Salivary Detection of Dengue Virus NS1 Protein with a Label-Free Immunosensor for Early Dengue Diagnosis
Abstract
:1. Introduction
2. Materials and Methods
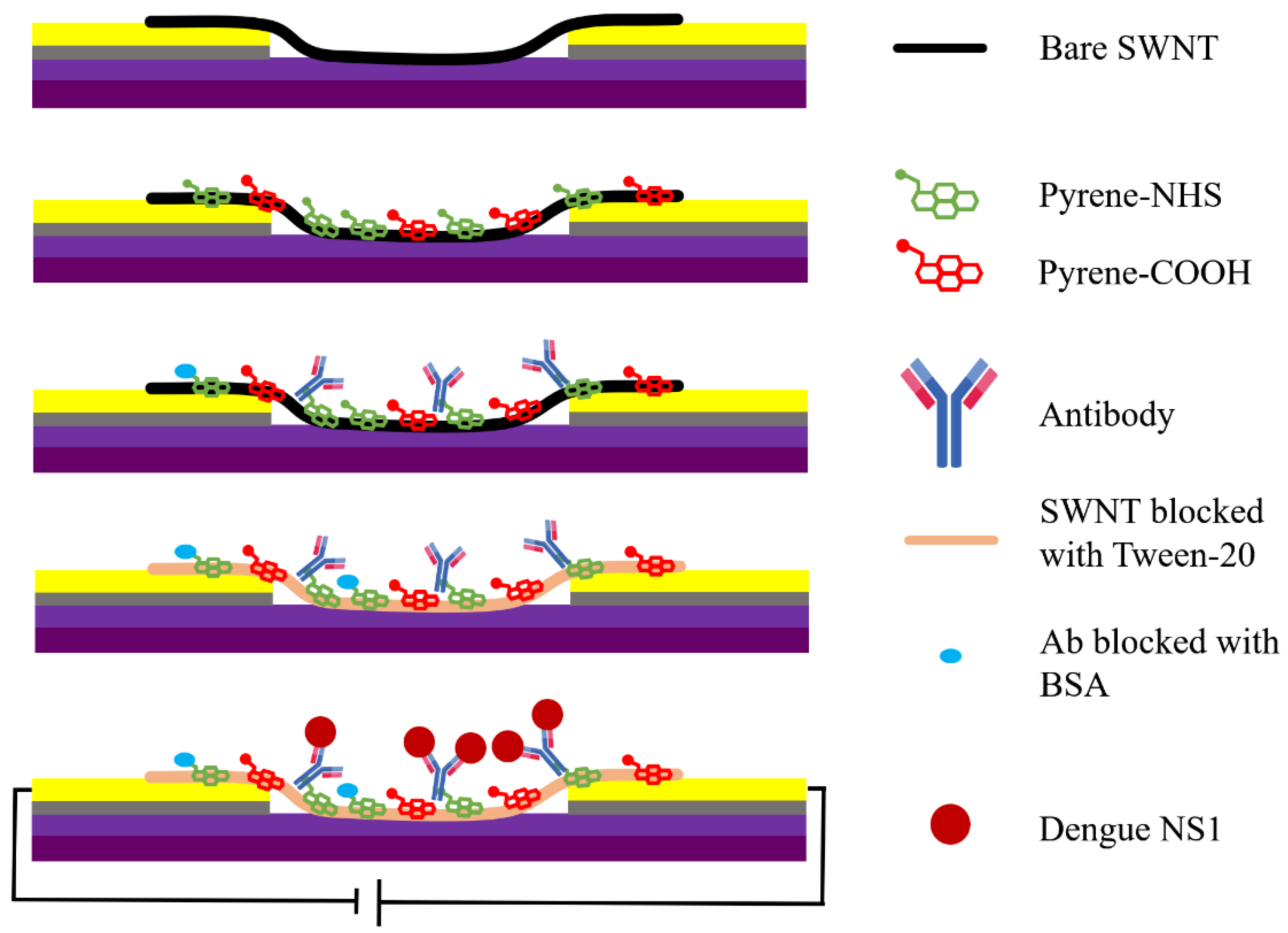
2.1. Fabrication of SWNT Immunosensors
2.1.1. Microelectrode Fabrication
2.1.2. Preparation of Anti-Dengue Virus NS1 Glycoprotein Antibody and NS1 Glycoprotein
2.1.3. Preparation of Adulterated PB and Artificial Human Saliva Samples
2.1.4. Functionalization of SWNT Networks with Anti-Dengue Virus NS1 Glycoprotein Antibody
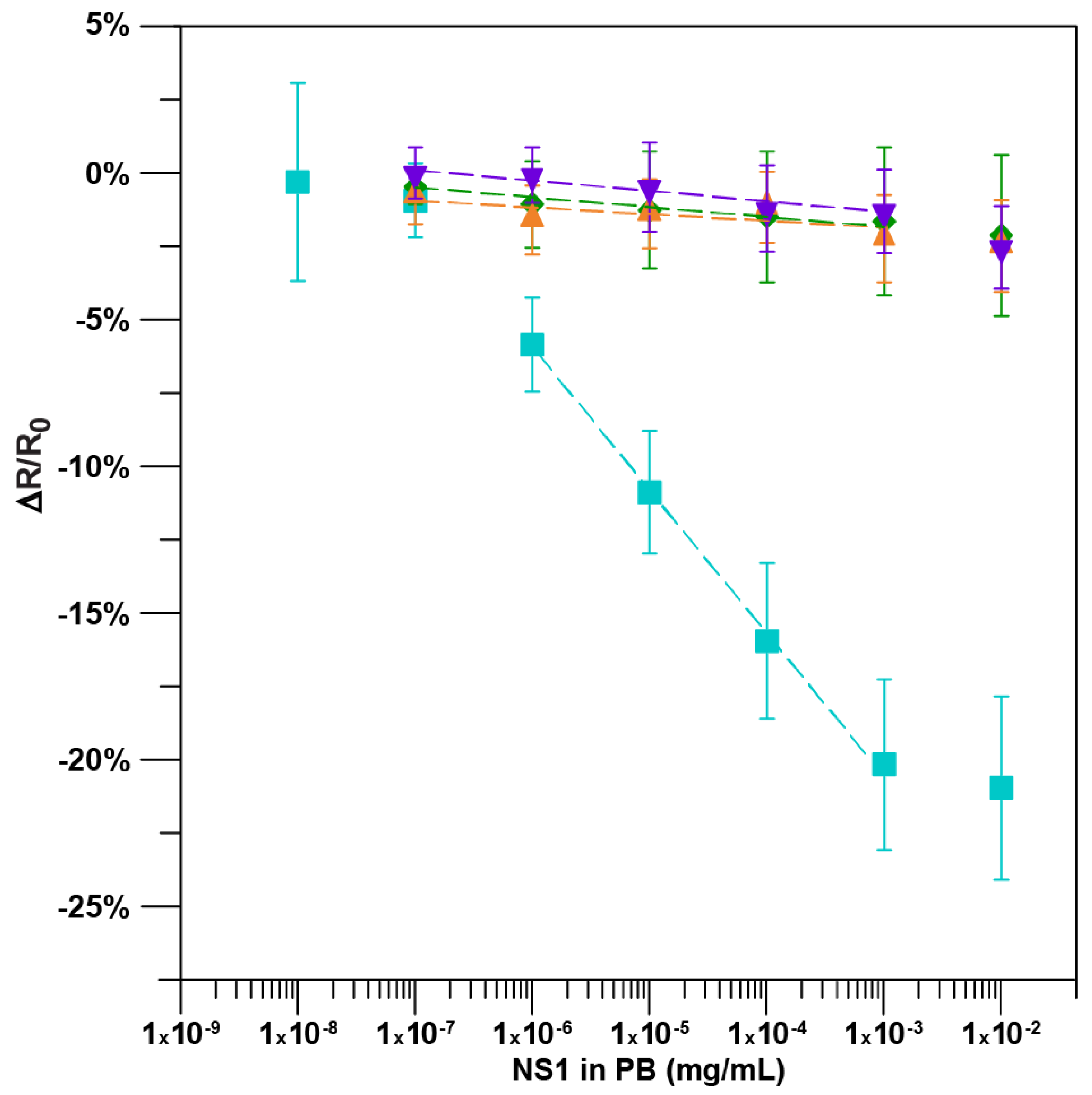
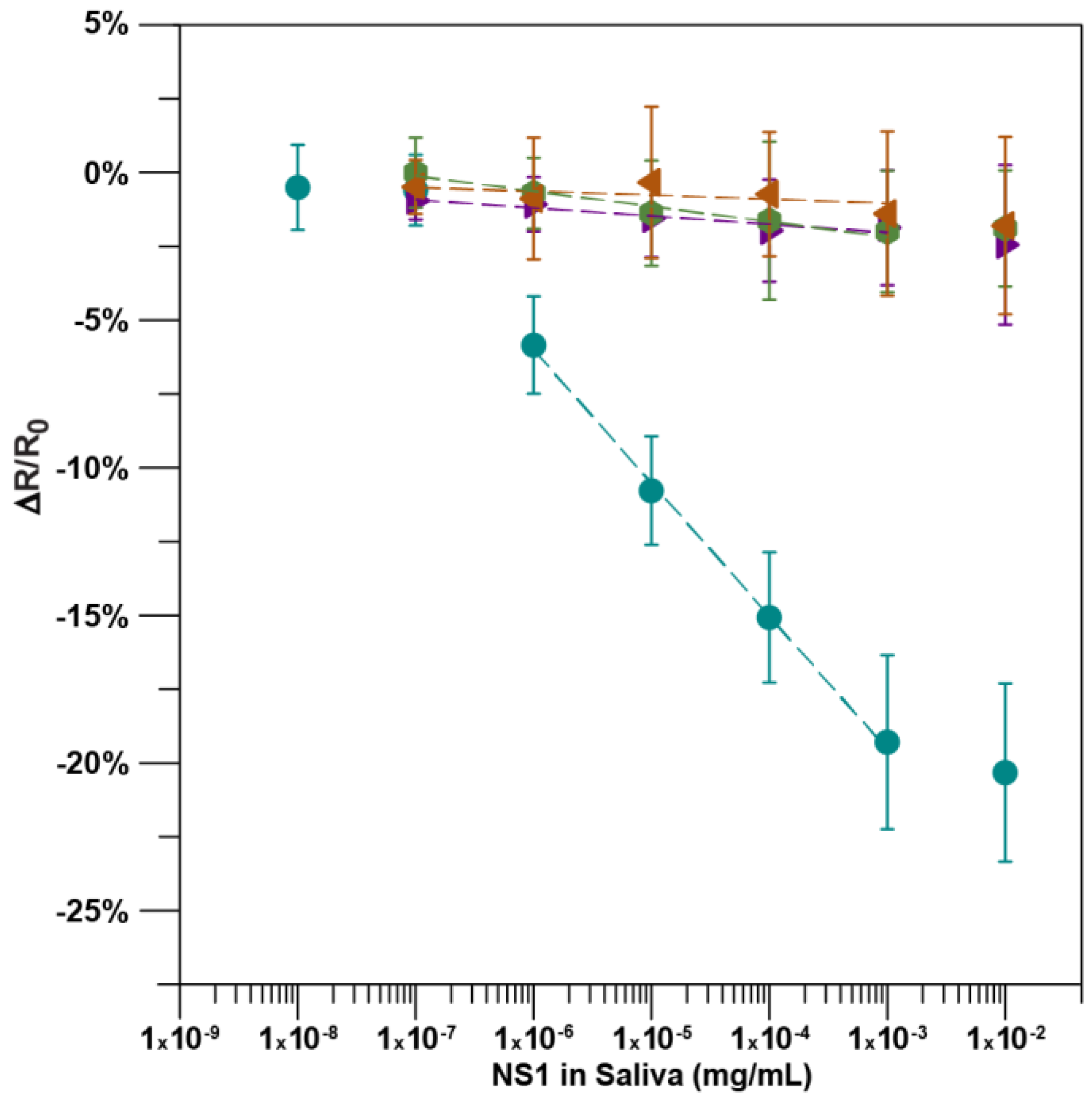
2.2. Detection of the NS1 Protein
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guzmán, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- Weaver, S.C.; Lecuit, M. Chikungunya virus and the global spread of a mosquito-borne disease. N. Engl. J. Med. 2015, 372, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Succo, T.; Leparc-Goffart, I.; Ferré, J.B.; Roiz, D.; Broche, B.; Maquart, M.; Noel, H.; Catelinois, O.; Entezam, F.; Caire, D.; et al. Autochthonous dengue outbreak in nimes, south of France, July to September 2015. Eurosurveill 2016, 21, 30240. [Google Scholar] [CrossRef] [PubMed]
- Teets, F.D.; Ramgopal, M.N.; Sweeney, K.D.; Graham, A.S.; Michael, S.F.; Isern, S. Origin of the dengue virus outbreak in martin county, Florida, USA 2013. Virol. Rep. 2014, 1, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Kutsuna, S.; Kato, Y.; Moi, M.L.; Kotaki, A.; Ota, M.; Shinohara, K.; Kobayashi, T.; Yamamoto, K.; Fujiya, Y.; Mawatari, M.; et al. Autochthonous dengue fever, Tokyo, Japan, 2014. Emerg. Infect. Dis. 2015, 21, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, C.P.; Farrar, J.J.; van Vinh Chau, N.; Wills, B. Dengue. N. Engl. J. Med. 2012, 366, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dengue: Guidelines for Diagnosis Treatment Prevention and Control: New Edition; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Shamala, D.S. Laboratory diagnosis of dengue: A review. Int. Med. J. Malays. 2015, 14, 17–28. [Google Scholar]
