Direct Detection of Candida albicans with a Membrane Based Electrochemical Impedance Spectroscopy Sensor
Abstract
1. Introduction
2. Materials and Methods
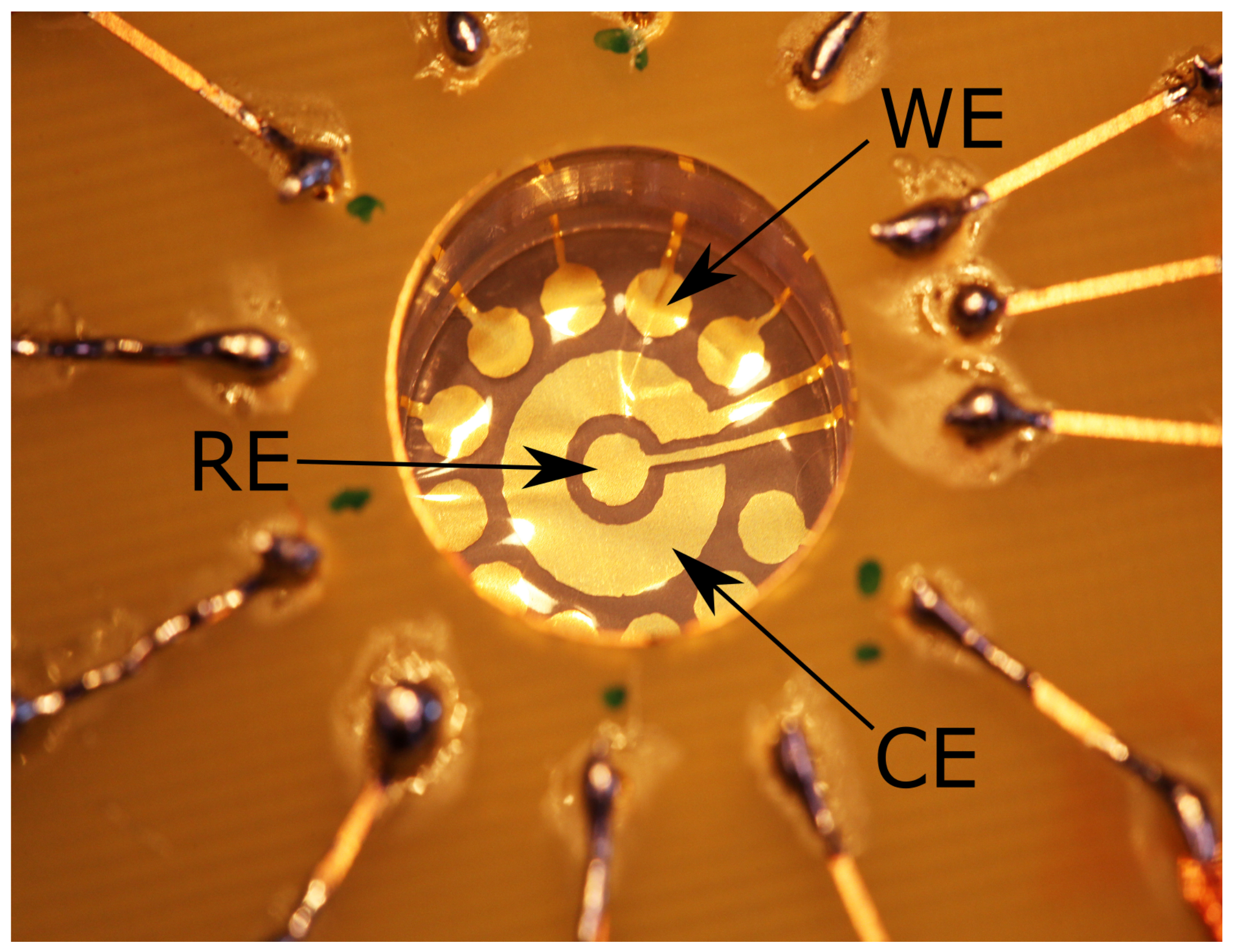
2.1. Membrane Electrodes Fabrication and Design
2.2. Membrane Electrodes Functionalization
2.3. Yeast Growth
2.4. Sensor Development and Sample Analysis
3. Results
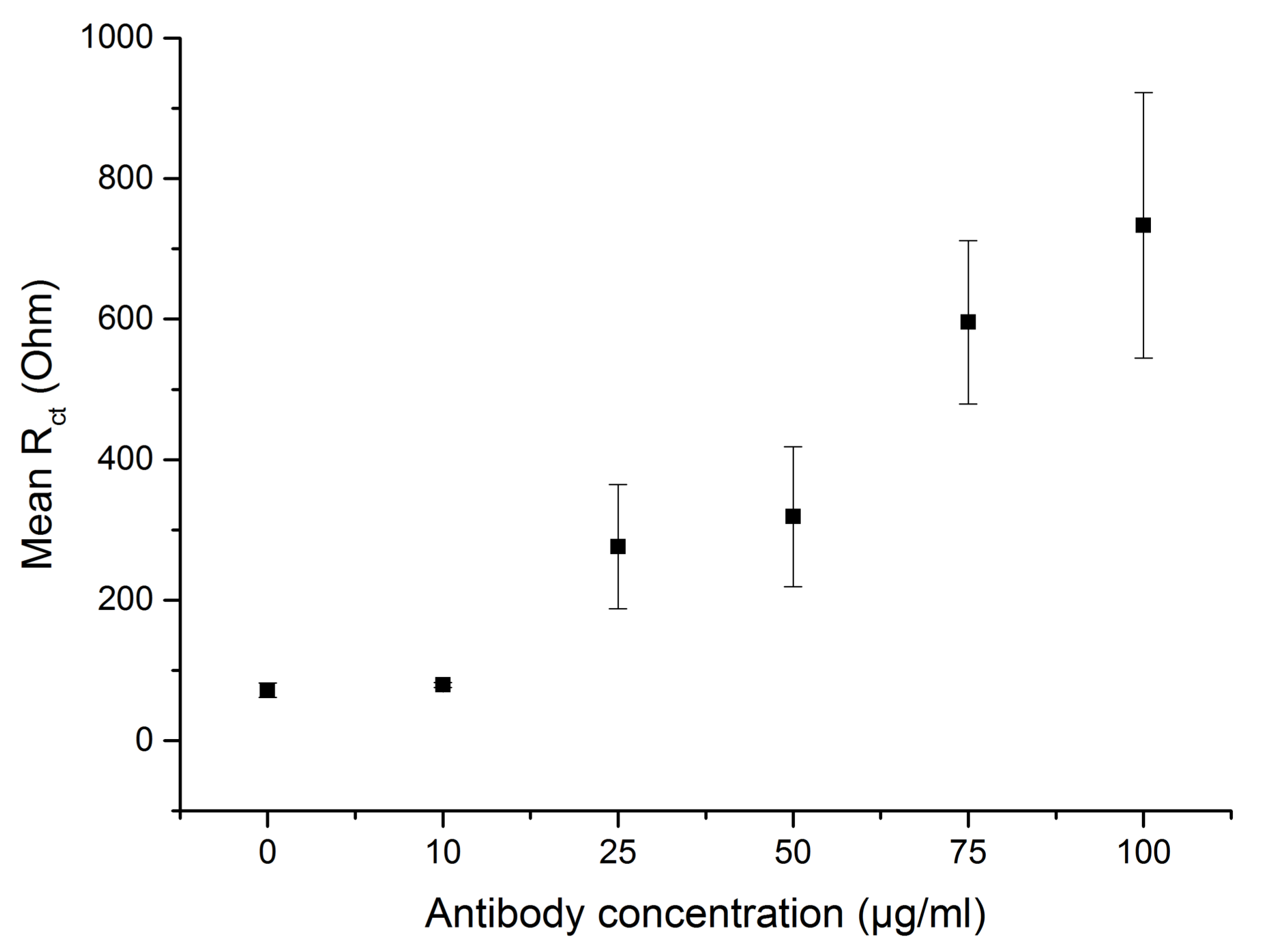
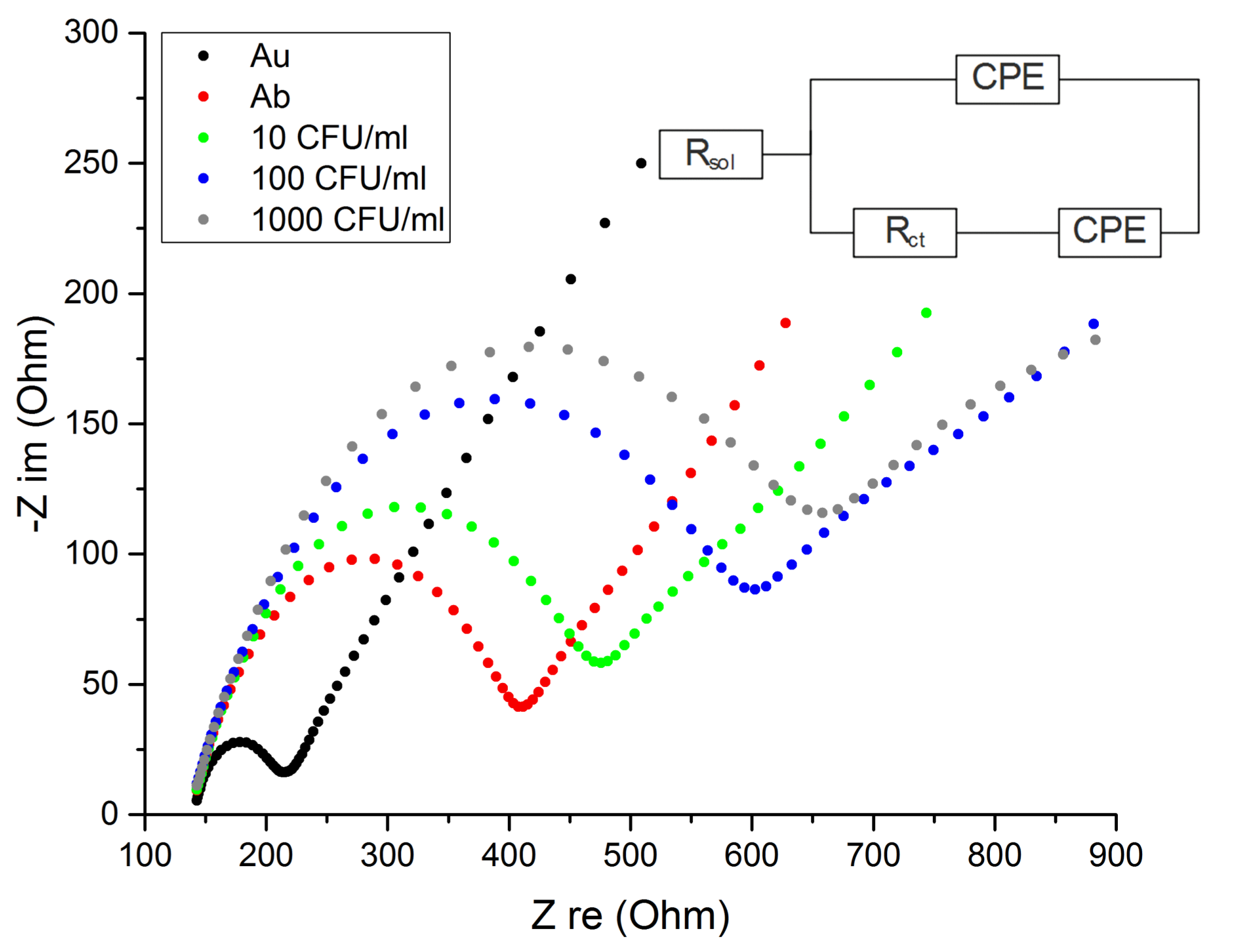
3.1. Characterization of the Antibody-Functionalized Electrodes
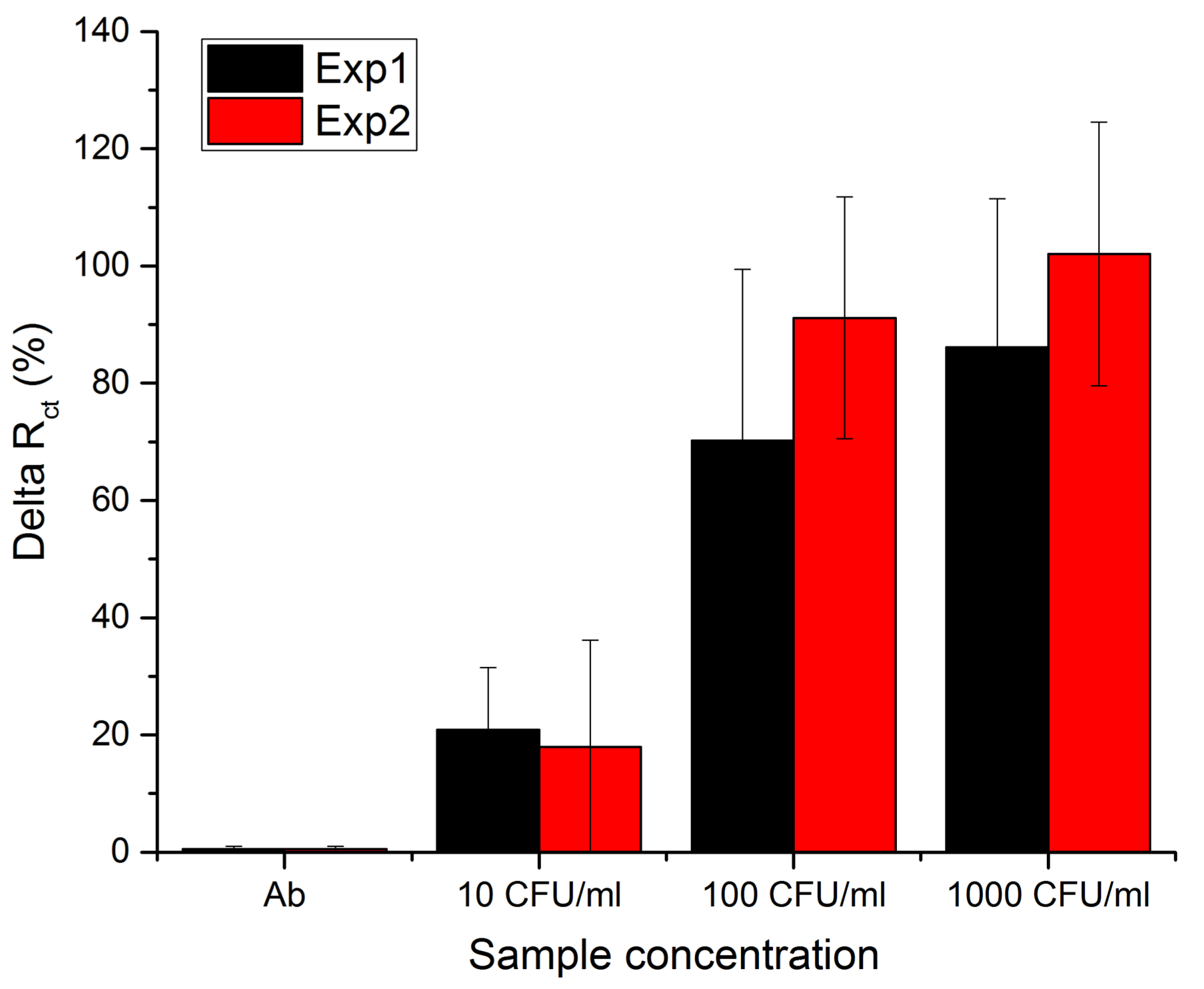
3.2. Sensor Sensitivity and Specificity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Safavieh, M.; Coarsey, C.; Esiobu, N.; Memic, A.; Vyas, J.M.; Shafiee, H.; Asghar, W. Advances in Candida detection platforms for clinical and point-of-care applications. Crit. Rev. Biotechnol. 2017, 37, 441–458. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.S.-Y.; Rosli, R.; Seow, H.F.; Chong, P.P. Candida and invasive candidiasis: Back to basics. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Sims, C.R.; Ostrosky-Zeichner, L.; Rex, J.H. Invasive Candidiasis in Immunocompromised Hospitalized Patients. Arch. Med. Res. 2005, 36, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-Q.; Ma, C.-F.; Shi, L.N.; Wang, Y.; Huang, M.; Kong, Q.