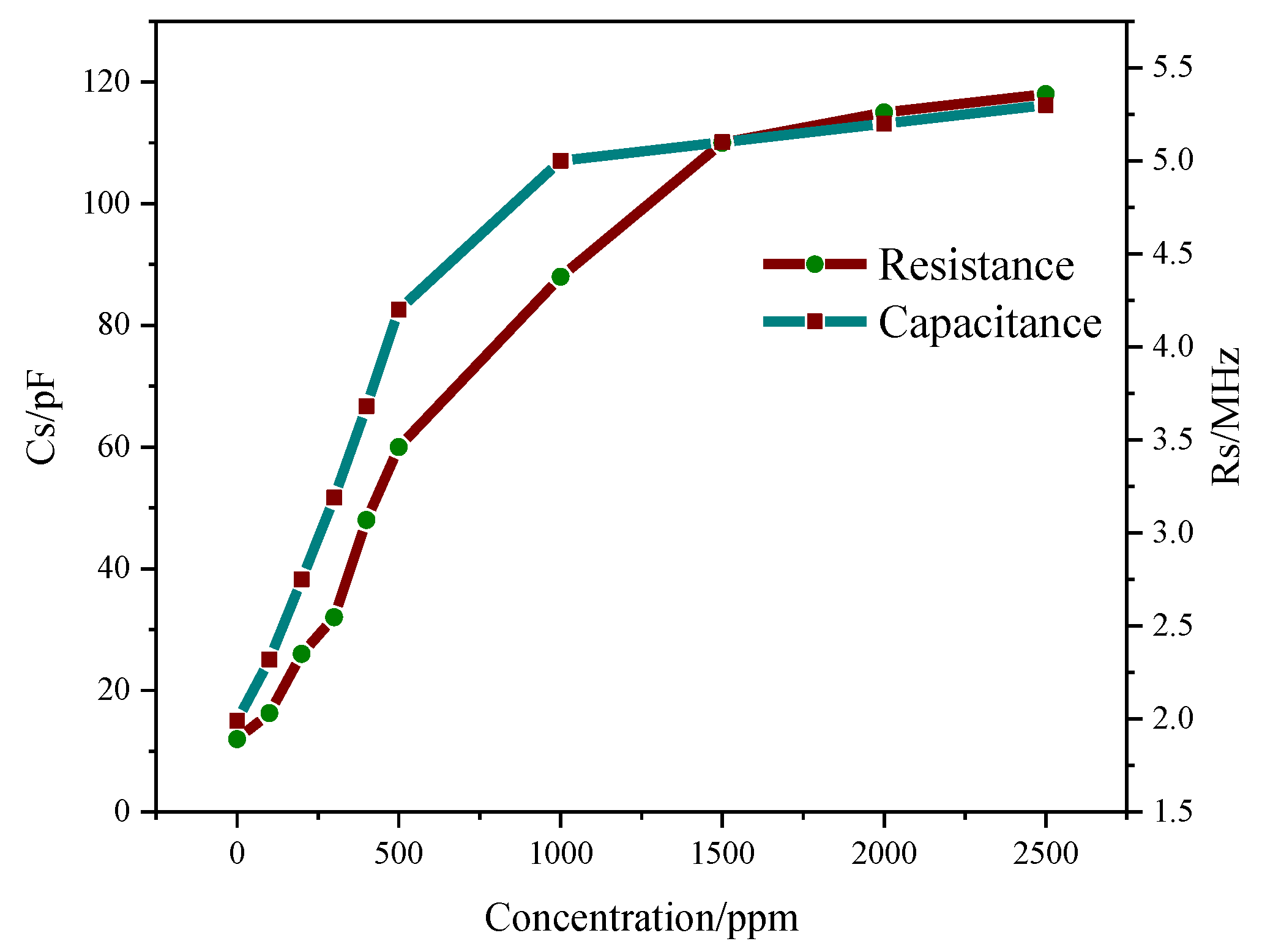
3.5.3. Gas Sensing Properties of Composite
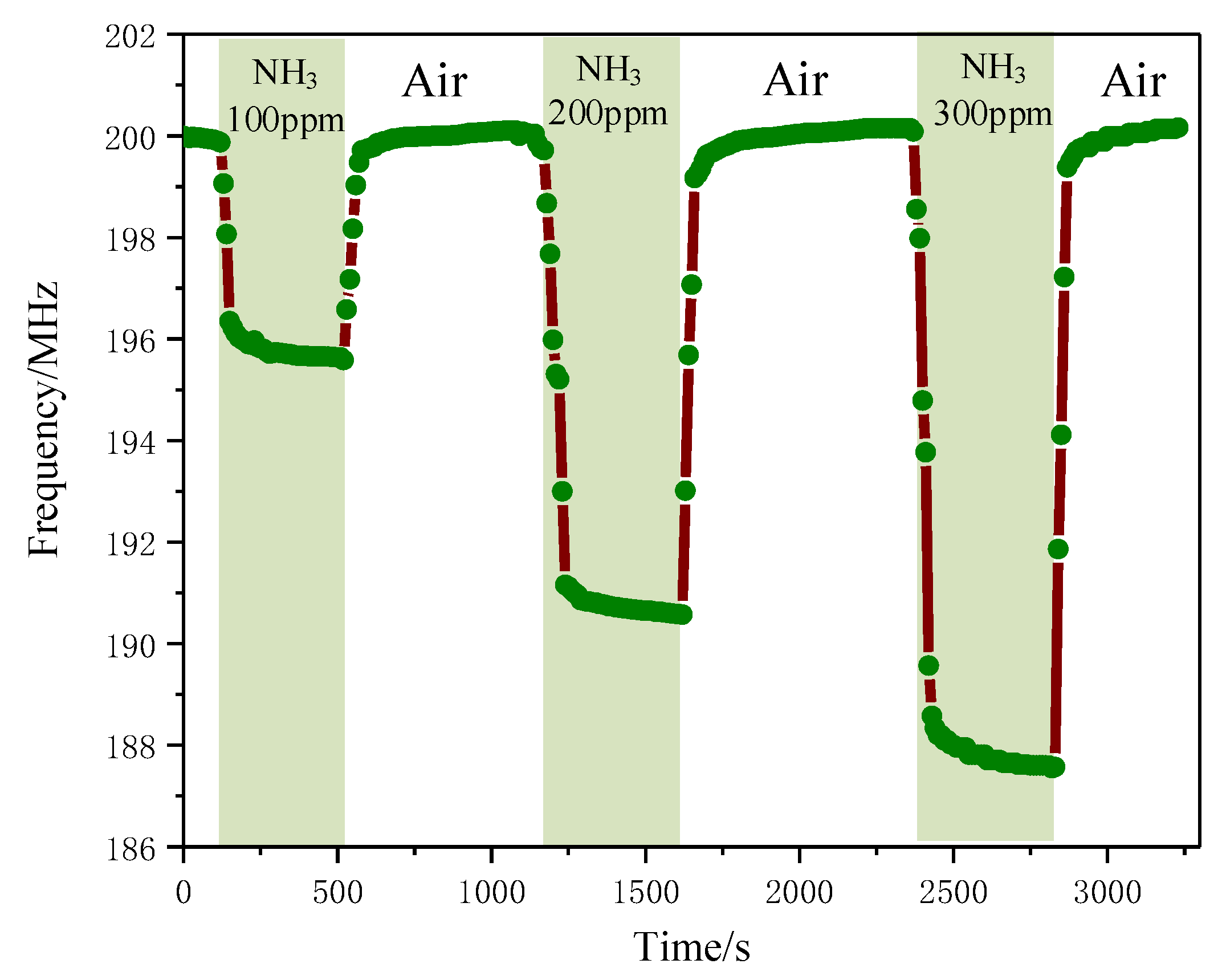
3.5.4. Relationship between Gas Concentration and Resonant Frequency
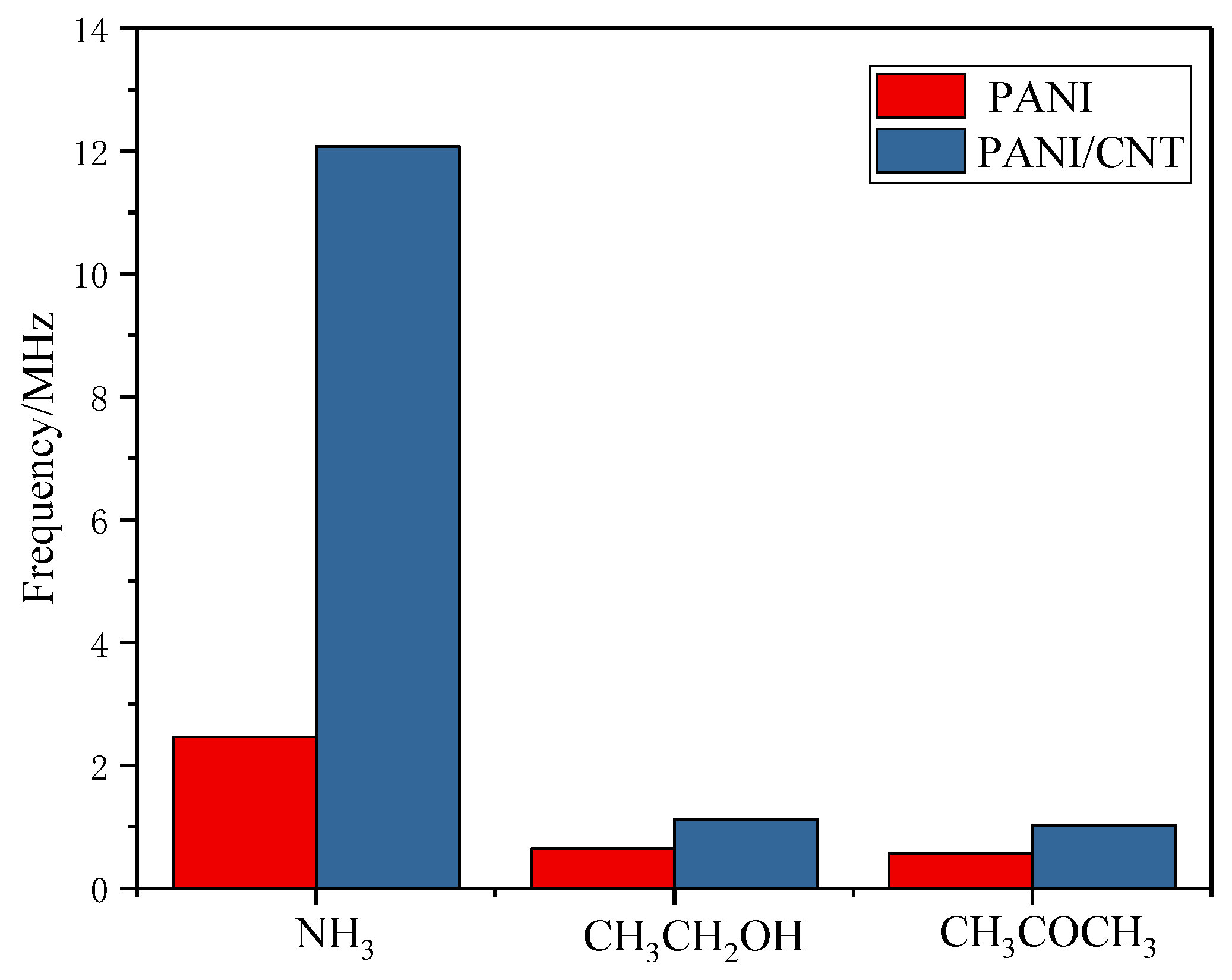
3.5.5. Sensor Specificity and Long-Term Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sharma, S.; Hussain, S.; Singh, S.; Islam, S.S. MWCNT-conducting polymer composite based ammonia gas sensors: A new approach for complete recovery process. Sens. Actuators B Chem. 2014, 194, 213–219. [Google Scholar] [CrossRef]
- Chen, W.; Li, F.; Ooi, P.C.; Ye, Y.; Kim, T.W.; Guo, T. Room temperature pH-dependent ammonia gas sensors using graphene quantum dots. Sens. Actuators B Chem. 2016, 222, 763–768. [Google Scholar] [CrossRef]
- Eising, M.; Cava, C.E.; Salvatierra, R.V.; Zarbin, A.J.G.; Roman, L.S. Doping effect on self-assembled films of polyaniline and carbon nanotube applied as ammonia gas sensor. Sens. Actuators B Chem. 2017, 245, 25–33. [Google Scholar] [CrossRef]
- Zhang, T.; Mubeen, S.; Yoo, B.; Myung, N.V.; Deshusses, M.A. A gas nanosensor unaffected by humidity. Nanotechnology 2009, 20, 255501. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Yang, Z.; Chen, Y.; Zhang, J.; Wang, Q.; Huang, F.; Wei, Q. A room temperature ammonia gas sensor based on cellulose/TiO2/PANI composite nanofibers. Colloids Surf. A Physicochem. Eng. Asp. 2016, 494, 248–255. [Google Scholar] [CrossRef]
