Evaluation of Hydrocarbon Soil Pollution Using E-Nose
Abstract
1. Introduction
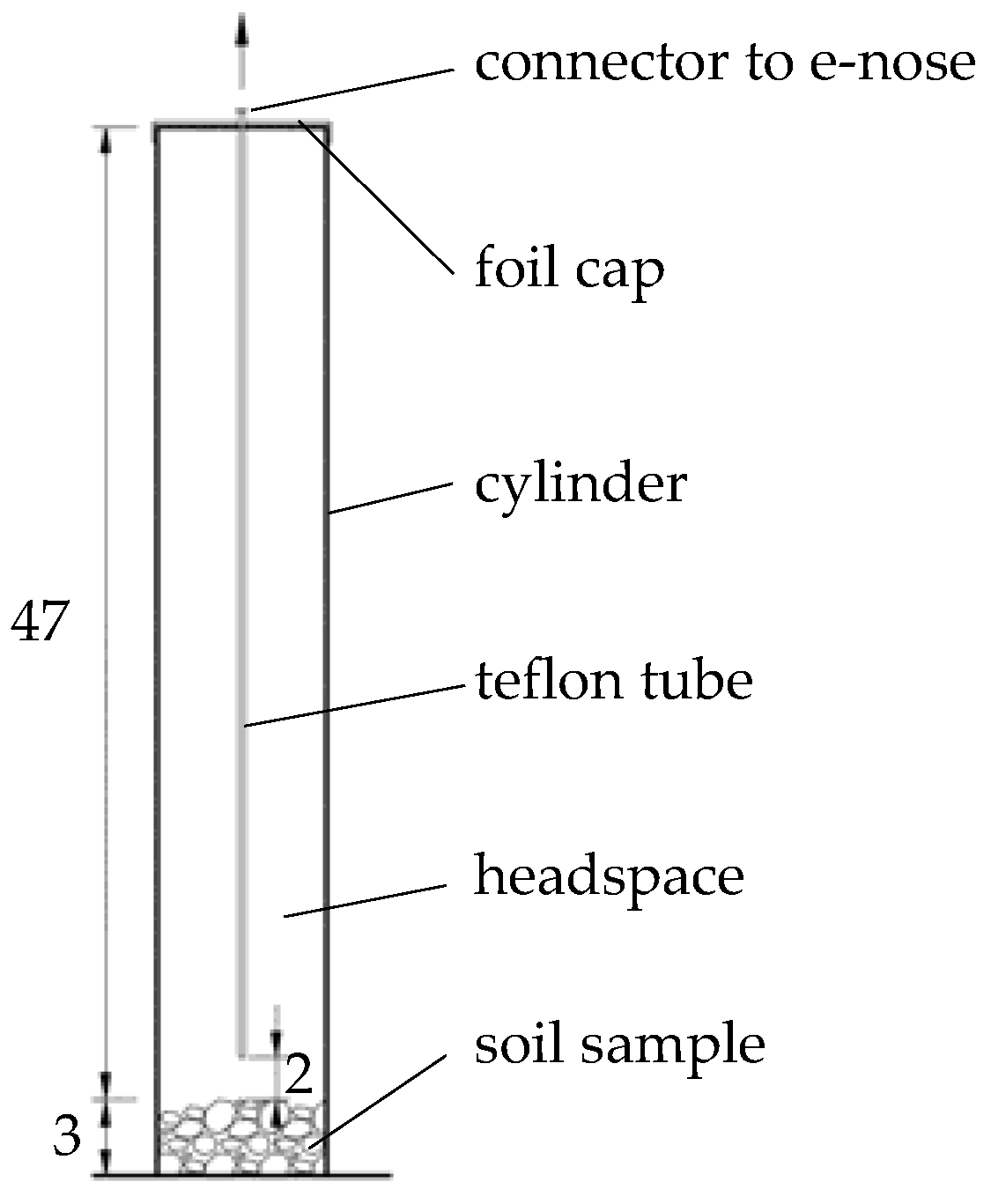
2. Materials and Methods
- (1)
- To find similarities and differences between the ANN classification of the samples using pattern recognition. Here, the architecture of the 10 networks used consisted of eight inputs, one hidden layer with 16 neurons, and two or three output neurons according to the number of target output classes. Training of the networks was performed using a scaled conjugate gradient backpropagation algorithm, and the error was estimated using cross entropy (CE).
- (2)
- ANN (two networks) function approximation and nonlinear regression were used to estimate the time lapse from when pollution was initiated. The network architecture consisted of eight inputs, one hidden layer with 16 neurons, and one output neuron. Five different networks were used in the case of petrol and ecodiesel pollutants. Training of the networks was performed using the Levenberg–Marquardt algorithm and the error was estimated using the mean squared error (MSE).