-Q. Diagnostic value of immunoglobulin G antibodies against Candida enolase and fructose-bisphosphate aldolase for candidemia. BMC Infect. Dis. 2013, 13, 253. [Google Scholar]
- He, Z.-X.; Shi, L.-C.; Ran, X.-Y.; Li, W.; Wang, X.-L.; Wang, F.-K. Development of a Lateral Flow Immunoassay for the Rapid Diagnosis of Invasive Candidiasis. Front. Microbiol. 2016, 7, 1451. [Google Scholar] [CrossRef] [PubMed]
- Villamizar, R.A.; Maroto, A.; Rius, F.X. Improved detection of Candida albicans with carbon nanotube field-effect transistors. Sens. Actuators B 2009, 136, 451–457. [Google Scholar] [CrossRef]
- Antinori, S.; Milazzo, L.; Sollima, S.; Galli, M.; Corbellino, M. Candidemia and invasive candidiasis in adults: A narrative review. Eur. J. Intern. Med. 2016, 34, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-L.; Hua, Y.; Guan, Q.; Yuan, C.-H. Improved detection of deeply invasive candidiasis with DNA aptamers specificbinding to (1→3)-β-D-glucans from Candida albicans. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Zand, F.; Moghaddami, M.; Davarpanah, M.A.; Masjedi, M.; Nikandish, R.; Amanati, A.; Afarid, M.; Nafarieh, L.; Nami, M. Invasive fungal infections in critically-ill patients: A literature review and position statement from the IFI-clinical forum, Shiraz, Iran. Biosci. Biotech. Res. Comm. 2016, 9, 371–381. [Google Scholar]
