A “Turn-On” Fluorescence Copper Biosensor Based on DNA Cleavage-Dependent Graphene Oxide-dsDNA-CdTe Quantum Dots Complex
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Apparatus
2.3. GO-dsDNA-CdTe QDs Complex Synthesis
3. Results and Discussion
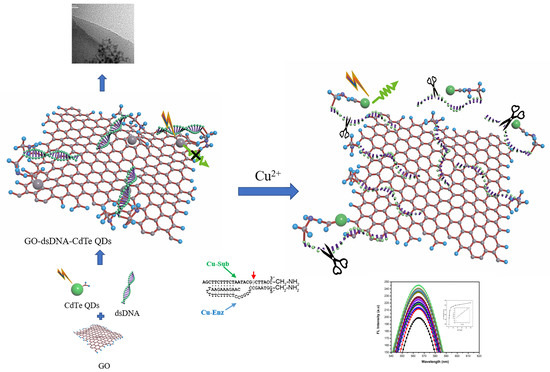
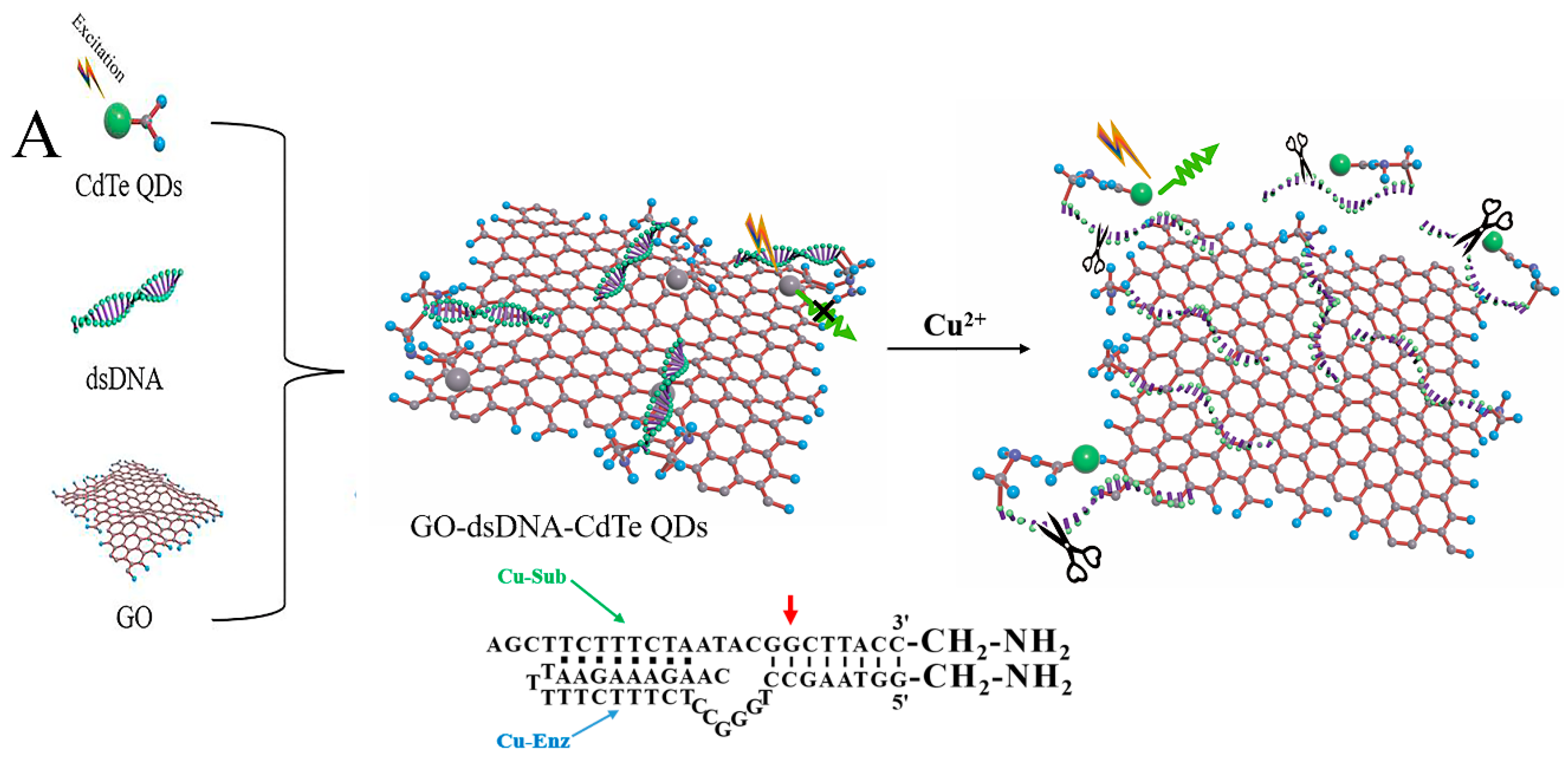
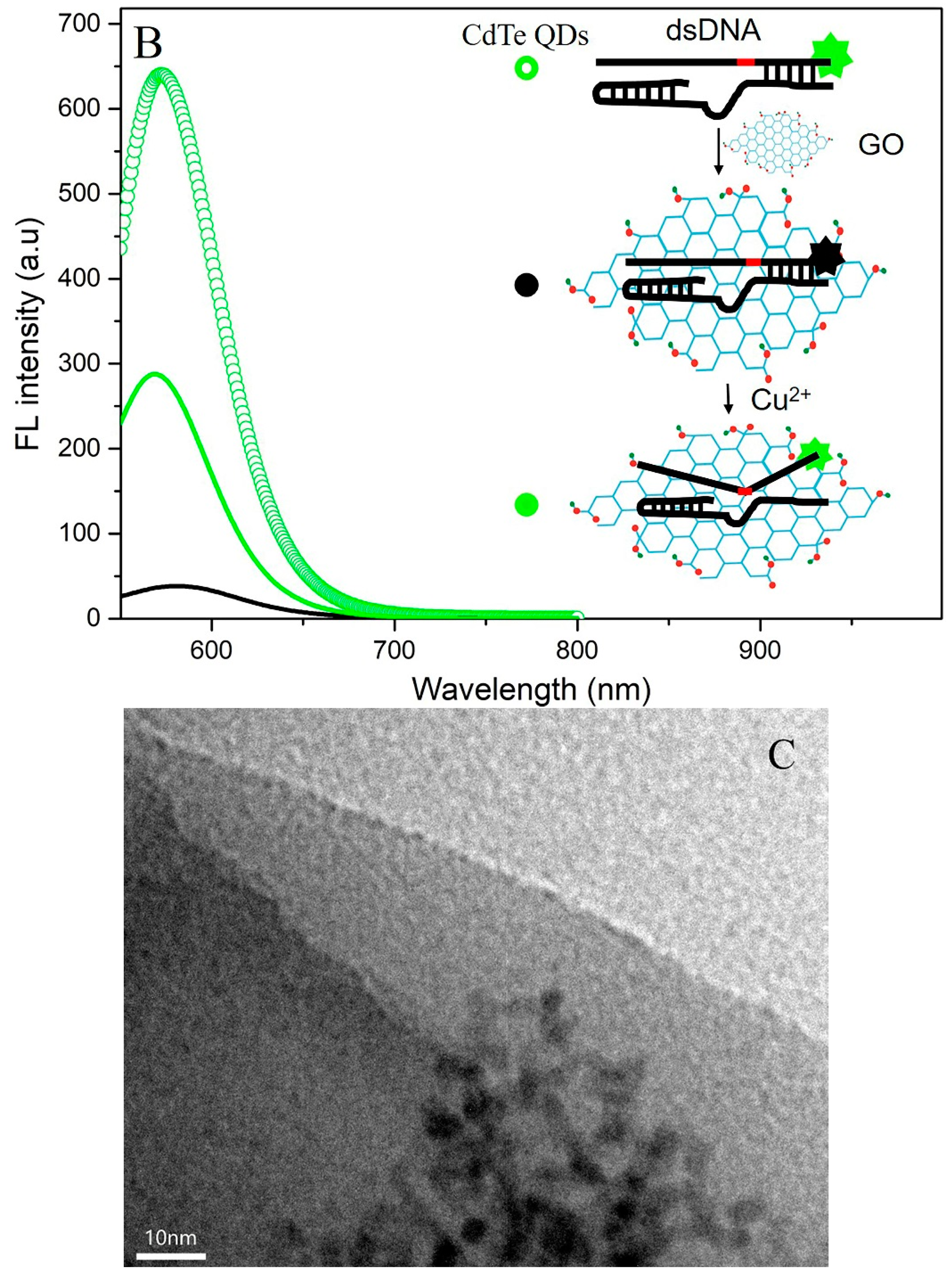
3.1. Principle of the Cu2+ Sensing System
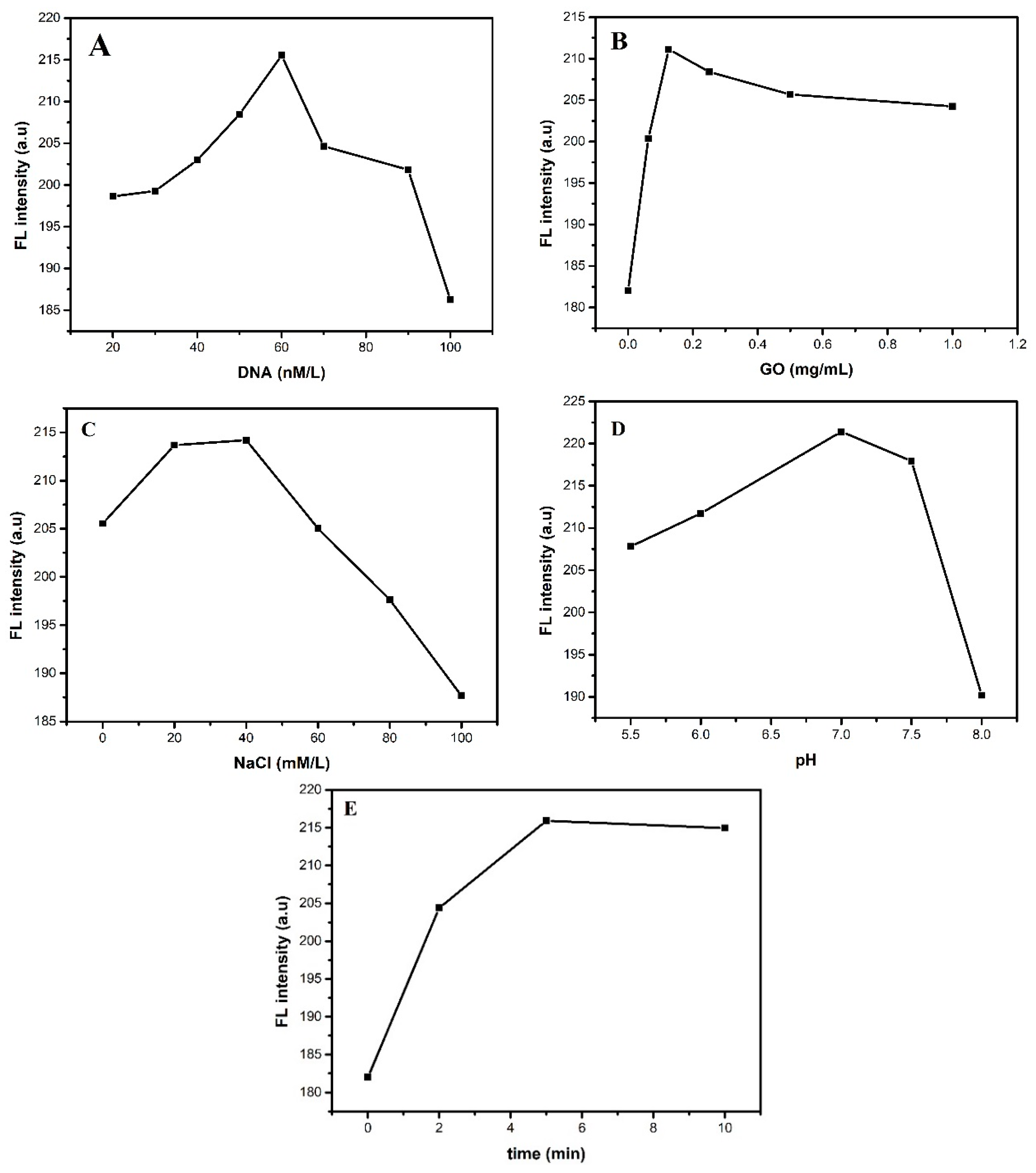
3.2. Optimization of Experimental Conditions
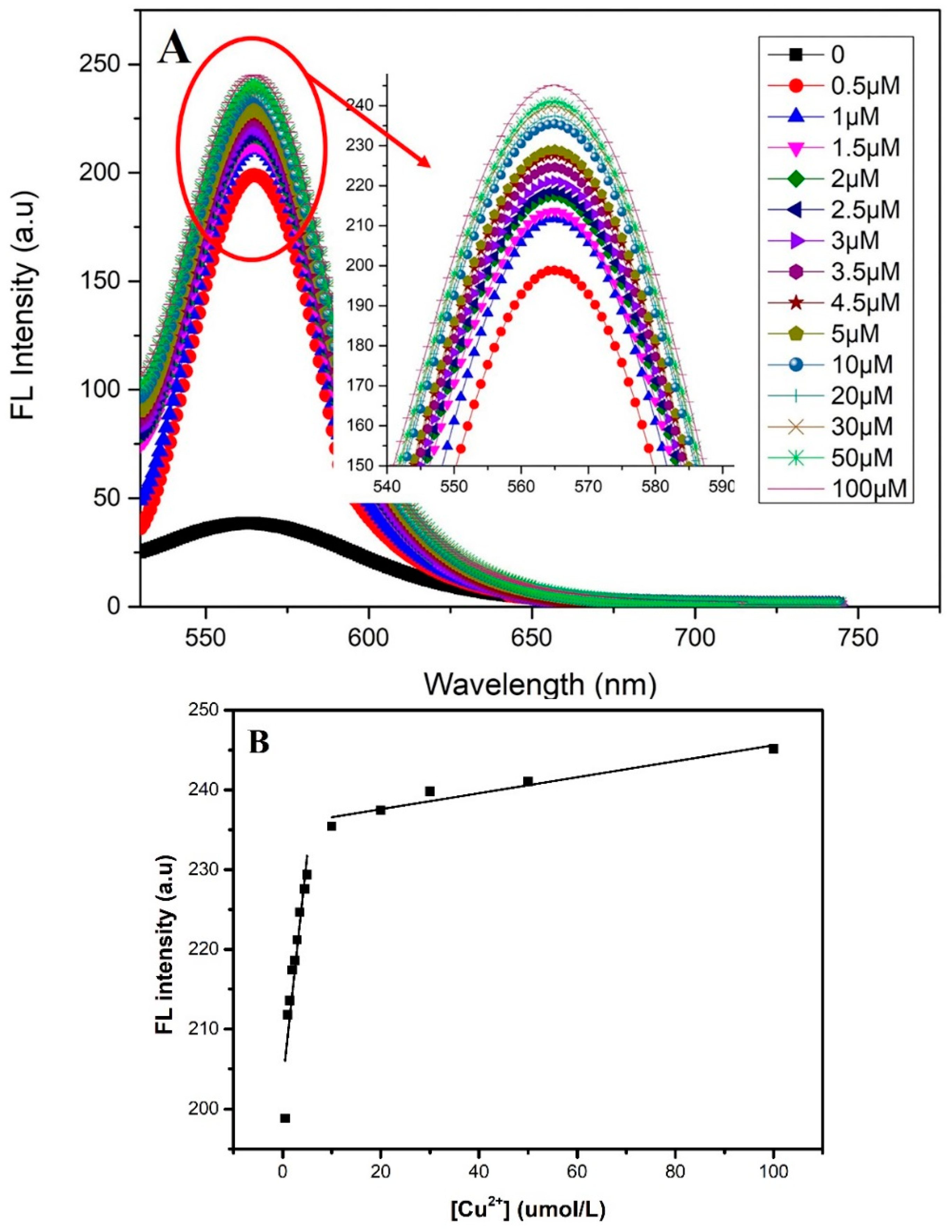
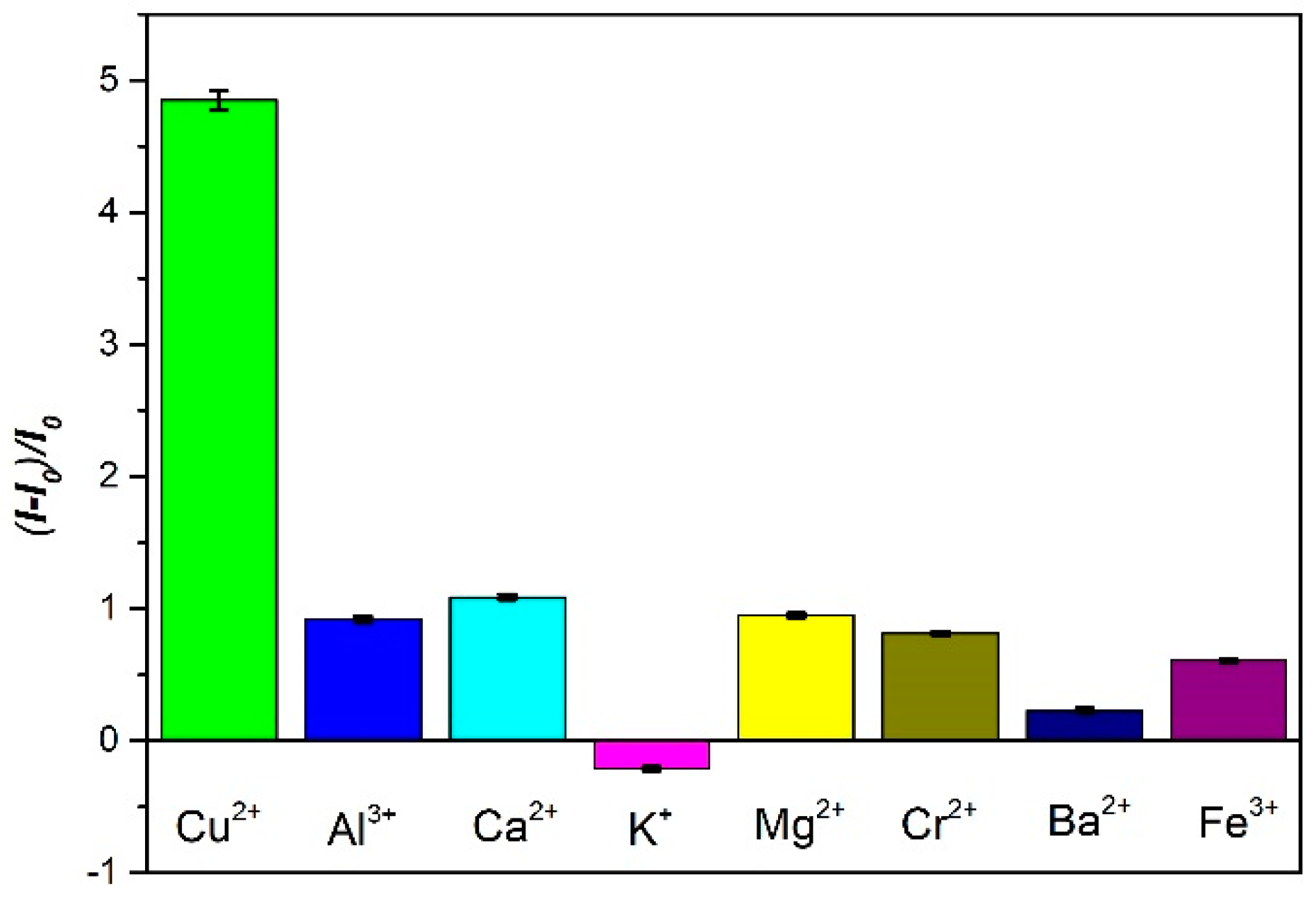
3.3. The Analytical Performance of the Sensor
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, S.P.; Huang, R.Y.; Du, K.J. Colorimetric sensing of Cu(ii) by 2-methyl-3-[(pyridin-2-ylmethyl)-amino]-1,4-naphthoquinone: Cu(ii) induced deprotonation of NH responsible for color changes. Dalton Trans. 2009, 1164, 4735–4740. [Google Scholar] [CrossRef] [PubMed]
- Sheline, C.T.; Choi, D.W. Cu2+ toxicity inhibition of mitochondrial dehydrogenases in vitro and in vivo. Ann. Neurol. 2004, 55, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Faghihian, H.; Hajishabani, A.; Dadfarnia, S.; Zamani, H. Use of clinoptilolite loaded with 1-(2-pyridylazo)-2-naphthol as a sorbent for preconcentration of Pb(II), Ni(II), Cd(II) and Cu(II) prior to their determination by flame atomic absorption spectroscopy. Int. J. Environ. Anal. Chem. 2009, 89, 223–231. [Google Scholar] [CrossRef]