- Mustafa, M.S.; Rasotgi, V.; Jain, S.; Gupta, V. Discovery of fifth serotype of dengue virus (DENV-5): A new public health dilemma in dengue control. Med. J. Armed Forces India 2015, 71, 67–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, L.J.; Pessanha, L.B.; Mansur, L.C.; de Souza, L.A.; Ribeiro, M.B.; da Silveira, M.d.V.; Souto Filho, J.T. Comparison of clinical and laboratory characteristics between children and adults with dengue. Braz. J. Infect. Dis. 2013, 17, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Parkash, O.; Shueb, R.H. Diagnosis of dengue infection using conventional and biosensor based techniques. Viruses 2015, 7, 5410–5427. [Google Scholar] [CrossRef] [PubMed]
- Hunsperger, E.A.; Yoksan, S.; Buchy, P.; Nguyen, V.C.; Sekaran, S.D.; Enria, D.A.; Vazquez, S.; Cartozian, E.; Pelegrino, J.L.; Artsob, H.; et al. Evaluation of commercially available diagnostic tests for the detection of dengue virus NS1 antigen and anti-dengue virus IgM antibody. PLoS Negl. Trop. Dis. 2014, 8, e3171. [Google Scholar] [CrossRef] [PubMed]
- Priyamvada, L.; Quicke, K.M.; Hudson, W.H.; Onlamoon, N.; Sewatanon, J.; Edupuganti, S.; Pattanapanyasat, K.; Chokephaibulkit, K.; Mulligan, M.J.; Wilson, P.C.; et al. Human antibody responses after dengue virus infection are highly cross-reactive to zika virus. Proc. Natl. Acad. Sci. USA 2016, 113, 7852–7857. [Google Scholar] [CrossRef] [PubMed]
- Winkler, G.; Maxwell, S.E.; Ruemmler, C.; Stollar, V. Newly synthesized dengue-2 virus nonstructural protein NS1 is a soluble protein but becomes partially hydrophobic and membrane-associated after dimerization. Virology 1989, 171, 302–305. [Google Scholar] [CrossRef]