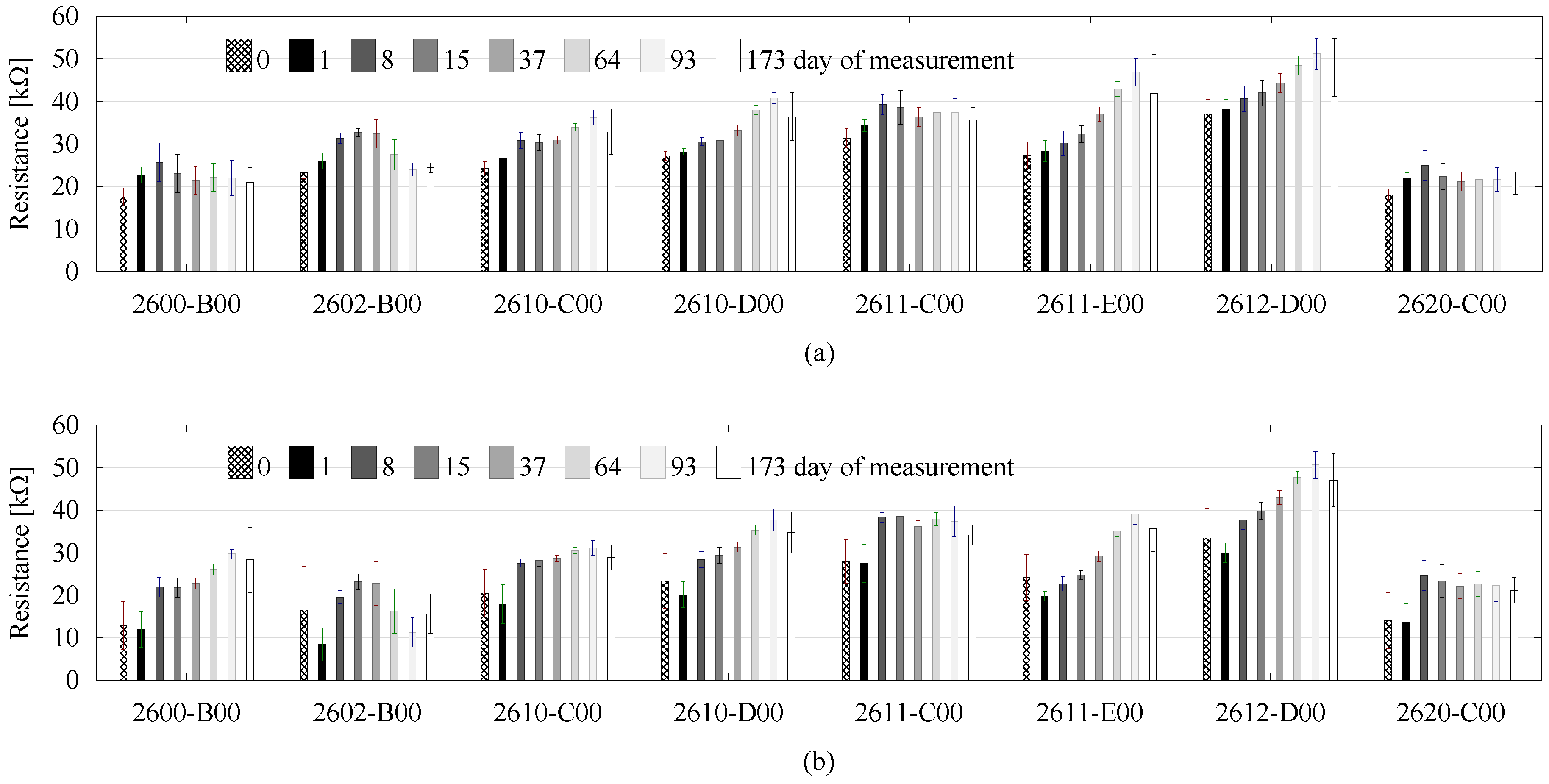
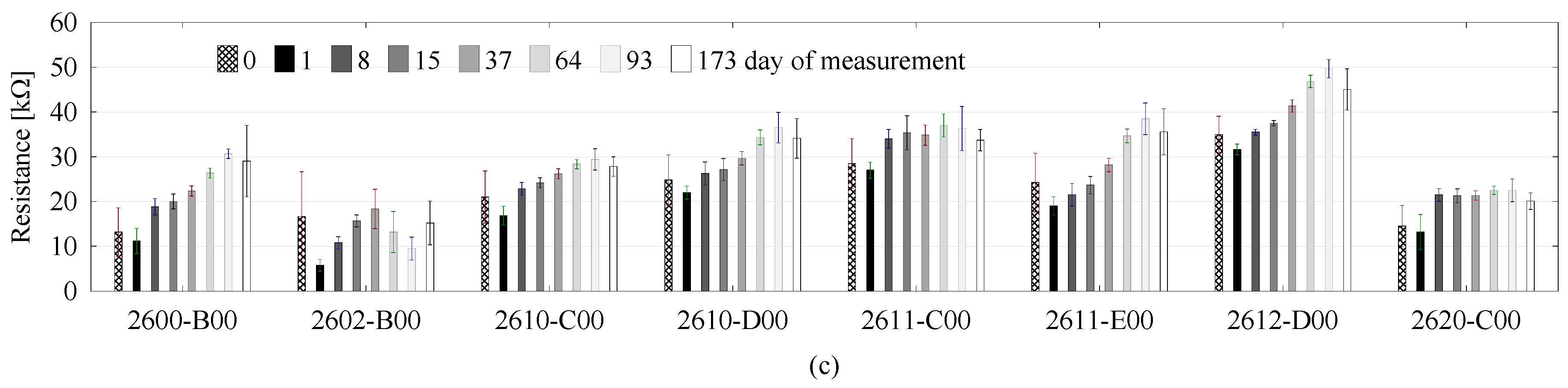
3. Results and Discussion

4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bandura, L.; Franus, M.; Józefaciuk, G.; Franus, W. Synthetic zeolites from fly ash as effective mineral sorbents for land-based petroleum spills cleanup. Fuel 2015, 147, 100–107. [Google Scholar] [CrossRef]
- Sadler, R.; Connell, D. Analytical Methods for the Determination of Total Petroleum Hydrocarbons in Soil. In Proceedings of the Fifth National Workshop on the Assessment of Site Contamination; Langley, A., Gilbey, M., Kennedy, B., Eds.; NEPC Service Corporation: Adelaide, SA, Australia, 2003; pp. 133–150. ISBN 0-642-32355-0. [Google Scholar]
- Szarlip, P.; Stelmach, W.; Jaromin-Gleń, K.; Bieganowski, A.; Brzezińska, M.; Trembaczowski, A.; Hałas, S.; Łagód, G. Comparison of the dynamics of natural biodegradation of petrol and diesel oil in soil. Desalin. Water Treat. 2014, 52, 3690–3697. [Google Scholar] [CrossRef]
- Tzing, S.H.; Chang, J.Y.; Ghule, A.; Chang, J.J.; Lo, B.; Ling, Y.C. A simple and rapid method for identifying the source of spilled oil using an electronic nose: Confirmation by gas chromatography with mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fingas, M.F. Development of oil hydrocarbon fingerprinting and identification techniques. Mar. Pollut. Bull. 2003, 47, 423–452. [Google Scholar] [CrossRef]
- Malley, D.F.; Hunter, K.N.; BarrieWebster, G.R. Analysis of diesel fuel contamination in soils by near-infrared reflectance spectrometry and solid phase microextraction–gas chromatography. J. Soil Contam. 1999, 8, 481–489. [Google Scholar] [CrossRef]
- Chakraborty, S.; Weindorf, D.C.; Morgan, C.L.S.; Ge, Y.; Galbraith, J.; Li, B.; Kahlon, C.S. Rapid identification of oil contaminated soils using visible near-infrared diffuse reflectance spectroscopy. J. Environ. Qual. 2010, 39, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Weindorf, D.C.; Li, B.; Abdalsatar, A.; Aldabaa, A.; Ghosh, R.K.; Sathi, P.; Ali, N. Development of a hybrid proximal sensing method for rapid identification of petroleum contaminated soils. Sci. Total Environ. 2015, 514, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.; Janik, L.; McLaughlin, M. An infrared spectroscopic test for total petroleum hydrocarbon (TPH) contamination in soils. In Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010; pp. 13–16. [Google Scholar]
- Hoerig, B.; Kuehn, F.; Oschuetz, F.; Lehmann, F. HyMap hyperspectral remote sensing to detect hydrocarbons. Int. J. Remote Sens. 2001, 8, 1413–1422. [Google Scholar] [CrossRef]
