Effectiveness of Sensors Contact Metallization (Ti, Au, and Ru) and Biofunctionalization for Escherichia coli Detection
Abstract
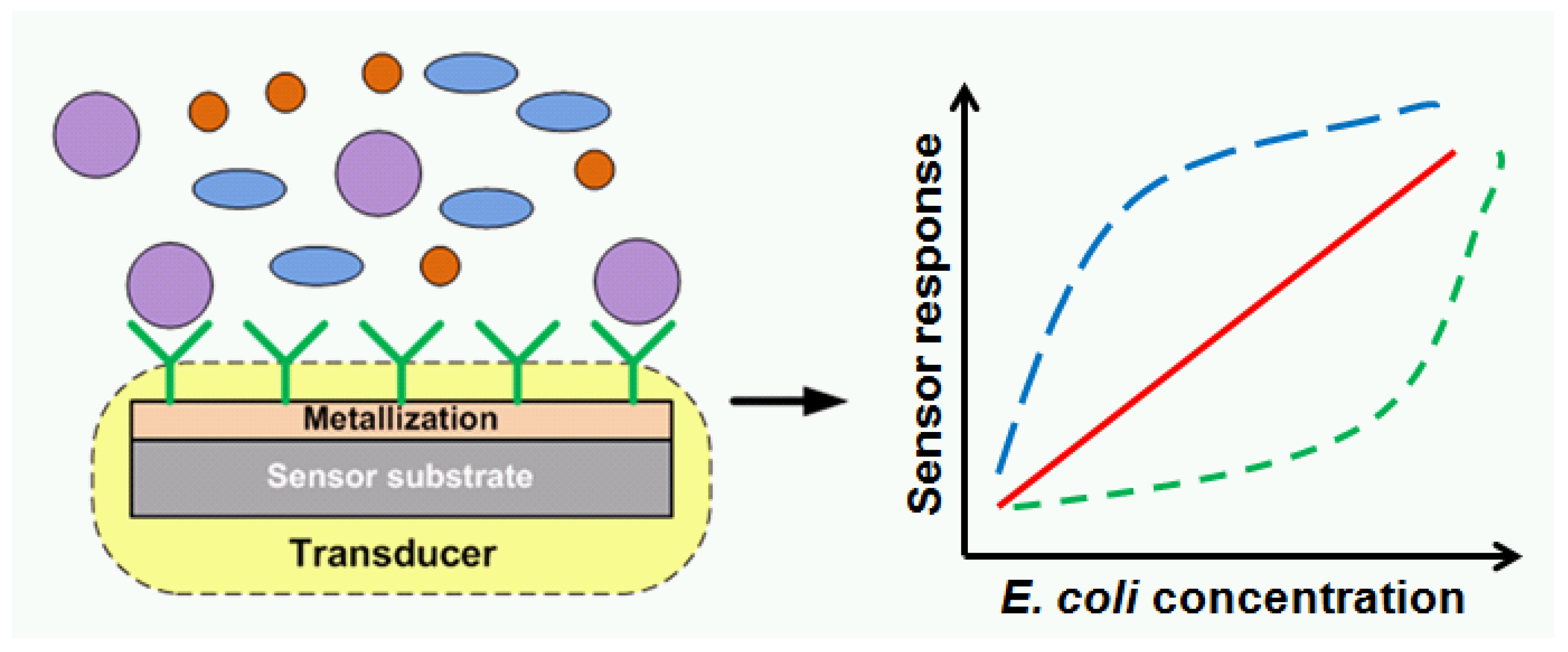
1. Introduction
2. Materials and Methods
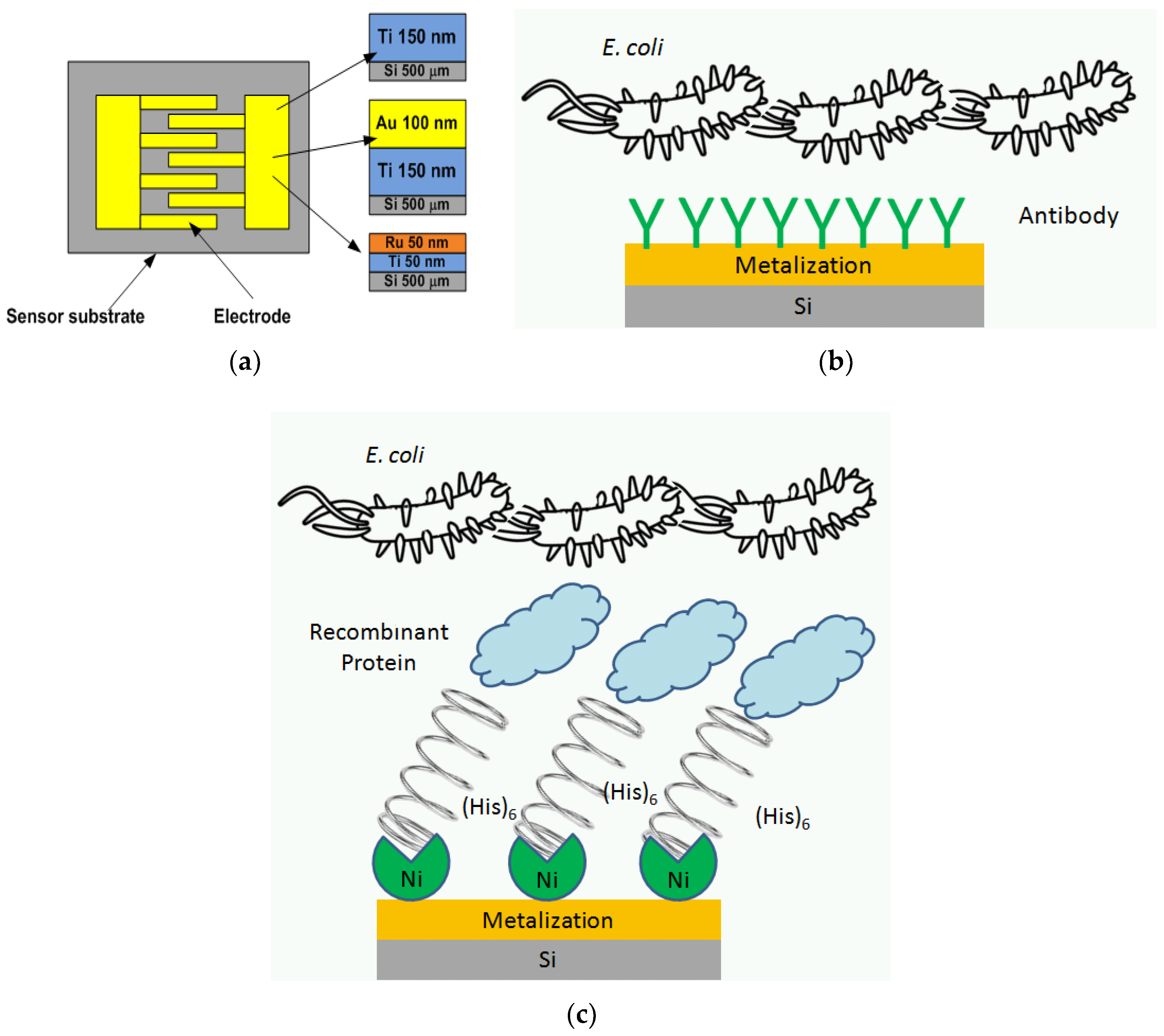
2.1. Fabrication Process
2.2. Characterization
2.3. Bacteria Growth Condition
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4.1 Whole-Bacterial Cell ELISA
2.4.2. Protein ELISA
2.5. Biofunctionalization
2.5.1. Biofunctionalization by His-Tagged LBP Protein and Bacteriophage His-Tagged gp37 Adhesin
2.5.2. Biofunctionalization by Polyclonal Anti-Escherichia coli Antibody
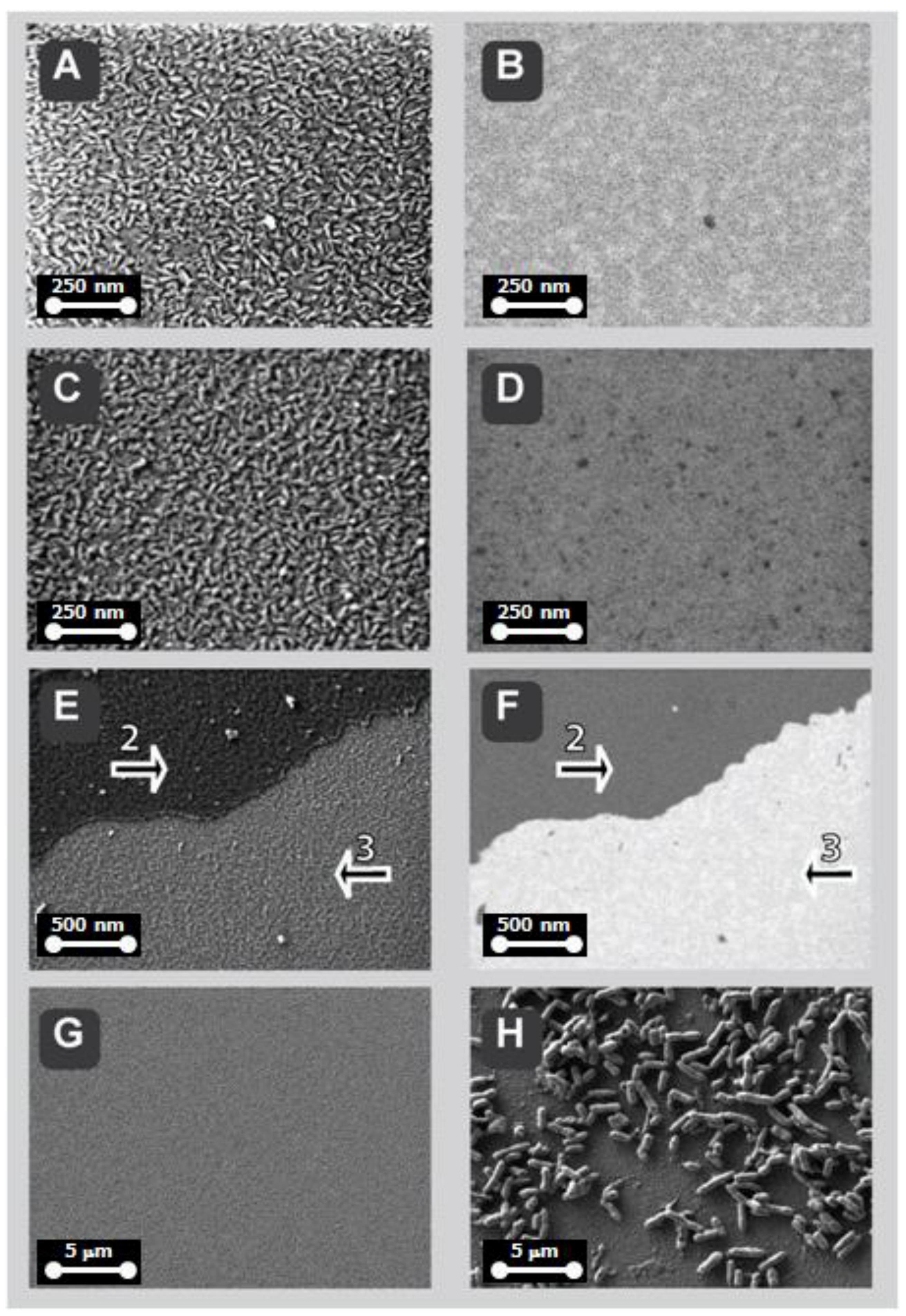
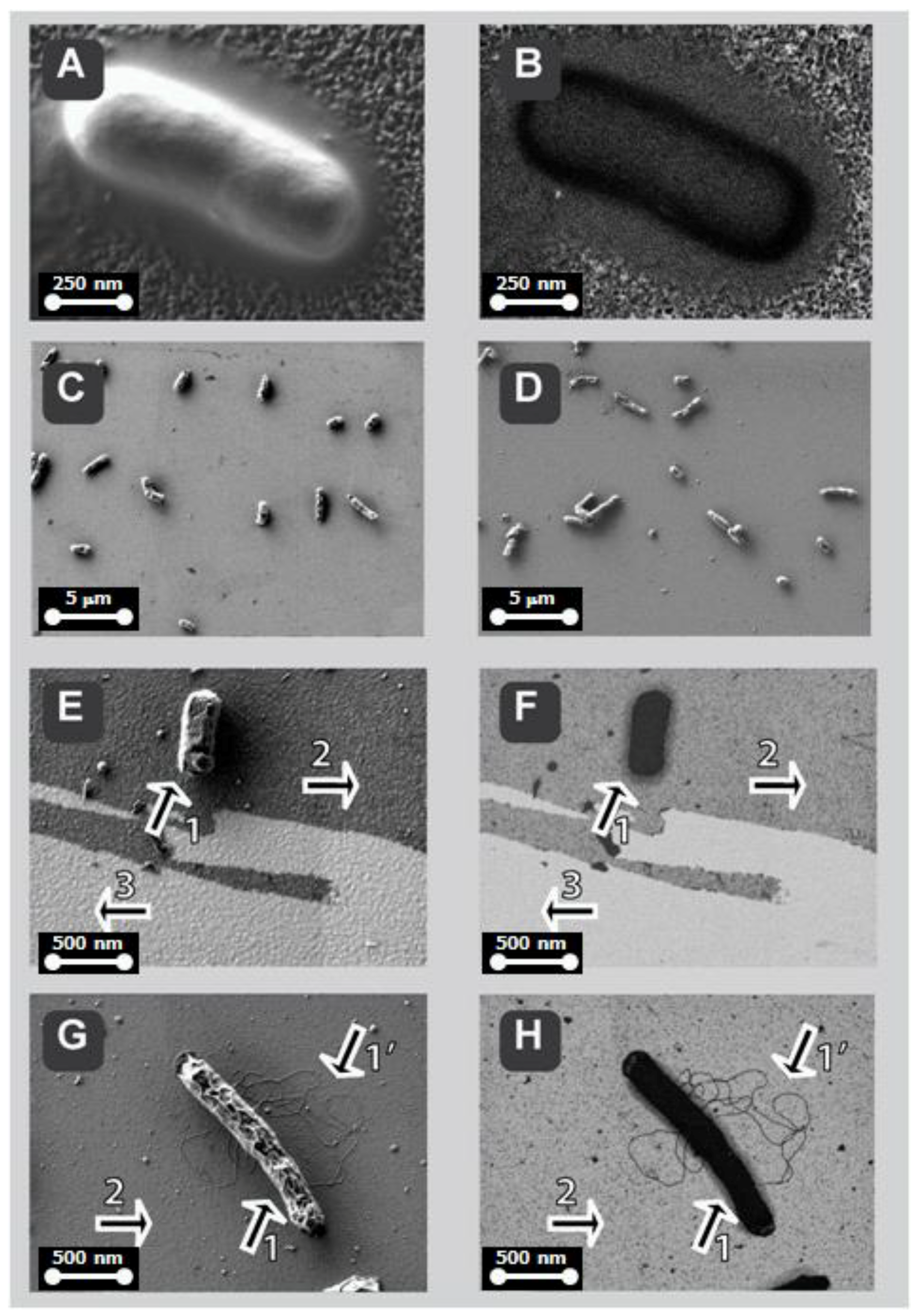
2.6. Low-Voltage Field-Emission Scanning Electro Microscopy LV-FESEM and Real-Time Energy Filtering Mapping
3. Results
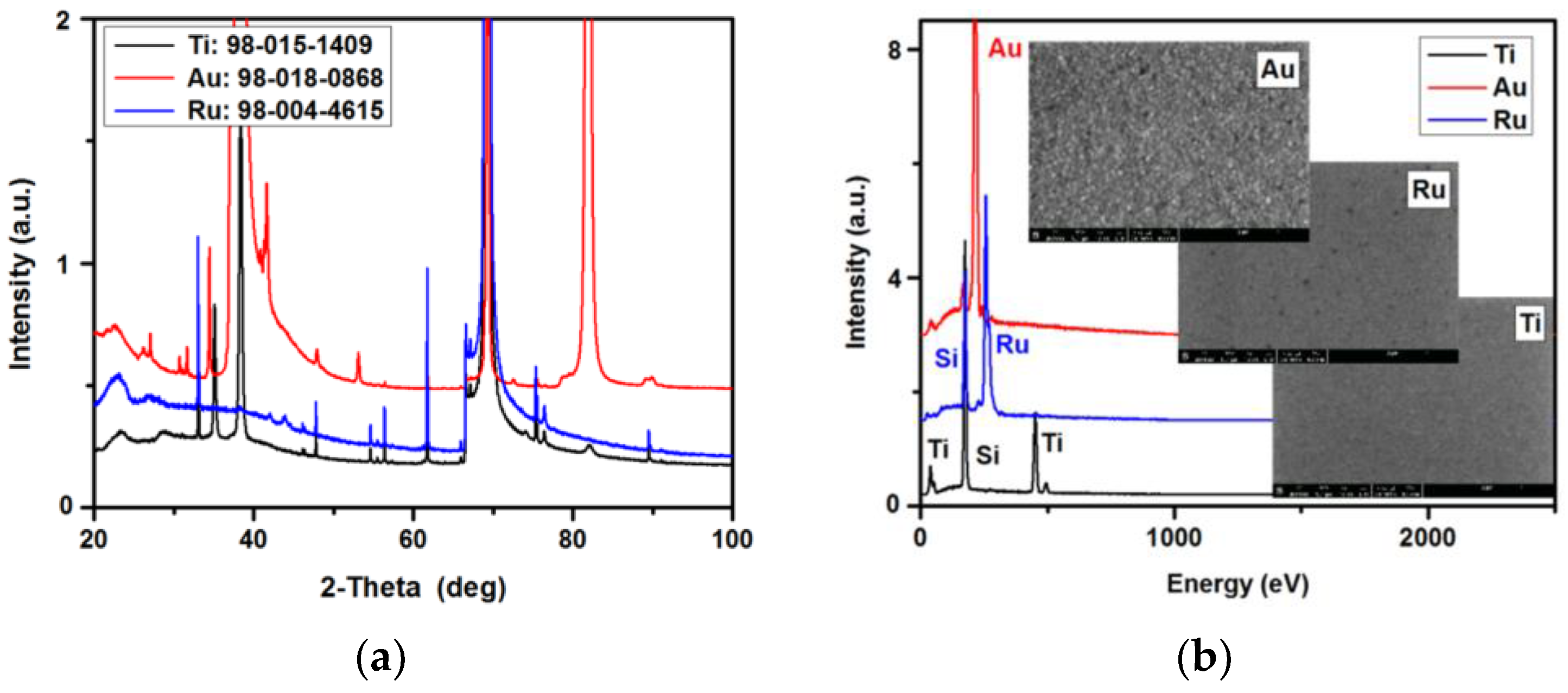
3.1. XRD/EDS
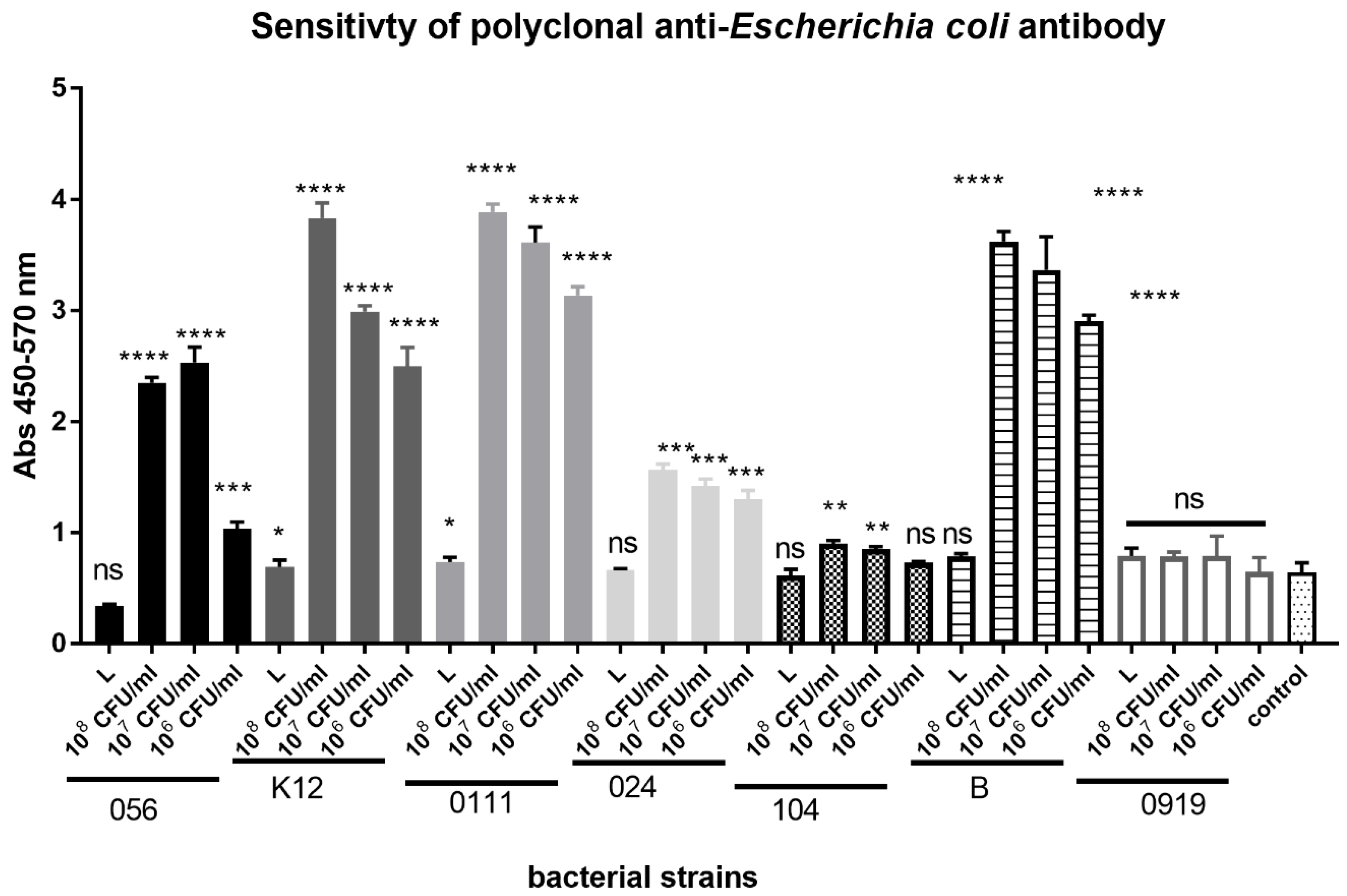
3.2. Sensitivity of Polyclonal Anti-Escherichia coli Antibody Conjugated with Horseradish Peroxidase
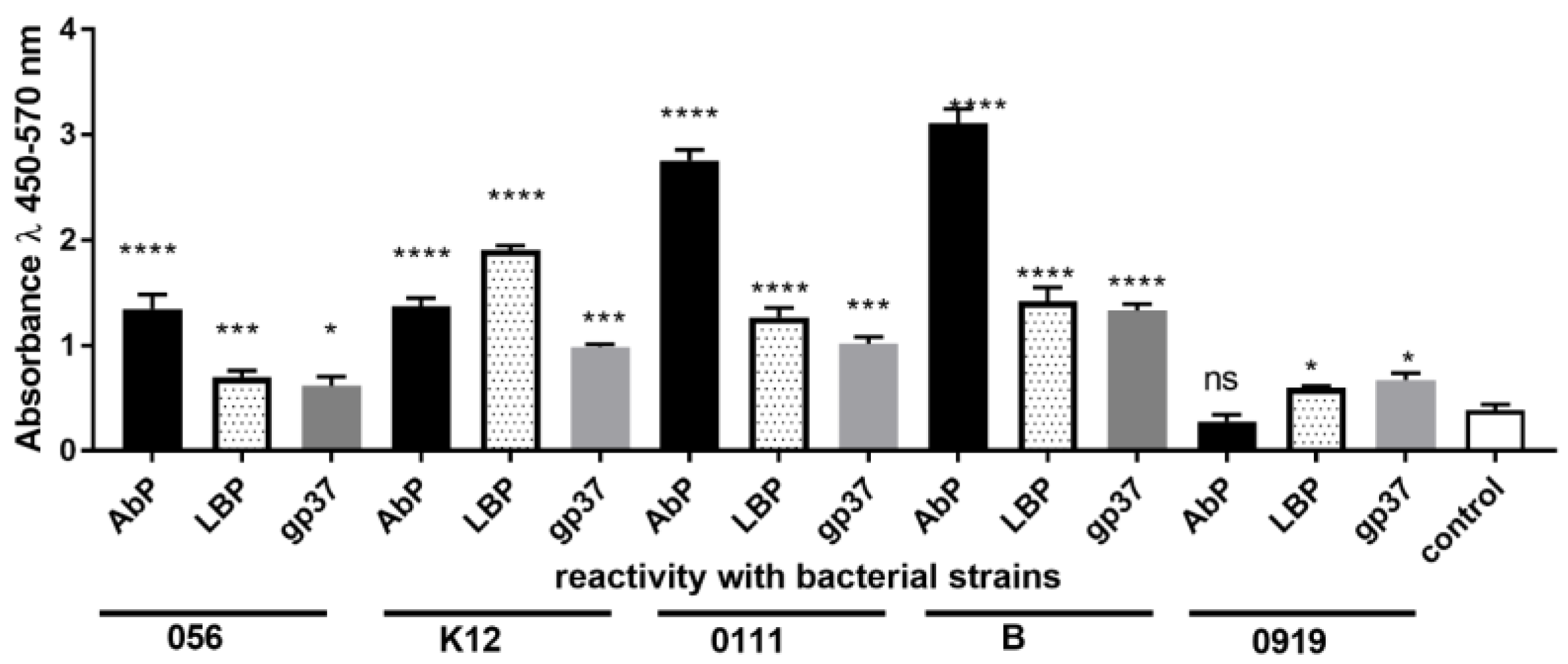
3.3. Specificity and Selectivity of LBP, gp37 Adhesin and Polyclonal Anti-E. coli Antibody
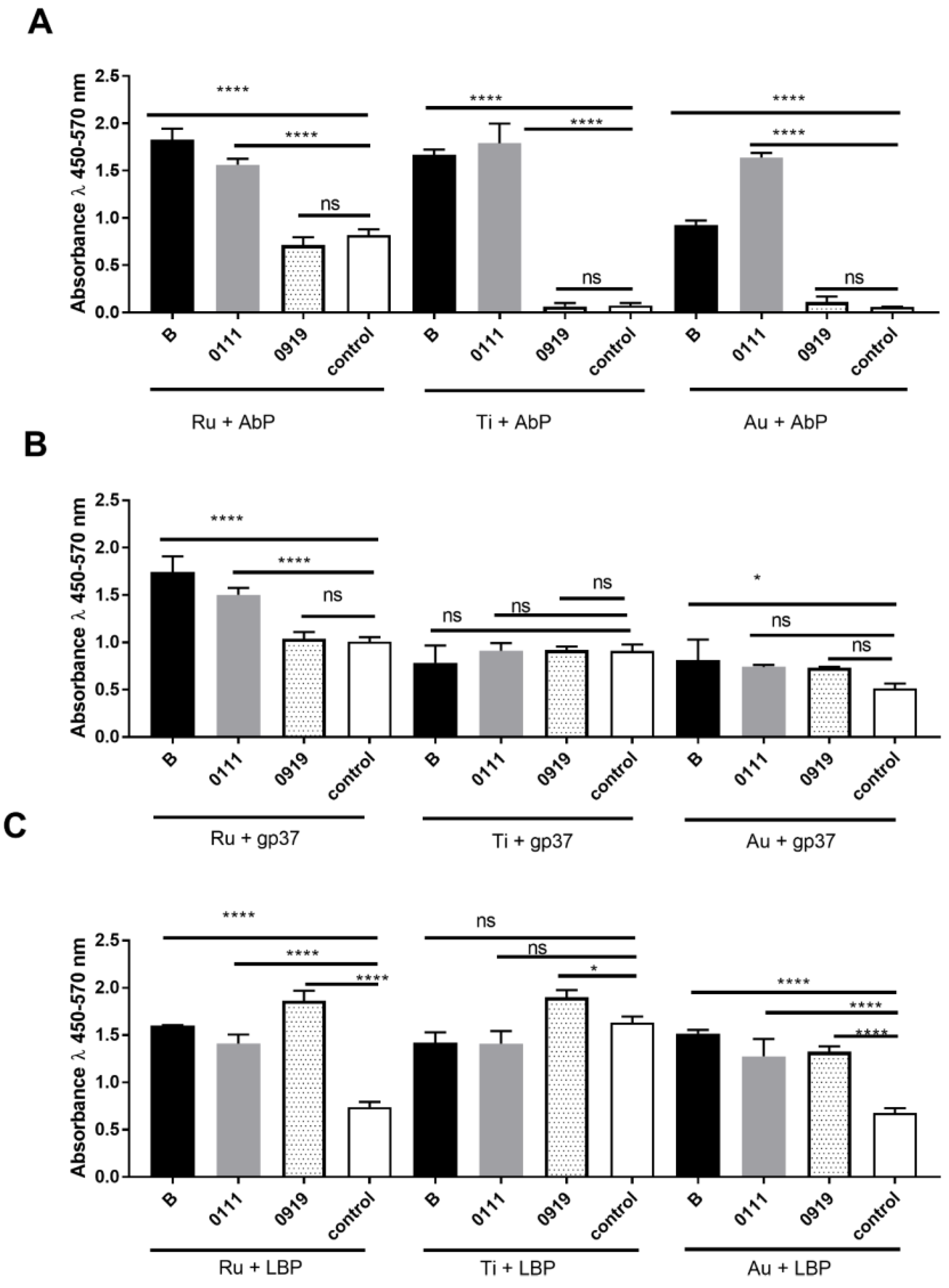
3.4. Evaluation of Escherichia coli Sensitivity and Specificity Detection on Various Protein-Modified Different Metal Surfaces
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, K.M.; Runyon, M.; Herrman, T.J.; Phillips, R.; Hsieh, J. Review of Salmonella detection and identification methods: Aspects of rapid emergency response and food safety. Food Control. 2015, 47, 264–276. [Google Scholar] [CrossRef]
- Mandal, P.K.; Biswas, A.K.; Choi, K.; Pal, U.K. Methods for Rapid Detection of Foodborne Pathogens: An Overview. Am. J. Food Technol. 2011, 6, 87–102. [Google Scholar] [CrossRef]