- Schell, W.A.; Benton, J.L.; Smith, P.B.; Poore, M.; Rouse, J.L.; Boles, D.J.; Johnson, M.D.; Alexander, B.D.; Pamula, V.K.; Eckhardt, A.E.; et al. Evaluation of a digital microfluidic real-time PCR platform to detect DNA of Candida albicans in blood. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.Y.A.; Bilitewski, U. Direct electrochemical determination of Candida albicans activity. Biosens. Bioelectron. 2013, 49, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, M.; Sharma, P.S.; Iskierko, Z.; Noworyta, K.; Cieplak, M.; Lisowski, W.; Oborska, S.; Kuhn, A.; Kutner, W. Early diagnosis of fungal infections using piezomicrogravimetric and electric chemosensors based on polymers molecularly imprinted with D-arabitol. Biosens. Bioelectron. 2016, 79, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Nugaeva, N.; Gfeller, K.Y.; Backmann, N.; Lang, H.P.; Dğgelin, M.; Hegner, M. Micromechanical cantilever array sensors for selective fungal immobilization and fast growth detection. Biosens. Bioelectron. 2005, 21, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Mulero, R.; Lee, D.H.; Kutzler, M.A.; Jacobson, J.M.; Kim, M.J. Ultra-Fast Low Concentration Detection of Candida Pathogens Utilizing High Resolution Micropore Chips. Sensors 2009, 9, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Kwak, D.H.; Punihaole, D.; Hong, Z.; Velankar, S.S.; Liu, X.; Asher, S.A. A photonic crystal protein hydrogel sensor for Candida albicans. Angew. Chem. Int. Ed. 2015, 54, 13036–13040. [Google Scholar] [CrossRef] [PubMed]
- Bakmand, T.; Kwasny, D.; Dimaki, M.; Svendsen, W.E. Fabrication and Characterization of Membrane-Based Gold Electrodes. Electroanalysis 2014, 27, 217–224. [Google Scholar] [CrossRef]
- Alatraktchi, F.A.; Bakmand, T.; Dimaki, M.; Svendsen, W.E. Novel Membrane-Based Electrochemical Sensor for Real-Time Bio-Applications. Sensors 2014, 14, 22128–22139. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, W.E.; Alatraktchi, F.A.; Bakmand, T.; Waagepetersen, H.; Dimaki, M. Novel culturing platform for brain slices and neuronal cells. In Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 346–349. [Google Scholar] [CrossRef]
- Bache, M.; Bosco, F.G.; Brøgger, A.L.; Frøhling, K.B.; Alstrøm, T.S.; Hwu, E.T.; Chen, C.H.; Eugen-Olsen, J.; Hwang, I.S.; Boisen, A. Nanomechanical recognition of prognostic biomarker suPAR with DVD-ROM optical technology. Nanotechnology 2013, 24, 444011. [Google Scholar] [CrossRef] [PubMed]