- Paíga, P.; Mendes, L.; Albergaria, J.T.; Delerue-Matos, C.M. Determination of total petroleum hydrocarbons in soil from different locations using infrared spectrophotometry and gas chromatography. Chem. Pap. 2012, 66, 711–721. [Google Scholar] [CrossRef]
- Cozzolino, D. Near infrared spectroscopy as a tool to monitor contaminants in soil, sediments and water—State of the art, advantages and pitfalls. Trends Environ. Anal. Chem. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Gancarz, M.; Wawrzyniak, J.; Gawrysiak-Witulska, M.; Wiącek, D.; Nawrocka, A.; Tadla, M.; Rusinek, R. Application of electronic nose with MOS sensors to prediction of rapeseed quality. Measurement 2017, 103, 227–234. [Google Scholar] [CrossRef]
- Gancarz, M.; Wawrzyniak, J.; Gawrysiak-Witulska, M.; Wiacek, D.; Nawrocka, A.; Rusinek, R. Electronic nose with polymer-composite sensors for monitoring fungal deterioration of stored rapeseed. Int. Agrophys. 2017, 31, 317–325. [Google Scholar] [CrossRef]
- Majchrzak, T.; Wojnowski, W.; Dymerski, T.; Gebicki, J.; Namiesnik, J. Electronic noses in classification and quality control of edible oils: A review. Food Chem. 2018, 246, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Gebicki, J.; Namiesnik, J. Electronic noses: Powerful tools in meat quality assessment. Meat Sci. 2017, 131, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Baby, R.E.; Cabezas, M.; Walsoe de Reca, E.N. Electronic nose: A useful tool for monitoring environmental contamination. Sens. Actuators B Chem. 2000, 69, 214–218. [Google Scholar] [CrossRef]
- Wilson, A.D.; Baietto, M. Applications and Advances in Electronic-Nose Technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [PubMed]
- Gębicki, J.; Bylinski, H.; Namiesnik, J. Measurement techniques for assessing the olfactory impact of municipal sewage treatment plants. Environ. Monit. Assess. 2016, 188, 32. [Google Scholar] [CrossRef] [PubMed]
- Krivetskiy, V.; Malkov, I.; Garshev, A.; Mordvinova, N.; Lebedev, O.I.; Dolenko, S.; Efitorov, A.; Grigoriev, T.; Rumyantseva, M.; Gaskov, A. Chemically modified nanocrystalline SnO2-based materials for nitrogen-containing gases detection using gas sensor array. J. Alloy Compd. 2017, 691, 514–523. [Google Scholar] [CrossRef]
- Szulczyński, B.; Wasilewski, T.; Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Namieśnik, J.; Gębicki, J. Different Ways to Apply a Measurement Instrument of E-Nose Type to Evaluate Ambient Air Quality with Respect to Odour Nuisance in a Vicinity of Municipal Processing Plants. Sensors 2017, 17, 2671. [Google Scholar] [CrossRef] [PubMed]