- Paranavitane, S.A.; Gomes, L.; Kamaladasa, A.; Adikari, T.N.; Wickramasinghe, N.; Jeewandara, C.; Shyamali, N.L.; Ogg, G.S.; Malavige, G.N. Dengue NS1 antigen as a marker of severe clinical disease. BMC Infect. Dis. 2014, 14, 570. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J. NS1: A corner piece in the dengue pathogenesis puzzle? Sci. Transl. Med. 2015, 7, 304fs337. [Google Scholar] [CrossRef] [PubMed]
- Blacksell, S.D.; Jarman, R.G.; Gibbons, R.V.; Tanganuchitcharnchai, A.; Mammen, M.P., Jr.; Nisalak, A.; Kalayanarooj, S.; Bailey, M.S.; Premaratna, R.; de Silva, H.J.; et al. Comparison of seven commercial antigen and antibody enzyme-linked immunosorbent assays for detection of acute dengue infection. Clin. Vaccine Immunol. 2012, 19, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Shu, P.-Y.; Yang, C.-F.; Kao, J.-F.; Su, C.-L.; Chang, S.-F.; Lin, C.-C.; Yang, W.-C.; Shih, H.; Yang, S.-Y.; Wu, P.-F.; et al. Application of the dengue virus NS1 antigen rapid test for on-site detection of imported dengue cases at airports. Clin. Vaccine Immunol. 2009, 16, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Tarigan, L.H.; Cifuentes, M.; Quinn, M.; Kriebel, D. Prevention of needle-stick injuries in healthcare facilities: A meta-analysis. Infect. Control Hosp. Epidemiol. 2015, 36, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Inal, S.; Kelleci, M. Distracting children during blood draw: Looking through distraction cards is effective in pain relief of children during blood draw. Int. J. Nurs. Pract. 2012, 18, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Rahim, F. Acquired dengue virus infection by needlestick injury: A case report. Case Study Case Rep. 2017, 7, 22–24. [Google Scholar]
- Lee, C.; Jang, E.J.; Kwon, D.; Choi, H.; Park, J.W.; Bae, G.-R. Laboratory-acquired dengue virus infection by needlestick injury: A case report, South Korea, 2014. Ann. Occup. Environ. Med. 2016, 28, 16. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Walt, D.R. Salivary diagnostics using a portable point-of-service platform: A review. Clin. Ther. 2015, 37, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C.; Pedersen, A.M.; Villa, A.; Ekstrom, J.; Proctor, G.B.; Vissink, A.; Aframian, D.; McGowan, R.; Aliko, A.; Narayana, N.; et al. The functions of human saliva: A review sponsored by the world workshop on oral medicine VI. Arch. Oral Biol. 2015, 60, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, E.M.; Huhtamo, E.; Virtala, A.-M.; Kantele, A.; Vapalahti, O. Approach to non-invasive sampling in dengue diagnostics: Exploring virus and NS1 antigen detection in saliva and urine of travelers with dengue. J. Clin. Virol. 2014, 61, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Radzol, A.R.M.; Lee, K.Y.; Mansor, W. Classification of salivary based NS1 from Raman spectroscopy with support vector machine. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 1835–1838. [Google Scholar]
- Radzol, A.R.M.; Lee, K.Y.; Mansor, W. Nonstructural protein 1 characteristic peak from NS1-saliva mixture with surface-enhanced Raman spectroscopy. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 2396–2399. [Google Scholar]
- Andries, A.-C.; Duong, V.; Ly, S.; Cappelle, J.; Kim, K.S.; Lorn Try, P.; Ros, S.; Ong, S.; Huy, R.; Horwood, P.; et al. Value of routine dengue diagnostic tests in urine and saliva specimens. PLoS Negl. Trop. Dis. 2015, 9, e0004100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, F.; Saucedo, N.M.; Ramnani, P.; Mulchandani, A. Label-free electrical immunosensor for highly sensitive and specific detection of microcystin-LR in water samples. Environ. Sci. Technol. 2015, 49, 9256–9263. [Google Scholar] [CrossRef] [PubMed]
- Tlili, C.; Myung, N.V.; Shetty, V.; Mulchandani, A. Label-free, chemiresistor immunosensor for stress biomarker cortisol in saliva. Biosens. Bioelectron. 2011, 26, 4382–4386. [Google Scholar] [CrossRef] [PubMed]
- Tlili, C.; Cella, L.N.; Myung, N.V.; Shetty, V.; Mulchandani, A. Single-walled carbon nanotube chemoresistive label-free immunosensor for salivary stress biomarkers. Analyst 2010, 135, 2637–2642. [Google Scholar] [CrossRef] [PubMed]