- Sibley, C.D.; Peirano, G.; Church, D.L. Molecular methods for pathogen and microbial community detection and characterization: Current and potential application in diagnostic microbiology. Infect. Genet. Evol. 2012, 12, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Sindhu, A.; Dilbaghi, N.; Chaudhury, A. Biosensors as innovative tools for the detection of food borne pathogens. Biosens. Bioelectron. 2011, 28, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fathi, F.; Rahbarghazi, R.; Rashidi, M.R. Label-free biosensors in the field of stem cell biology. Biosens. Bioelectron. 2018, 101, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Khansili, N.; Rattu, G.; Krishna, P.M. Label-free optical biosensors for food and biological sensor applications. Sens. Actuators B Chem. 2018, 265, 35–49. [Google Scholar] [CrossRef]
- Farkas, E.; Szekacs, A.; Kovacs, B.; Olah, M.; Horvath, R.; Szekacs, I. Label-free optical biosensor for real-time monitoring the cytotoxicity of xenobiotics: A proof of principle study on glyphosate. J. Hazard. Mater. 2018, 351, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Doornbos, M.L.; Van der Linden, I.; Vereyken, L.; Tresadern, G.; IJzerman, A.P.; Lavreysen, H.; Heitman, L.H. Constitutive activity of the metabotropic glutamate receptor 2 explored with a whole-cell label-free biosensor. Biochem. Pharmacol. 2018, 152, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Haensch, C.; Hoeppener, S.; Schubert, U.S. Chemical modification of self-assembled silane based monolayers by surface reactions. Chem. Soc. Rev. 2010, 39, 2323–2334. [Google Scholar] [CrossRef] [PubMed]
- Windmiller, J.R.; Wang, J. Wearable electrochemical sensors and biosensors: A review. Electroanalysis 2013, 25, 29–46. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Gooding, J.; Ciampi, S. The molecular level modification of surfaces: From self-assembled monolayers to complex molecular assemblies. Chem. Soc. Rev. 2011, 45, 2704–2718. [Google Scholar] [CrossRef] [PubMed]
- Welch, N.; Scoble, J.; Muir, B.; Pigram, P. Orientation and characterization of immobilized antibodies for improved immunoassays. Biointerphases 2017, 12, 02D301. [Google Scholar] [CrossRef] [PubMed]