- Brattoli, M.; de Gennaro, G.; de Pinto, V.; Loiotile, A.D.; Lovascio, S.; Penza, M. Odour detection methods: Olfactometry and chemical sensors. Sensors 2011, 11, 5290–5322. [Google Scholar] [CrossRef] [PubMed]
- Guz, Ł.; Łagód, G.; Jaromin-Gleń, K.; Guz, E.; Sobczuk, H. Assessment of batch bioreactor odour nuisance using an e-nose. Desal. Water Treat. 2016, 57, 1327–1335. [Google Scholar] [CrossRef]
- Capelli, L.; Sironi, S.; Del Rosso, R. Electronic Noses for Environmental Monitoring Applications. Sensors 2014, 14, 19979–20007. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D. Review of Electronic-nose Technologies and Algorithms to Detect Hazardous Chemicals in the Environment. Proc. Technol. 2012, 1, 453–463. [Google Scholar] [CrossRef]
- Tonacci, A.; Corda, D.; Tartarisco, G.; Pioggia, G.; Domenici, C. A Smart Sensor System for Detecting Hydrocarbon Volatile Organic Compounds in Sea Water. CLEAN Soil Air Water 2014, 43, 147–152. [Google Scholar] [CrossRef]
- Chandler, R.; Das, A.; Gibson, T. Detection of oil pollution in seawater: Biosecurity prevention using electronic nose technology. In Proceedings of the 2015 31st IEEE International Conference on Data Engineering Workshops (ICDEW), Seoul, Korea, 13–17 April 2015; IEEE: Piscataway, NJ, USA, 2015. ISBN 978-1-4799-8442-8. [Google Scholar] [CrossRef]
- Bourgeois, W.; Stuetz, R.M. Use of a chemical sensor array for detecting pollutants in domestic wastewater. Water Res. 2002, 36, 4505–4512. [Google Scholar] [CrossRef]
- Fenner, R.A.; Stuetz, R.M. The application of electronic nose technology to environmental monitoring of water and wastewater treatment. Water Environ. Res. 1998, 71, 282–289. [Google Scholar] [CrossRef]
- Deshmukh, S.; Bandyopadhyay, R.; Bhattacharyya, N.; Pandey, R.A.; Jana, A. Application of electronic nose for industrial odors and gaseous emissions measurement and monitoring—An overview. Talanta 2015, 144, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Warneke, C.; de Gouw, J.; Yuan, B. Airborne Measurements near the Deepwater Horizon Oil Spill. In Proceedings of the 5th International Conference on Proton Transfer Reaction Mass Spectrometry and its Applications, Obergurgl, Austria, 26 January–2 February 2011; Hansel, A., Dunkl, J., Eds.; Innsbruck University Press: Innsbruck, Austria, 2011; pp. 65–68, ISBN 978-3-902719-88-1. [Google Scholar]