- Hallil, H.; Ménini, P.; Aubert, H. Novel Microwave Gas Sensor using Dielectric Resonator with SnO2, Sensitive Layer. Procedia Chem. 2009, 1, 935–938. [Google Scholar] [CrossRef]
- Kumar, L.; Rawal, I.; Kaur, A.; Annapoorni, S. Flexible room temperature ammonia sensor based on polyaniline. Sens. Actuators B Chem. 2017, 240, 408–416. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, Q.; Xie, G.; Su, Y.; Zhao, K.; Du, H.; Jiang, Y. Gas sensors based on polyaniline/zinc oxide hybrid film for ammonia detection at room temperature. Chem. Phys. Lett. 2016, 665, 147–152. [Google Scholar] [CrossRef]
- Bahoumina, P.; Hallil, H.; Lachaud, J.L.; Abdelghani, A.; Frigui, K.; Bila, S.; Baillargeat, D.; Ravichandran, A.; Coquet, P.; Paragua, C.; et al. Microwave flexible gas sensor based on polymer multi wall carbon nanotubes sensitive layer. Sens. Actuators B Chem. 2017, 249, 708–714. [Google Scholar] [CrossRef]
- Chu, V.T.; Mai, A.T.; Hieu, N.V.; Trung, T. Electrochemical synthesis of polyaniline nanowires on Pt interdigitated microelectrode for room temperature NH3, gas sensor application. Curr. Appl. Phys. 2012, 12, 1011–1016. [Google Scholar]