- Oliverio, M.; Perotto, S.; Messina, G.S.; Lovato, L.; De Angelis, F. Chemical Functionalization of Plasmonic Surface Biosensors: A Tutorial Review on Issues, Strategies, and Costs. ACS Appl. Mater. Interfaces 2017, 9, 29394–29411. [Google Scholar] [CrossRef] [PubMed]
- Brianda, E.; Salmainb, M.; Compère, C.; Pradier, C.M. Immobilization of Protein A on SAMs for the elaboration of immunosensors. Colloids Surf. B 2006, 53, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Wadu-Mesthrige, K.; Amro, N.A.; Liu, G.Y. Immobilization of proteins on self-assembled monolayers. Scanning 2000, 22, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Dohyun, K.; Herr, A. Protein immobilization techniques for microfluidic assays. Biomicrofluidics 2013, 7, 041501. [Google Scholar]
- Brzozowska, E.; Śmietana, M.; Koba, M.; Górska, S.; Pawlik, K.; Gamian, A.; Bock, W.J. Recognition of bacterial lipopolysaccharide using bacteriophage-adhesin-coated long-period gratings. Biosens. Bioelectron. 2015, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Smietana, M.; Koba, M.; Brzozowska, E.; Krogulski, K.; Nakonieczny, J.; Wachnicki, L.; Mikulic, P.; Godlewski, M.; Bock, J.W. Label-free sensitivity of long-period gratings enhanced by atomic layer deposited TiO2 nano-overlays. Optics. Express 2015, 23, 8441–8453. [Google Scholar] [CrossRef] [PubMed]
- Rydosz, A.; Brzozowska, E.; Gorska, S.; Wincza, K.; Gamian, A.; Gruszczynski, S. A broadband capacitive method for label-free bacterials LPS detection. Biosens. Bioelectron. 2016, 75, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Drab, M. Phage Aggregation-Dispersion by Ions: Striving beyond Antibacterial Therapy. Trends Biotechnol. 2018, 36, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Drab, M.; Krajniak, J.; Grzelakowski, K.P. The New Methodology and Chemical Contrast Observation by Use of the Energy-Selective Back-Scattered Electron Detector. Microsc. Microanal. 2016, 2, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Charoen-In, U.; Wanthong, A.; Harnsoongnoen, S. Determination of glucose concentration with resonant coplanar microwave sensor. In Proceedings of the International Electrical Engineering Congress (iEECON), Pattaya, Thailand, 8–10 March 2017; pp. 1–3. [Google Scholar]