- Reddy, D.K.; Anil, G.; Reddy, M.R.P.; Rao, T.G. A DRC ICP-MS Based Screening Method for Heavy Metals Contamination in Proton Pump Inhibitor Compounds and Their Intermediates. At. Spectrosc. 2008, 29, 201–209. [Google Scholar]
- Zhang, J.F.; Zhou, Y.; Yoon, J.; Kim, Y.; Kim, S.J.; Kim, J.S. Naphthalimide Modified Rhodamine Derivative: Ratiometric and Selective Fluorescent Sensor for Cu2+ Based on Two Different Approaches. Org. Lett. 2010, 12, 3852–3855. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Niu, J.; Yu, C.; Zhao, L.; Ge, J. Highly luminescent water-soluble CdTe nanowires as fluorescent probe to detect copper(II). Chem. Commun. 2005, 33, 4184–4186. [Google Scholar] [CrossRef] [PubMed]
- Berchmans, S.; Vergheese, T.M.; Kavitha, A.L.; Veerakumar, M.; Yegnaraman, V. Electrochemical preparation of copper–dendrimer nanocomposites: Picomolar detection of Cu2+, ions. Anal. Bioanal. Chem. 2008, 390, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Mülazımoğlu, I.E.; Solak, A.O. A novel apigenin modified glassy carbon sensor electrode for the determination of copper ions in soil samples. Anal. Methods 2011, 3, 2534–2539. [Google Scholar] [CrossRef]