- Ramnani, P.; Gao, Y.; Ozsoz, M.; Mulchandani, A. Electronic detection of microrna at attomolar level with high specificity. Anal. Chem. 2013, 85, 8061–8064. [Google Scholar] [CrossRef] [PubMed]
- Stefansson, S.; Kwon, H.H.; Ahn, S.N. Targeting antibodies to carbon nanotube field effect transistors by pyrene hydrazide modification of heavy chain carbohydrates. J. Nanotechnol. 2012, 2012, 490175. [Google Scholar] [CrossRef]
- Kim, J.P.; Lee, B.Y.; Lee, J.; Hong, S.; Sim, S.J. Enhancement of sensitivity and specificity by surface modification of carbon nanotubes in diagnosis of prostate cancer based on carbon nanotube field effect transistors. Biosens. Bioelectron. 2009, 24, 3372–3378. [Google Scholar] [CrossRef] [PubMed]
- Gruner, G. Carbon nanotube transistors for biosensing applications. Anal. Bioanal. Chem. 2006, 384, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Wasik, D.; Mulchandani, A.; Yates, M.V. A heparin-functionalized carbon nanotube-based affinity biosensor for dengue virus. Biosens. Bioelectron. 2017, 91, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Schipper, R.G.; Silletti, E.; Vingerhoeds, M.H. Saliva as research material: Biochemical, physicochemical and practical aspects. Arch. Oral Biol. 2007, 52, 1114–1135. [Google Scholar] [CrossRef] [PubMed]
- Radzol, A.R.M.; Lee, K.Y.; Mansor, W.; Azmin, N.H.R. SVM-RBF model PCA criterion selection for detection of NS1 molecule from Raman spectra of salivary mixture. In Proceedings of the 2015 IEET International Conference on Biomedical Image and Signal Processing (ICBISP 2015), Beijing, China, 19 November 2015; pp. 1–6. [Google Scholar]
- Radzol, A.R.M.; Lee, K.Y.; Mansor, W.; Othman, N.H. Principal component analysis for detection of NS1 molecules from Raman spectra of saliva. In Proceedings of the 2015 IEEE 11th International Colloquium on Signal Processing & Its Applications (CSPA), Kuala Lumpur, Malaysia, 6–8 March 2015; pp. 168–173. [Google Scholar]