- Abdolrazzaghi, M.; Daneshmand, M.; Iyer, A.K. Strongly Enhanced Sensitivity in Planar Microwave Sensors Based on Metamaterial Coupling. IEEE Trans. Microwave Theory Tech. 2017, 99, 1–13. [Google Scholar] [CrossRef]
- Liu, W.; Sun, H.; Xu, L. A Microwave Method for Dielectric Characterization Measurement of Small Liquids Using a Metamaterial-Based Sensor. Sensors 2018, 18, 1438. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.W. Label-Free and Antibody-Free Wideband Microwave Biosensor for Identifying the Cancer Cells. IEEE Trans. Microwave Theory Tech. 2016, 64, 982–990. [Google Scholar] [CrossRef]
- Vélez, P.; Bonache, J.; Martín, F. Dual and broadband power dividers at microwave frequencies based on composite right/left handed (CRLH) lattice networks. Photonics Nanostruct. Fundam. Appl. 2014, 12, 269–278. [Google Scholar] [CrossRef]
- Huang, H.; Xia, H.; Xie, W.; Guo, Z.; Li, H.; Xie, D. Design of broadband graphene-metamaterial absorbers for permittivity sensing at mid-infrared regions. Sci. Rep. 2018, 8, 4183. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, Y.; Wang, H.; Li, Y.; Huang, W. Tribocorrosion behavior of Ti-30Zr alloy for dental implants. Mater. Lett. 2018, 218, 190–192. [Google Scholar] [CrossRef]
- Weinmann, M.; Schnitter, C.; Stenzel, M.; Markhoff, J.; Schulze, C.; Bader, R. Development of bio-compatible refractory Ti/Nb(/Ta) alloys for application in patient-specific orthopaedic implants. Int. J. Refract. Met. Hard Mater. 2018, 75, 126–136. [Google Scholar] [CrossRef]
- Yan, J.; Chang, B.; Hu, X.; Cao, C.; Zhao, L.; Zhang, Y. Titanium implant functionalized with antimiR-138 delivered cell sheet for enhanced peri-implant bone formation and vascularization. Mater. Sci. Eng. C 2018, 89, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Li, Y.; Li, W.; Chen, X.; Zeng, X. Prepration of a coated Ti anode for producing acidic electrolyzed oxidizing water. LWT Food Sci. Technol. 2016, 66, 606–614. [Google Scholar] [CrossRef]