- Shao, X.; Gu, H.; Wang, Z.; Chai, X.; Tian, Y.; Shi, G. Highly selective electrochemical strategy for monitoring of cerebral Cu2+ based on a carbon Dot-TPEA hybridized surface. Anal. Chem. 2013, 85, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.C.; Wu, J.; Li, J.; Xie, C.G.; Liu, Y.S.; Zhong, Y.; Liu, J.H. Electrochemical determination of trace copper(II) with enhanced sensitivity and selectivity by gold nanoparticle/single-wall carbon nanotube hybrids containing three-dimensional l -cysteine molecular adapters. Sens. Actuators B Chem. 2013, 182, 382–389. [Google Scholar] [CrossRef]
- Guo, C.; Wang, J.; Cheng, J.; Dai, Z. Determination of trace copper ions with ultrahigh sensitivity and selectivity utilizing CdTe quantum dots coupled with enzyme inhibition. Biosens. Bioelectron. 2012, 36, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Khaing Oo, M.K.; Fan, X. Highly sensitive multiplexed heavy metal detection using quantum-dot-labeled DNAzymes. ACS Nano 2010, 4, 5897–5904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ye, B.C. Colorimetric chiral recognition of enantiomers using the nucleotide-capped silver nanoparticles. Anal. Chem. 2011, 83, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, B.; Zhang, L.; Jiang, H. An optical sensor for Cu(II) detection with upconverting luminescent nanoparticles as an excitation source. Chem. Commun. 