- Anders, K.L.; Nguyet, N.M.; Quyen, N.T.; Ngoc, T.V.; Tram, T.V.; Gan, T.T.; Tung, N.T.; Dung, N.T.; Chau, N.V.; Wills, B.; et al. An evaluation of dried blood spots and oral swabs as alternative specimens for the diagnosis of dengue and screening for past dengue virus exposure. Am. J. Trop. Med. Hyg. 2012, 87, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Allonso, D.; Meneses, M.D.; Fernandes, C.A.; Ferreira, D.F.; Mohana-Borges, R. Assessing positivity and circulating levels of NS1 in samples from a 2012 dengue outbreak in Rio de Janeiro, Brazil. PLoS ONE 2014, 9, e113634. [Google Scholar] [CrossRef] [PubMed]
- Andries, A.-C.; Duong, V.; Ong, S.; Ros, S.; Sakuntabhai, A.; Horwood, P.; Dussart, P.; Buchy, P. Evaluation of the performances of six commercial kits designed for dengue NS1 and anti-dengue IgM, IgG and IgA detection in urine and saliva clinical specimens. BMC Infect. Dis. 2016, 16, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helmerhorst, E.J.; Dawes, C.; Oppenheim, F.G. The complexity of oral physiology and its impact on salivary diagnostics. Oral Dis. 2018, 24, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, J.; Ying, J.Y. A stacking flow immunoassay for the detection of dengue-specific immunoglobulins in salivary fluid. Lab Chip 2015, 15, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Justino, A.B.; Teixeira, R.R.; Peixoto, L.G.; Jaramillo, O.L.B.; Espindola, F.S. Effect of saliva collection methods and oral hygiene on salivary biomarkers. Scand. J. Clin. Lab. Investig. 2017, 77, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Shirtcliff, E.A.; Granger, D.A.; Schwartz, E.; Curran, M.J. Use of salivary biomarkers in biobehavioral research: Cotton-based sample collection methods can interfere with salivary immunoassay results. Psychoneuroendocrinology 2001, 26, 165–173. [Google Scholar] [CrossRef]