- Grønbeck, H.; Curioni, A.; Andreoni, W. Thiols and Disulfides on the Au(111) Surface: The Headgroup-Gold Interaction. J. Am. Chem. Soc. 2000, 122, 3839–3842. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwasny, D.; Tehrani, S.E.; Almeida, C.; Schjødt, I.; Dimaki, M.; Svendsen, W.E. Direct Detection of Candida albicans with a Membrane Based Electrochemical Impedance Spectroscopy Sensor. Sensors 2018, 18, 2214. https://doi.org/10.3390/s18072214
Kwasny D, Tehrani SE, Almeida C, Schjødt I, Dimaki M, Svendsen WE. Direct Detection of Candida albicans with a Membrane Based Electrochemical Impedance Spectroscopy Sensor. Sensors. 2018; 18(7):2214. https://doi.org/10.3390/s18072214
Chicago/Turabian StyleKwasny, Dorota, Sheida Esmail Tehrani, Catarina Almeida, Ida Schjødt, Maria Dimaki, and Winnie E. Svendsen. 2018. "Direct Detection of Candida albicans with a Membrane Based Electrochemical Impedance Spectroscopy Sensor" Sensors 18, no. 7: 2214. https://doi.org/10.3390/s18072214
APA StyleKwasny, D., Tehrani, S. E., Almeida, C., Schjødt, I., Dimaki, M., & Svendsen, W. E. (2018). Direct Detection of Candida albicans with a Membrane Based Electrochemical Impedance Spectroscopy Sensor. Sensors, 18(7), 2214. https://doi.org/10.3390/s18072214