2012, 48, 4860–4862. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Shi, W.; Xie, J.; Huang, Y. Highly sensitive and selective fluorescent assay for quantitative detection of divalent copper ion in environmental water samples. Anal. Methods 2011, 3, 2102–2107. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, N.; Bhalla, V.; Sharma, P.R.; Kaur, T. Highly selective fluorescence turn-on chemodosimeter based on rhodamine for nanomolar detection of copper ions. Org. Lett. 2012, 14, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Li, J.; Zhang, S.; Wei, C.; Hao, E.; Vicente, M.G.H. A selective fluorescent sensor for imaging Cu2+ in living cells. New J. Chem. 2009, 33, 1888–1893. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, H.; He, Y.; Cao, Q. Colorimetric detection of Cu2+ using 4-mercaptobenzoic acid modified silver nanoparticles. Colloids Surf. A 2011, 391, 179–183. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Shi, L.L.; Shang, Z.B.; Zhang, Z.; Jin, W.J. Colorimetric and fluorescence sensing of Cu2+ in water using 1,8-dihydroxyanthraquinone-β-cyclodextrin complex with the assistance of ammonia. Talanta 2012, 94, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.F.; Lin, B.T. Application of a nanoporous gold electrode for the sensitive detection of copper via mercury-free anodic stripping voltammetry. Analyst 2009, 134, 2306–2313. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, L.; Mu, X.; Sun, T.; Guo, L. A reagentless signal-on architecture for electronic, real-time copper sensors based on self-cleavage of DNAzymes. Anal. Methods 2010, 2, 627–630. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, H.; Chen, S.; Yu, H.; Zhang, Y.; Quan, X. A “turn-on” fluorescent copper biosensor based on DNA cleavage-dependent graphene-quenched DNAzyme. Biosens. Bioelectron. 2011, 26, 4111–4116. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, Y. Colorimetric Cu2+ detection with a ligation DNAzyme and nanoparticles. Chem. Commun. 2007, 68, 4872–4874. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. Accelerated color change of gold nanoparticles assembled by DNAzymes for simple and fast colorimetric Pb2+ detection. J. Am. Chem. Soc. 2004, 126, 12298–12305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, B.; Li, T.; Wang, E. Label-free DNAzyme-based fluorescing molecular switch for sensitive and selective detection of lead ions. Chem. Commun. 2011, 47, 3099–3101. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Xiang, Y.; Xing, H.; Zhou, Z.; Tong, A.; Lu, Y. Label-free catalytic and molecular beacon containing an abasic site for sensitive fluorescent detection of small inorganic and organic molecules. Anal. Chem. 2012, 84, 2916–2922. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Q.; Cai, Y.; Kong, D.M.; Shen, H.X. Single-stranded DNAzyme-based Pb2+, fluorescent sensor that can work well over a wide temperature range. Biosens. Bioelectron. 2012, 34, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jin, Y.; Deng, J.; Chen, G. Gold nanorods-based FRET assay for sensitive detection of Pb2+ using 8-17DNAzyme. Analyst 2011, 136, 5169–5174. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Dong, S.; Wang, E. Label-free colorimetric detection of aqueous mercury ion (Hg2+) using Hg2+-modulated G-quadruplex-based DNAzymes. Anal. Chem. 2009, 81, 2144–2149. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Li, H.; Zhao, J.; Lin, F.; Zhang, L.; Zhang, Y.; Yao, S. A novel label-free fluorescent sensor for the detection of potassium ion based on DNAzyme. Talanta 2012, 89, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wu, J.; Li, J.; Ju, H. Electrochemical Sensor for Lead Cation Sensitized with a DNA Functionalized Porphyrinic Metal-Organic Framework. Anal. Chem. 2015, 87, 10635–106341. [Google Scholar] [CrossRef] [PubMed]