- Nemati, A.; Saghafi, M.; Khamesh, S.; Alibakhshi, E.; Zarrintaj, P.; Reza, S.M. Magnetron-sputtered TixNy thin films applied on titanium-based alloys for biomedical applications: Composition-microstructure-property relationships. Surf. Coat. Technol. 2018, 349, 251–259. [Google Scholar] [CrossRef]
- Varshney, M.; Li, Y. Interdigitated array microelectrodes based impedance biosensors for detection of bacterial cells. Biosens. Bioelectron. 2009, 24, 2951–2960. [Google Scholar] [CrossRef] [PubMed]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wendy, C.; Foroushani, A.D.; Wang, H.; Li, D.; Liu, J.; Barrow, C.; Wang, X.; Yang, W. New Gold Nanostructures for Sensor Applications: A Review. Materials 2014, 7, 5169–5201. [Google Scholar] [CrossRef] [PubMed]
- Bafadir, E.B.; Sezgintürk, M.K. A review on impedimetric biosensors. Artif. Cells Nanomed. Biotechnol. 2014, 44, 1–15. [Google Scholar]
- Muhammad, A.; Chaudhary, V.A.; Mulla, I.S.; Sainkar, S.R.; Mandale, A.B.; Belhekar, A.A.; Pillai, V.A. Highly Selective Ammonia Gas Sensor Using Surface-Ruthenated Zinc Oxide. Sens. Actuators A Phys. 1999, 75, 162–167. [Google Scholar]
- Jia, S.; Matsuda, S.; Tamura, S.; Shironita, S.; Umeda, M. Study of CO2 reduction at Pt-Ru electrocatalyst in polymer electrolyte cell by differential electrochemical mass spectrometry and liquid chromatography. Electrochim. Acta 2018, 261, 340–345. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Y.; Cao, D.; Xiao, K.; Guo, T.; Zhao, X. Enhanced photoelectrocatalytic degradation of 2,4-dichlorophenol by TiO2/Ru-IrO2 bifacial electrode. Chem. Eng. J. 2018, 343, 69–77. [Google Scholar] [CrossRef]
- Kwok, Y.H.; Wang, Y.F.; Tsang, A.C.H.; Leung, D.Y.C. Graphene-carbon nanotube composite aerogel with Ru@Pt nanoparticle as a porous electrode for direct methanol microfluidic fuel cell. Appl. Energy 2018, 217, 258–265. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, S.; Xu, Y.; Xue, H.; Pang, H. Ruthenium based materials as electrode materials for supercapacitors. Chem. Eng. J. 2018, 333, 505–518. [Google Scholar] [CrossRef]