- Aubert, H.; Chebila, F.; Jatlaoui, M.; Thai, T.; Hallil, H.; Traille, A.; Bouaziz, S.; Rifaï, A.; Pons, P.; Menini, P.; et al. Wireless sensing and identification based on radar cross section variability measurement of passive electromagnetic sensors. Ann. Telecommun. 2013, 68, 425–435. [Google Scholar] [CrossRef]
- Vyas, R.; Lakafosis, V.; Lee, H.; Shaker, G.S.A.; Yang, L.; Orecchini, G.; Traille, A.N.; Tentzeris, M.M.; Roselli, L. Inkjet Printed, Self Powered, Wireless Sensors for Environmental, Gas, and Authentication-Based Sensing. IEEE Sens. J. 2011, 11, 3139–3152. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Lee, J.D.; Lee, S.M.; Lee, Y.H. Adsorption of NH3 and NO2 molecules on carbon nanotubes. Appl. Phys. Lett. 2001, 79, 3863–3865. [Google Scholar] [CrossRef]
- da Cunha, M.P.; Maskay, A.; Lad, R.J.; Coyle, T.; Harkay, G. Langasite 2.45 GHz ISM band SAW resonator for harsh environment wireless applications. In Proceedings of the 2016 IEEE International Conference on Ultrasonics Symposium (IUS), Tours, France, 18–21 September 2016; pp. 1–4. [Google Scholar]
- Deshpande, N.G.; Gudage, Y.G.; Sharma, R.; Vyas, J.C.; Kim, J.B.; Lee, Y.P. Studies on tin oxide-intercalated polyaniline nanocomposite for ammonia gas sensing applications. Sens. Actuators B Chem. 2009, 138, 76–84. [Google Scholar] [CrossRef]