- Cuenoud, B.; Szostak, J.W. A DNA metalloenzyme with DNA ligase activity. Nature 1995, 375, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lu, Y. Label-free fluorescent aptamer sensor based on regulation of malachite green fluorescence. Anal. Chem. 2010, 82, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, J.; Engelhard, M.; Wang, C.; Lin, Y. Facile and controllable electrochemical reduction of graphene oxide and its applications. J. Mater. Chem. 2010, 20, 743–748. [Google Scholar] [CrossRef]
- Dong, H.; Gao, W.; Yan, F.; Ji, H.; Ju, H. Fluorescence resonance energy transfer between quantum dots and graphene oxide for sensing biomolecules. Anal. Chem. 2010, 82, 5511–5517. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Ni, S.; Wang, X. One-pot synthesis of strongly luminesencing CdTe quantum dots and their conjugation with mouse antibody to alpha-fetoprotein. Colloid J. 2010, 72, 710–715. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S. Graphene and its derivative-based sensing materials for analytical devices. J. Mater. Chem. 2011, 21, 18503–18516. [Google Scholar] [CrossRef]
- Patil, A.J.; Vickery, J.L.; Scott, T.B.; Mann, S. Aqueous Stabilization and Self-Assembly of Graphene Sheets into Layered Bio-Nanocomposites using DNA. Adv. Mater. 2009, 21, 3159–3164. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, H.; Wu, N.; Khakani, M.A.E.; Ma, D. Tuning the Charge-Transfer Property of PbS-Quantum Dot/TiO2-Nanobelt Nanohybrids via Quantum Confinement. J. Phys. Chem. Lett. 2010, 1, 1030–1035. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; Xu, B.; Li, T.; Huang, J.; Bai, W. A “Turn-On” Fluorescence Copper Biosensor Based on DNA Cleavage-Dependent Graphene Oxide-dsDNA-CdTe Quantum Dots Complex. Sensors 2018, 18, 2605. https://doi.org/10.3390/s18082605
Ding L, Xu B, Li T, Huang J, Bai W. A “Turn-On” Fluorescence Copper Biosensor Based on DNA Cleavage-Dependent Graphene Oxide-dsDNA-CdTe Quantum Dots Complex. Sensors. 2018; 18(8):2605. https://doi.org/10.3390/s18082605
Chicago/Turabian StyleDing, Liyun, Bing Xu, Tao Li, Jun Huang, and Wei Bai. 2018. "A “Turn-On” Fluorescence Copper Biosensor Based on DNA Cleavage-Dependent Graphene Oxide-dsDNA-CdTe Quantum Dots Complex" Sensors 18, no. 8: 2605. https://doi.org/10.3390/s18082605
APA StyleDing, L., Xu, B., Li, T., Huang, J., & Bai, W. (2018). A “Turn-On” Fluorescence Copper Biosensor Based on DNA Cleavage-Dependent Graphene Oxide-dsDNA-CdTe Quantum Dots Complex. Sensors, 18(8), 2605. https://doi.org/10.3390/s18082605