- Laik, B.; Bourg, S.; Pereira-Ramos, J.P.; Bruyere, S.; Pierson, J.F. Electrochemical reaction of lithium with ruthenium nitride thin films prepared by pulsed-DC magnetron sputtering. Electrochimi. Acta 2015, 164, 12–20. [Google Scholar] [CrossRef]
- Rosestolato, D.; Battaglin, G.; Ferro, S. Electrochemical properties of stoichiometric RuN film prepared by RF-magnetron sputtering: A preliminary study. Electrochem. Commun. 2014, 49, 9–13. [Google Scholar] [CrossRef]
- Tian, H.Y.; Wang, Y.; Chan, H.L.W.; Choy, C.L.; No, K.S. Structural and electrical characteristics of highly textured oxidation-free Ru thin films by DC magnetron sputtering. J. Alloys Compd. 2005, 392, 231–236. [Google Scholar] [CrossRef]
- Hossain, M.N.; Chen, S.; Chen, A. Fabrication and electrochemical study of ruthenium-ruthenium oxide/activated carbon nanocomposites for enhanced energy storage. J. Alloys Compd. 2018, 751, 138–147. [Google Scholar] [CrossRef]
- Yang, S.; Yu, J.; Jiang, T.; Zhu, L.; Xu, X. High performance symmetric solid state supercapacitor based on electrode of RuxNi1−xCo2O4 grown on nickel foam. J. Alloys Compd. 2018, 764, 767–775. [Google Scholar] [CrossRef]
- Nosheen, E.; Shah, S.M.; Iqbal, Z. Ru-dye grafted CdS and reduced graphene oxide Ru/CdS/rGO composite: An efficient and photo tuneable electrode material for solid state dye sensitized polymer solar cells. J. Photochem. Photobiol. B 2017, 167, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.; Navaeipour, F.; Khalilzadeh, B.; Tajalli, H.; Mollabashi, M.; Johari, A.; Rashidi, M. Highly sensitive electrochemiluminescence detection of p53 protein using functionalized Ru-silica nanoporous@gold nanocomposite. Biosens. Bioelectron. 2016, 80, 146–153. [Google Scholar]