- Saxena, V.; Choudhury, S.; Gadkari, S.C.; Yakhmi, J. Room temperature operated ammonia gas sensor using polycarbazole Langmuir–Blodgett film. Sens. Actuators B Chem. 2005, 107, 277–282. [Google Scholar] [CrossRef]
- Airoudj, A.; Debarnot, D.; Bêche, B.; Poncin-Epaillard, F. New sensitive layer based on pulsed plasma-polymerized aniline for integrated optical ammonia sensor. Anal. Chim. Acta 2008, 626, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Vena, A.; Sydanheimo, L.; Tentzeris, M.M.; Ukkonen, L. A Fully Inkjet-Printed Wireless and Chipless Sensor for CO2, and Temperature Detection. IEEE Sens. J. 2017, 15, 89–99. [Google Scholar] [CrossRef]
- Bailly, G.; Rossignol, J.; de Fonseca, B.; Pribetich, P.; Stuerga, D. Microwave gas sensing with hematite: Shape effect on ammonia detection using pseudocubic, rhombohedral, and spindlelike particles. ACS Sens. 2016, 1, 656–662. [Google Scholar] [CrossRef]
- Talwar, V.; Singh, O.; Singh, R.C. ZnO assisted polyaniline nanofibers and its application as ammonia gas sensor. Sens. Actuators B Chem. 2014, 191, 276–282. [Google Scholar] [CrossRef]
- Zhang, C. Theoretical and Experimental Research of LC-Type Passive Wireless Humidity Sensor; Southeast University: Nanjing, China, 2014. [Google Scholar]
- Zhang, L.; Rahimabady, M.; Tan, S.Y.; Tan, C.Y.; Chen, S.; Chen, Y.F.; Yao, K.; Humbert, A.; Soccol, D.; Zang, K.; et al. P (VDF-HFP) Polymer as Sensing Material for Capacitive Carbon Dioxide Sensors. IEEE Sens. J. 2017, 17, 4349–4356. [Google Scholar] [CrossRef]