- Lavanya, S.; Deepika, B.; Narayanan, S.; Krishna Murthy, V.; Uma, M.V. Indicative extent of humic and fulvic acids in soils determined by electronic nose. Comput. Electron. Agric. 2017, 139, 198–203. [Google Scholar] [CrossRef]
- Pobkrut, T.; Kerdcharoen, T. Soil sensing survey robots based on electronic nose. In Proceedings of the 2014 14th International Conference on Control, Automation and Systems (ICCAS), Seoul, Korea, 22–25 October 2014; IEEE: Piscataway, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Lauf, R.J.; Hoffheins, B.S. Analysis of Liquid Fuels Using a Gas Sensor Array. Fuel 1991, 70, 935–940. [Google Scholar] [CrossRef]
- Shurmer, H.V. The fifth sense: On the scent of the electronic nose. IEE Rev. 1990, 36, 95–98. [Google Scholar] [CrossRef]
- Pace, C.; Khalaf, W.; Latino, M.; Donato, N.; Neri, G. E-nose Development for Safety Monitoring Applications in Refinery Environment. Procedia Eng. 2012, 47, 1267–1270. [Google Scholar] [CrossRef]
- Ferreiro-González, M.; Barbero, G.F.; Palma, M.; Ayuso, J.; Álvarez, J.A.; Barroso, C.G. Characterization and Differentiation of Petroleum-Derived Products by E-Nose Fingerprints. Sensors 2017, 17, 2544. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro-González, M.; Barbero, G.F.; Palma, M.; Ayuso, J.; Álvarez, J.A.; Barroso, C.G. Determination of Ignitable Liquids in Fire Debris: Direct Analysis by Electronic Nose. Sensors 2016, 16, 695. [Google Scholar] [CrossRef] [PubMed]
- Autelitano, F.; Garilli, E.; Pinalli, R.; Montepara, A.; Giuliani, F. The odour fingerprint of bitumen. Road Mater. Pavements Des. 2017, 18, 178–188. [Google Scholar] [CrossRef]
- Getino, J.; Ares, L.; Rodrigo, J.; Robla, J.I.; Horrillo, M.C.; Sayago, I.; Fernandez, M.J.; Gutierrez, J. Environmental applications of gas sensor arrays: Combustion atmospheres and contaminated soils. Sens. Actuators B Chem. 1999, 59, 249–254. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Horrillo, M.C. Advances in artificial olfaction: Sensors and applications. Talanta 2014, 124, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Cesare, F.; Pantalei, S.; Zampetti, E.; Macagnano, A. Electronic nose and SPME techniques to monitor phenanthrene biodegradation in soil. Sens. Actuators B Chem. 2008, 131, 63–70. [Google Scholar] [CrossRef]
- Bieganowski, A.; Jaromin-Glen, K.; Guz, Ł.; Łagód, G.; Jozefaciuk, G.; Franus, W.; Suchorab, Z.; Sobczuk, H. Evaluating Soil Moisture Status Using an e-Nose. Sensors 2016, 16, 886. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, W.; Gardey, G.; Servieres, M.; Stuetz, R.M. A chemical sensor array based system for protecting wastewater treatment plants. Sens. Actuators B Chem. 2003, 91, 109–116. [Google Scholar] [CrossRef]