- Peres, J.C.; Rouquette, J.L.; Miocevic, O.; Warner, M.C.; Slowey, P.D.; Shirtcliff, E.A. New techniques for augmenting saliva collection: Bacon rules and lozenge drools. Clin. Ther. 2015, 37, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Navazesh, M. Methods for collecting saliva. Ann. N. Y. Acad. Sci. 1993, 694, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Zohaib, S.; Najeeb, S.; Zafar, M.S.; Slowey, P.D.; Almas, K. Human saliva collection devices for proteomics: An update. Int. J. Mol. Sci. 2016, 17, 846. [Google Scholar] [CrossRef] [PubMed]
- Young, P.R.; Hilditch, P.A.; Bletchly, C.; Halloran, W. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J. Clin. Microbiol. 2000, 38, 1053–1057. [Google Scholar] [PubMed]
- Nguyen, M.T.; Ho, T.N.; Nguyen, V.V.; Nguyen, T.H.; Ha, M.T.; Ta, V.T.; Nguyen, L.D.; Phan, L.; Han, K.Q.; Duong, T.H.; et al. An evidence-based algorithm for early prognosis of severe dengue in the outpatient setting. Clin. Infect. Dis. 2017, 64, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Wattal, C. Dengue NS1 antigen detection: A useful tool in early diagnosis of dengue virus infection. Indian J. Med. Microbiol. 2010, 28, 107–110. [Google Scholar] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasik, D.; Mulchandani, A.; Yates, M.V. Salivary Detection of Dengue Virus NS1 Protein with a Label-Free Immunosensor for Early Dengue Diagnosis. Sensors 2018, 18, 2641. https://doi.org/10.3390/s18082641
Wasik D, Mulchandani A, Yates MV. Salivary Detection of Dengue Virus NS1 Protein with a Label-Free Immunosensor for Early Dengue Diagnosis. Sensors. 2018; 18(8):2641. https://doi.org/10.3390/s18082641
Chicago/Turabian StyleWasik, Daniel, Ashok Mulchandani, and Marylynn V. Yates. 2018. "Salivary Detection of Dengue Virus NS1 Protein with a Label-Free Immunosensor for Early Dengue Diagnosis" Sensors 18, no. 8: 2641. https://doi.org/10.3390/s18082641
APA StyleWasik, D., Mulchandani, A., & Yates, M. V. (2018). Salivary Detection of Dengue Virus NS1 Protein with a Label-Free Immunosensor for Early Dengue Diagnosis. Sensors, 18(8), 2641. https://doi.org/10.3390/s18082641