- Guo, L.; Lv, G.; Qiu, L.; Yang, H.; Zhang, L.; Yu, H.; Zou, M.; Lin, J. Insights into anticancer activity and mechanism of action of a ruthenium(II) complex in human esophageal squamous carcinoma EC109 cells. Eur. J. Pharmacol. 2016, 786, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiong, H.; Chen, M.; Zhang, X.; Wang, S. Surface-enhanced molecularly imprinted electrochemiluminescence sensor based on Ru@SiO2 for ultrasensitive detection of fumonisin B1. Biosens. Bioelectron. 2017, 96, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, M.; Rieu, M.; Stambouli, V.; Tournier, G.; Viricelle, J.P.; Pijolat, C. Selective ammonia gas sensor based on SnO2-APTES modification. Procedia Eng. 2016, 168, 280–283. [Google Scholar] [CrossRef]
- Lin, J.J.; Hsu, P.Y.; Wu, Y.L.; Jhuang, J.J. Characteristics of polysilicon wire glucose sensors with a surface modified by silica nanoparticles/γ-APTES nanocomposite. Sensors 2011, 11, 2796–2808. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Hsu, P.Y.; Hsu, C.P.; Liu, W.C. Polysilicon wire for the detection label free DNA. J. Electrochem. Soc. 2010, 157, 191–195. [Google Scholar] [CrossRef]
- Wu, Y.L.; Hsu, P.Y.; Lin, J.J. Polysilicon wire glucose sensor highly immune to interference. Biosens. Bioelectron. 2011, 26, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Gunda, N.S.K.; Singh, M.; Norman, L.; Kaur, K.; Mitra, S.K. Optimization and characterization of biomolecule immobilization on silicon substrates using (3-aminopropyl)triethoxysilane (APTES) and glutaraldehyde linker. Appl. Surf. Sci. 2014, 305, 522–530. [Google Scholar] [CrossRef]
- Rajh, T.; Dimitrijevic, N.M.; Bissonnette, M.; Koritarov, T.; Konda, V. Titanium dioxide in the service of the biomedical revolution. Chem. Rev. 2014, 114, 10177–10216. [Google Scholar] [CrossRef] [PubMed]