- Bourgeois, W.; Hogben, P.; Pike, A.; Stuetz, R.M. Development of a sensor array based measurement system for continuous monitoring of water and wastewater. Sens. Actuators B Chem. 2003, 88, 312–319. [Google Scholar] [CrossRef]
- Lieberman, S.H.; Theriault, G.A.; Cooper, S.S.; Malone, P.G.; Olsen, R.S.; Lurk, P.W. Rapid, subsurface, in situ field screening of petroleum hydrocarbon contamination using laser induced fluorescence over optical fibers. In Proceedings of the Second International Symposium on Field Screening Methods for Hazardous Wastes and Toxic Chemicals, Las Vegas, NV, USA, 12–14 February 1991; Air and Waste Management Association: Pittsburgh, PA, USA, 1991; pp. 57–63. [Google Scholar]
- Apitz, S.E.; Borbridge, L.M.; Theriault, G.A.; Lieberman, S.H. Remote in situ determination of fuel products in soils: Field results and laboratory investigations. Analysis 1992, 20, 461–474. [Google Scholar]
- Kurup, P.U. Novel technologies for sniffing soil and ground water contaminants. Curr. Sci. 2009, 97, 1212–1219. [Google Scholar]
- Bourgeois, W.; Romain, A.C.; Nicolas, J.; Stuetz, R.M. The use of sensor arrays for environmental monitoring: Interests and limitations. J. Environ. Monit. 2003, 5, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Guz, Ł.; Łagód, G.; Jaromin-Gleń, K.; Suchorab, Z.; Sobczuk, H.; Bieganowski, A. Application of Gas Sensor Arrays in Assessment of Wastewater Purification Effects. Sensors 2015, 15, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Krzanowski, W.J. Principles of Multivariate Analysis: A User's Perspective, 2nd ed.; Oxford University Press Inc.: New York, NY, USA, 2000; pp. 477–503. ISBN 978-0-19-850708-6. [Google Scholar]
- Di Natale, C.; Martinelli, E.; Pennazza, G.; Orsini, A. Data analysis for chemical sensor arrays. In Advances in Sensing With Security Applications; Byrnes, J., Ostheimer, G., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 147–169. ISBN 13 978-1-4020-4295-9. [Google Scholar]
- Gardner, M.W.; Dorling, S.R. Artificial Neural Networks (the Multilayer Perceptron)—A Review of Applications in the Atmospheric Sciences. Atmos. Environ. 1998, 32, 2627–2636. [Google Scholar] [CrossRef]
- Fu, J.; Li, G.; Qin, Y.; Freeman, W.J. A Pattern Recognition Method for Electronic Noses Based on an Olfactory Neural Network. Sens. Actuators B Chem. 2007, 125, 489–497. [Google Scholar] [CrossRef]