- Xiong, J.; Li, Y.; Hong, Y.; Zhang, B.; Cui, T.; Tan, Q.; Zheng, S.; Liang, T. Wireless LTCC-Based Capacitive Pressure Sensor for Harsh Environment. Sens. Actuators A Phys. 2013, 197, 30–37. [Google Scholar] [CrossRef]
- Ma, M.; Khan, H.; Shan, W.; Wang, Y.; Ou, J.Z.; Liu, Z.; Kalantar-zadeh, K.; Li, Y. A Novel Wireless Gas Sensor Based on LTCC Technology. Sens. Actuators B Chem. 2017, 239, 711–717. [Google Scholar] [CrossRef]
- Qiulin, T.; Hao, K.; Li, Q.; Jijun, X.; Jun, L.; Chenyang, X.; Wendong, Z.; Tao, L. High Temperature Characteristic for Wireless Pressure LTCC-Based Sensor. Microsyst. Technol. 2015, 21, 209–214. [Google Scholar] [CrossRef]
- Jow, U.M.; Ghovanloo, M. Design and Optimization of Printed Spiral Coils for Efficient Transcutaneous Inductive Power Transmission. IEEE Trans. Biomed. Circuits Syst. 2007, 1, 193–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.Y.; Tee, B.C.-K.; Chortos, A.L.; Schwartz, G.; Tse, V.; Lipomi, D.J.; Wong, H.-S.P.; McConnell, M.V.; Bao, Z. Continuous Wireless Pressure Monitoring and Mapping with Ultra Small Passive Sensors for Health Monitoring and Critical Care. Nat. Commun. 2014, 5, 5028. [Google Scholar] [CrossRef] [PubMed]
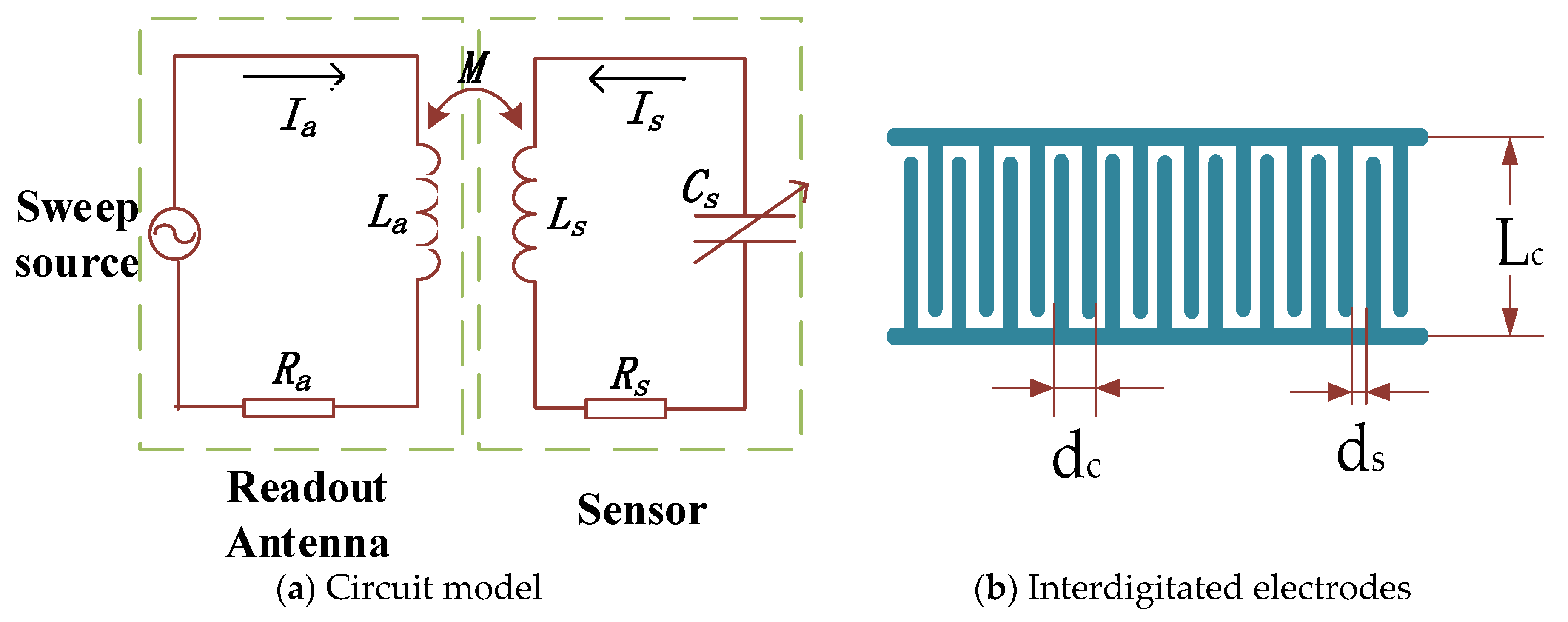