- Meroni, D.; Lo Presti, L.; Di Liberto, G.; Ceotto, M.; Acres, R.G.; Prince, K.C.; Bellani, R.; Soliveri, G.; Ardizzone, S. A close look at the structure of the TiO2-APTES interface in hybrid nanomaterials and its degradation pathway: An experimental and theoretical study. J. Phys. Chem. C 2017, 121, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Klug, J.; Pérez, L.A.; Coronado, E.A.; Lacconi, G.I.J. Chemical and wectrochemical oxidation of silicon surfaces functionalized with APTES: The role of surface roughness in the AuNPs anchoring kinetics. J. Phys. Chem. C 2013, 117, 11317–11327. [Google Scholar] [CrossRef]
- Liu, Y.C.C.; Rieben, N.; Iversen, L.; Sørensen, B.S.; Park, J.; Nygard, J.; Martinez, K.L. Specific and reversible immobilization of histidine-tagged proteins on functionalized silicon nanowires. Nanotechnology 2010, 21, 245105. [Google Scholar] [CrossRef] [PubMed]
- Staszek, K.; Piekarz, I.; Sorocki, J.; Koryciak, S.; Wincza, K.; Gruszczynski, S. Low-cost microwave vector system for liquid properties monitoring. IEEE Trans. Ind. Electron. 2018, 65, 1665–1674. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górska, S.; Rydosz, A.; Brzozowska, E.; Drab, M.; Wincza, K.; Gamian, A.; Gruszczyński, S. Effectiveness of Sensors Contact Metallization (Ti, Au, and Ru) and Biofunctionalization for Escherichia coli Detection. Sensors 2018, 18, 2912. https://doi.org/10.3390/s18092912
Górska S, Rydosz A, Brzozowska E, Drab M, Wincza K, Gamian A, Gruszczyński S. Effectiveness of Sensors Contact Metallization (Ti, Au, and Ru) and Biofunctionalization for Escherichia coli Detection. Sensors. 2018; 18(9):2912. https://doi.org/10.3390/s18092912
Chicago/Turabian StyleGórska, Sabina, Artur Rydosz, Ewa Brzozowska, Marek Drab, Krzysztof Wincza, Andrzej Gamian, and Sławomir Gruszczyński. 2018. "Effectiveness of Sensors Contact Metallization (Ti, Au, and Ru) and Biofunctionalization for Escherichia coli Detection" Sensors 18, no. 9: 2912. https://doi.org/10.3390/s18092912
APA StyleGórska, S., Rydosz, A., Brzozowska, E., Drab, M., Wincza, K., Gamian, A., & Gruszczyński, S. (2018). Effectiveness of Sensors Contact Metallization (Ti, Au, and Ru) and Biofunctionalization for Escherichia coli Detection. Sensors, 18(9), 2912. https://doi.org/10.3390/s18092912