- Smolarz, A.; Kotyra, A.; Wojcik, W.; Ballester, J. Advanced diagnostics of industrial pulverized coal burner using optical methods and artificial intelligence. Exp. Therm. Fluid Sci. 2012, 43, 82–89. [Google Scholar] [CrossRef]
- Sobański, T.; Szczurek, A.; Nitsch, K.; Licznerski, B.W.; Radwan, W. Electronic nose applied to automotive fuel qualification. Sens. Actuators B Chem. 2006, 116, 207–212. [Google Scholar] [CrossRef]
- Brudzewski, K.; Osowski, S.; Markiewicz, T.; Ulaczyk, J. Classification of gasoline with supplement of bio-products by means of an electronic nose and SVM neural network. Sens. Actuators B Chem. 2006, 113, 135–141. [Google Scholar] [CrossRef]
- Wolińska, A.; Kuźniar, A.; Szafranek-Nakonieczna, A.; Jastrzębska, N.; Rogulska, E.; Stępniewska, Z. Biological Activity of Autochthonic Bacterial Community in Oil-Contaminated Soil. Water Air Soil Poll. 2016, 227, 130. [Google Scholar] [CrossRef] [PubMed]
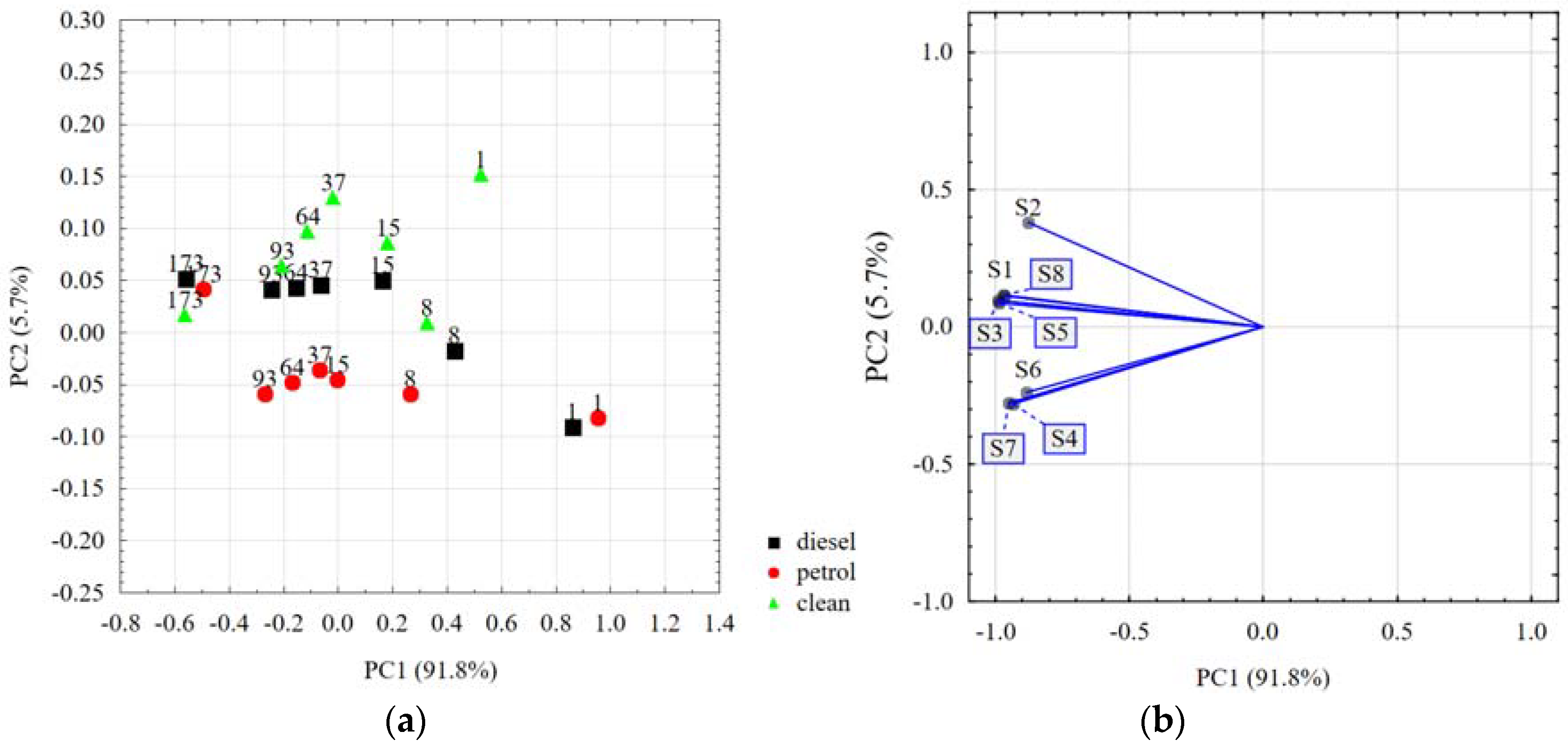
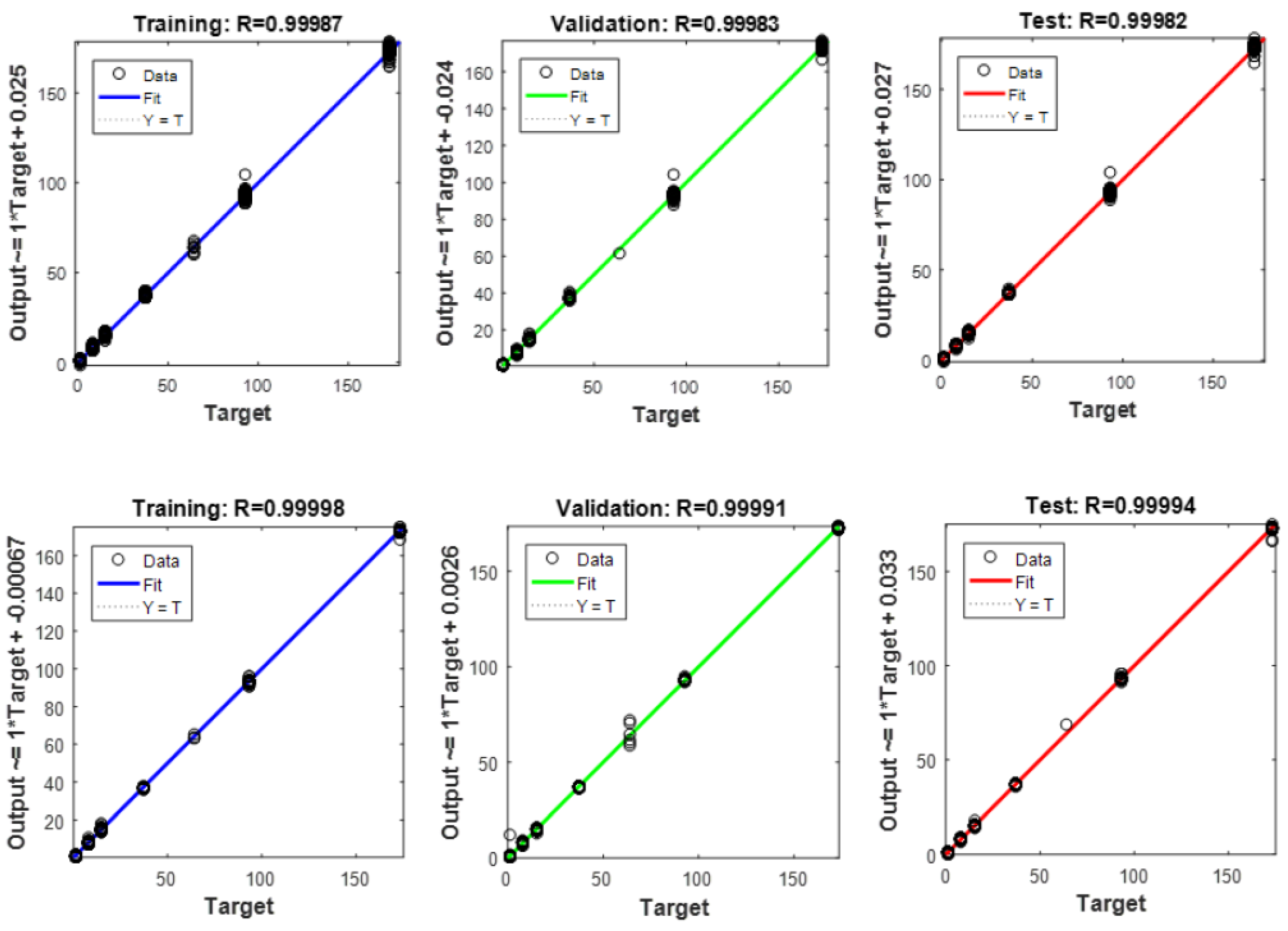
| No. | WRB Soil Group | Corg (%) | Particle Size Group |
|---|---|---|---|
| 1 | Brunic Arenosol | 0.86 | Sand |
| 2 | Stagnic Luvisol | 1.19 | Sandy loam |
| 3 | Haplic Cambisol | 0.57 | Sandy loam |
| 4 | Leptic Cambisol | 1.08 | Silt loam |
| 5 | Mollic Stagnic Fluvisol | 1.14 | Silt loam |
| 6 | Stagnic Phaeozem (Siltic) | 1.97 | Silt |
| 7 | Haplic Chernozem (Siltic) | 1.11 | Silt loam |
| 8 | Haplic Luvisol (Siltic) | 1.06 | Silt |
| 9 | Leptic Skeletic Dystric Cambisol | 0.90 | Silt loam |
| 10 | Haplic Fluvisol (Clayic) | 1.86 | Silt |
| %E * | |||
|---|---|---|---|
| Network ID | Training | Validation | Testing |
| 1 | 17.44 | 15.98 | 17.40 |
| 2 | 18.83 | 18.95 | 19.82 |
| 3 | 17.20 | 17.09 | 17.50 |
| 4 | 17.32 | 16.98 | 18.64 |
| 5 | 18.11 | 19.47 | 20.44 |
| 6 | 18.00 | 18.33 | 17.57 |
| 7 | 17.85 | 17.78 | 17.43 |
| 8 | 17.22 | 17.99 | 18.09 |
| 9 | 17.92 | 16.71 | 18.26 |
| 10 | 17.17 | 17.18 | 17.67 |
| Average | 17.71 | 17.65 | 18.28 |
| STEP1 | STEP2 | ||||||
|---|---|---|---|---|---|---|---|
| Network ID | %E * | Network ID | %E * | ||||
| Training | Validation | Testing | Training | Validation | Testing | ||
| 1 | 0.90 | 0.62 | 0.96 | 1 | 3.98 | 4.15 | 3.53 |
| 2 | 6.46 | 5.83 | 7.32 | 2 | 3.98 | 4.25 | 3.94 |
| 3 | 0.76 | 0.41 | 1.03 | 3 | 4.38 | 3.42 | 3.32 |
| 4 | 4.03 | 4.07 | 3.86 | 4 | 3.75 | 3.63 | 4.15 |
| 5 | 6.86 | 6.35 | 8.14 | 5 | 3.80 | 4.46 | 3.01 |
| 6 | 5.63 | 5.17 | 5.73 | 6 | 3.46 | 3.42 | 3.32 |
| 7 | 1.92 | 1.17 | 2.48 | 7 | 4.88 | 5.28 | 3.94 |
| 8 | 1.06 | 1.45 | 1.38 | 8 | 4.04 | 3.21 | 3.94 |
| 9 | 1.71 | 1.65 | 2.27 | 9 | 4.51 | 4.36 | 3.53 |
| 10 | 3.59 | 3.31 | 4.07 | 10 | 3.82 | 4.88 | 3.63 |