| Parameter | Value | |
|---|---|---|
| Number of finger pairs | Nc | 10 |
| Finger width | Lc | 6 mm |
| Gap between fingers | dc | 2 mm |
| Gap at ends of fingers | ds | 0.5 mm |
| Thickness of metal films | t | 25 μm |
| Number of inductor coil | N | 1 |
| f0/MHz | PANI | PANI/CNT | |
|---|---|---|---|
| Temperature/°C | |||
| 25 | 1.5 | 6.7 | |
| 35 | 2.2 | 9.8 | |
| 45 | 2.857 | 12.07 | |
| 55 | 2.94 | 10.68 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, S.; Fan, Z.; Deng, J.; Guo, X.; Zhang, L.; Liu, G.; Tan, Q.; Xiong, J. An LC Passive Wireless Gas Sensor Based on PANI/CNT Composite. Sensors 2018, 18, 3022. https://doi.org/10.3390/s18093022
Shen S, Fan Z, Deng J, Guo X, Zhang L, Liu G, Tan Q, Xiong J. An LC Passive Wireless Gas Sensor Based on PANI/CNT Composite. Sensors. 2018; 18(9):3022. https://doi.org/10.3390/s18093022
Chicago/Turabian StyleShen, Sanmin, Zhihong Fan, Jiahao Deng, Xiaowei Guo, Lei Zhang, Guanyu Liu, Qiulin Tan, and Jijun Xiong. 2018. "An LC Passive Wireless Gas Sensor Based on PANI/CNT Composite" Sensors 18, no. 9: 3022. https://doi.org/10.3390/s18093022
APA StyleShen, S., Fan, Z., Deng, J., Guo, X., Zhang, L., Liu, G., Tan, Q., & Xiong, J. (2018). An LC Passive Wireless Gas Sensor Based on PANI/CNT Composite. Sensors, 18(9), 3022. https://doi.org/10.3390/s18093022