| Average | 3.29 | 3.00 | 3.72 | Average | 4.06 | 4.11 | 3.63 |
| ANN ID | Day Known | Day Unknown | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 8 | 15 | 37 | 64 | 93 | 173 | 1–173 | |
| 1 | 7.2 | 8.9 | 10.8 | 12.0 | 10.6 | 13.6 | 15.6 | 21.7 |
| 2 | 6.1 | 11.1 | 9.9 | 8.4 | 13.8 | 19.1 | 17.4 | 20.7 |
| 3 | 8.3 | 14.4 | 14.8 | 14.4 | 14.2 | 14.0 | 13.9 | 23.7 |
| 4 | 7.6 | 11.9 | 12.2 | 13.3 | 13.3 | 13.3 | 12.8 | 15.4 |
| 5 | 6.1 | 9.8 | 14.1 | 9.2 | 14.5 | 19.4 | 15.0 | 19.1 |
| 6 | 6.3 | 8.8 | 10.7 | 12.0 | 13.8 | 18.6 | 17.2 | 24.7 |
| 7 | 8.3 | 10.7 | 12.2 | 14.6 | 12.9 | 17.9 | 15.0 | 24.4 |
| 8 | 7.4 | 8.8 | 14.7 | 8.4 | 14.3 | 15.5 | 12.2 | 18.0 |
| 9 | 7.7 | 8.7 | 8.2 | 8.0 | 13.3 | 17.7 | 12.2 | 23.3 |
| 10 | 9.7 | 8.2 | 9.2 | 13.3 | 11.6 | 16.4 | 14.8 | 21.5 |
| Average | 7.5 | 10.1 | 11.7 | 11.4 | 13.2 | 16.6 | 14.6 | 21.3 |
| Petrol | ||||||
| Network ID | Training | Validation | Testing | |||
| MSE | R | MSE | R | MSE | R | |
| 1 | 0.99 | 0.99 | 1.14 | 0.99 | 1.27 | 0.99 |
| 2 | 0.06 | 0.99 | 0.19 | 0.99 | 0.08 | 0.99 |
| 3 | 0.16 | 0.99 | 0.18 | 0.99 | 0.48 | 0.99 |
| 4 | 0.032 | 0.99 | 0.013 | 0.99 | 0.023 | 0.99 |
| 5 | 0.10 | 0.99 | 0.26 | 0.99 | 15.58 | 0.97 |
| average | 0.27 | 0.99 | 0.36 | 0.99 | 3.49 | 0.99 |
| Diesel | ||||||
| Network ID | Training | Validation | Testing | |||
| MSE * | R * | MSE | R | MSE | R | |
| 1 | 0.11 | 0.99 | 0.69 | 0.99 | 0.40 | 0.99 |
| 2 | 0.29 | 0.99 | 0.56 | 0.99 | 0.71 | 0.99 |
| 3 | 0.10 | 0.99 | 0.21 | 0.99 | 0.90 | 0.99 |
| 4 | 0.29 | 0.99 | 0.30 | 0.99 | 0.93 | 0.99 |
| 5 | 1.33 | 0.99 | 1.27 | 0.99 | 1.65 | 0.99 |
| average | 0.42 | 0.99 | 0.61 | 0.99 | 0.92 | 0.99 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bieganowski, A.; Józefaciuk, G.; Bandura, L.; Guz, Ł.; Łagód, G.; Franus, W. Evaluation of Hydrocarbon Soil Pollution Using E-Nose. Sensors 2018, 18, 2463. https://doi.org/10.3390/s18082463
Bieganowski A, Józefaciuk G, Bandura L, Guz Ł, Łagód G, Franus W. Evaluation of Hydrocarbon Soil Pollution Using E-Nose. Sensors. 2018; 18(8):2463. https://doi.org/10.3390/s18082463
Chicago/Turabian StyleBieganowski, Andrzej, Grzegorz Józefaciuk, Lidia Bandura, Łukasz Guz, Grzegorz Łagód, and Wojciech Franus. 2018. "Evaluation of Hydrocarbon Soil Pollution Using E-Nose" Sensors 18, no. 8: 2463. https://doi.org/10.3390/s18082463
APA StyleBieganowski, A., Józefaciuk, G., Bandura, L., Guz, Ł., Łagód, G., & Franus, W. (2018). Evaluation of Hydrocarbon Soil Pollution Using E-Nose. Sensors, 18(8), 2463. https://doi.org/10.3390/s18082463








