Investigation of A Slow-Light Enhanced Near-Infrared Absorption Spectroscopic Gas Sensor, Based on Hollow-Core Photonic Band-Gap Fiber
Abstract
1. Introduction
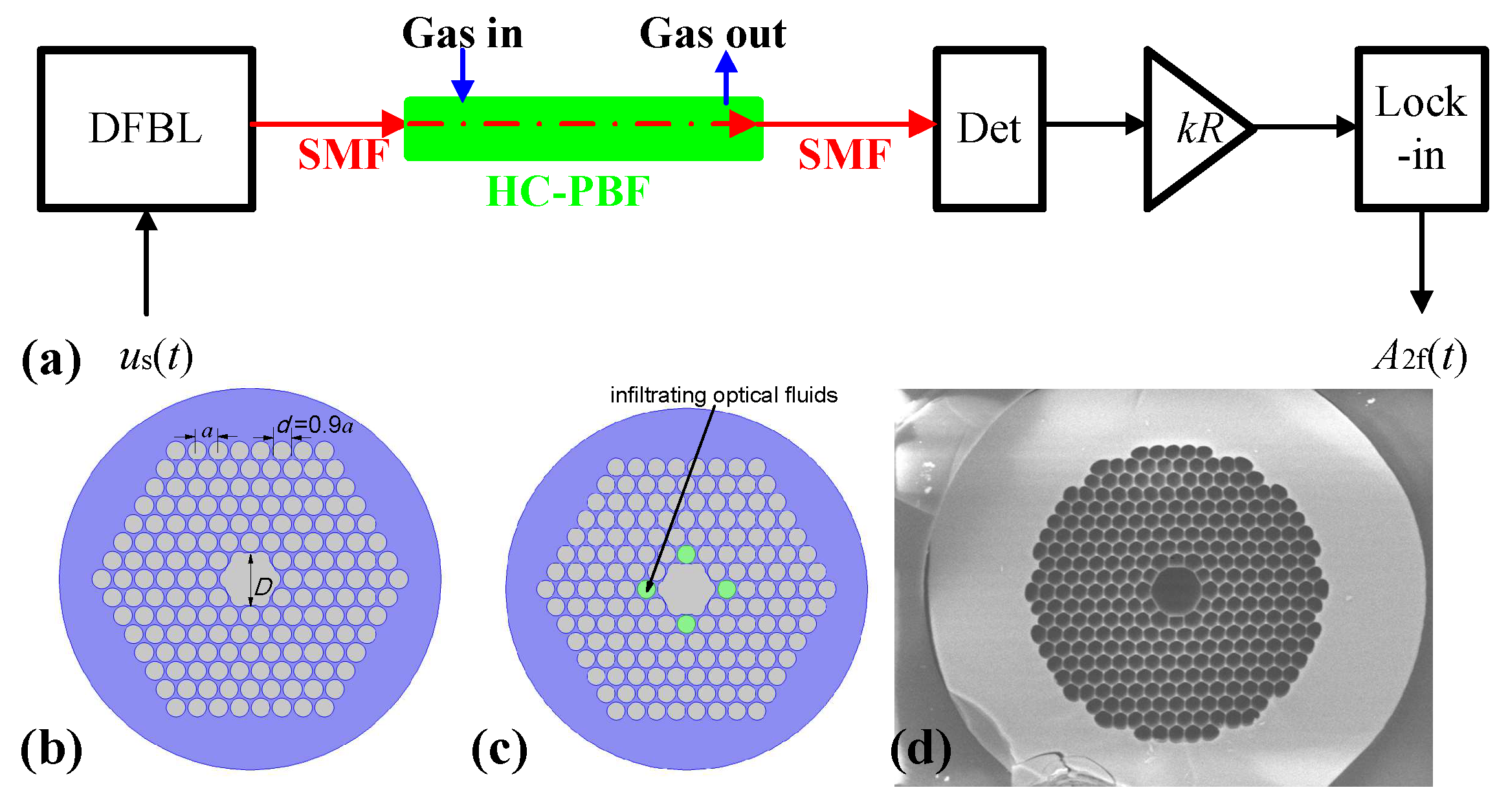
2. Modeling of the Hollow-Core Photonic Band-Gap Fiber-Based Spectroscopic Gas Sensor
3. Mode Analysis of Hollow-Core Photonic Band-Gap Fiber
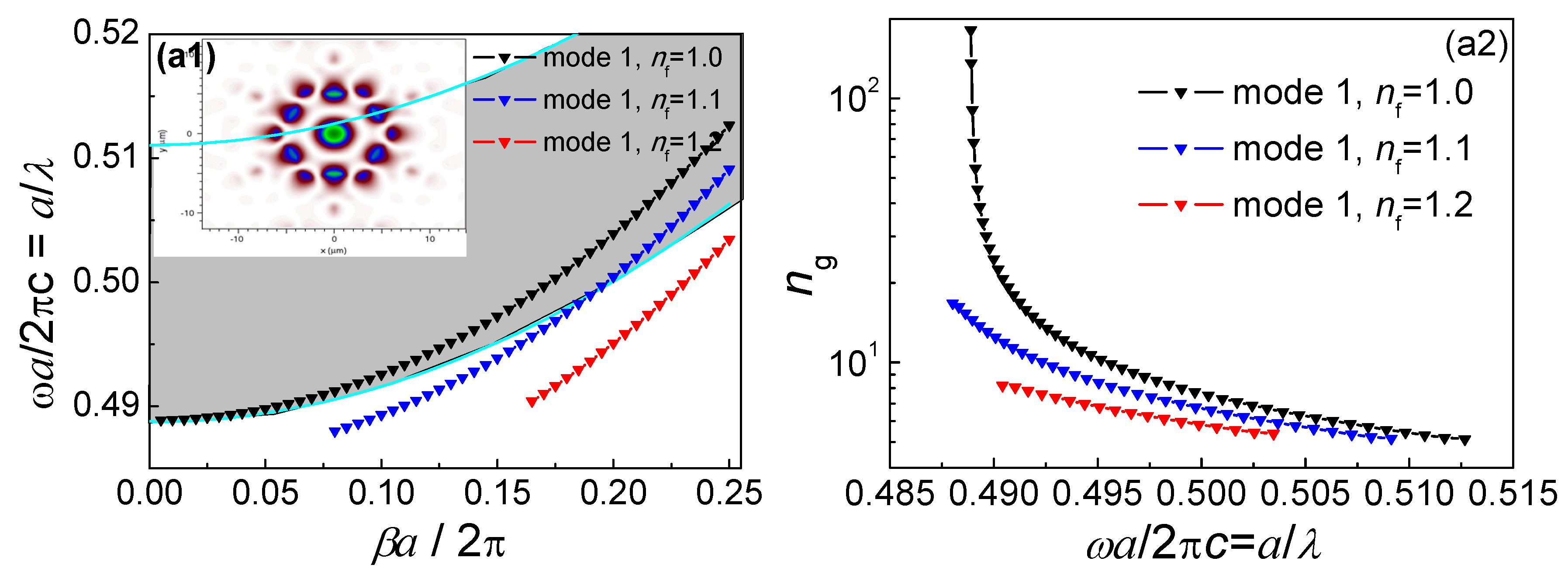
3.1. Uninfiltrated Hollow-Core Photonic Band-Gap Fiber Mode Analysis
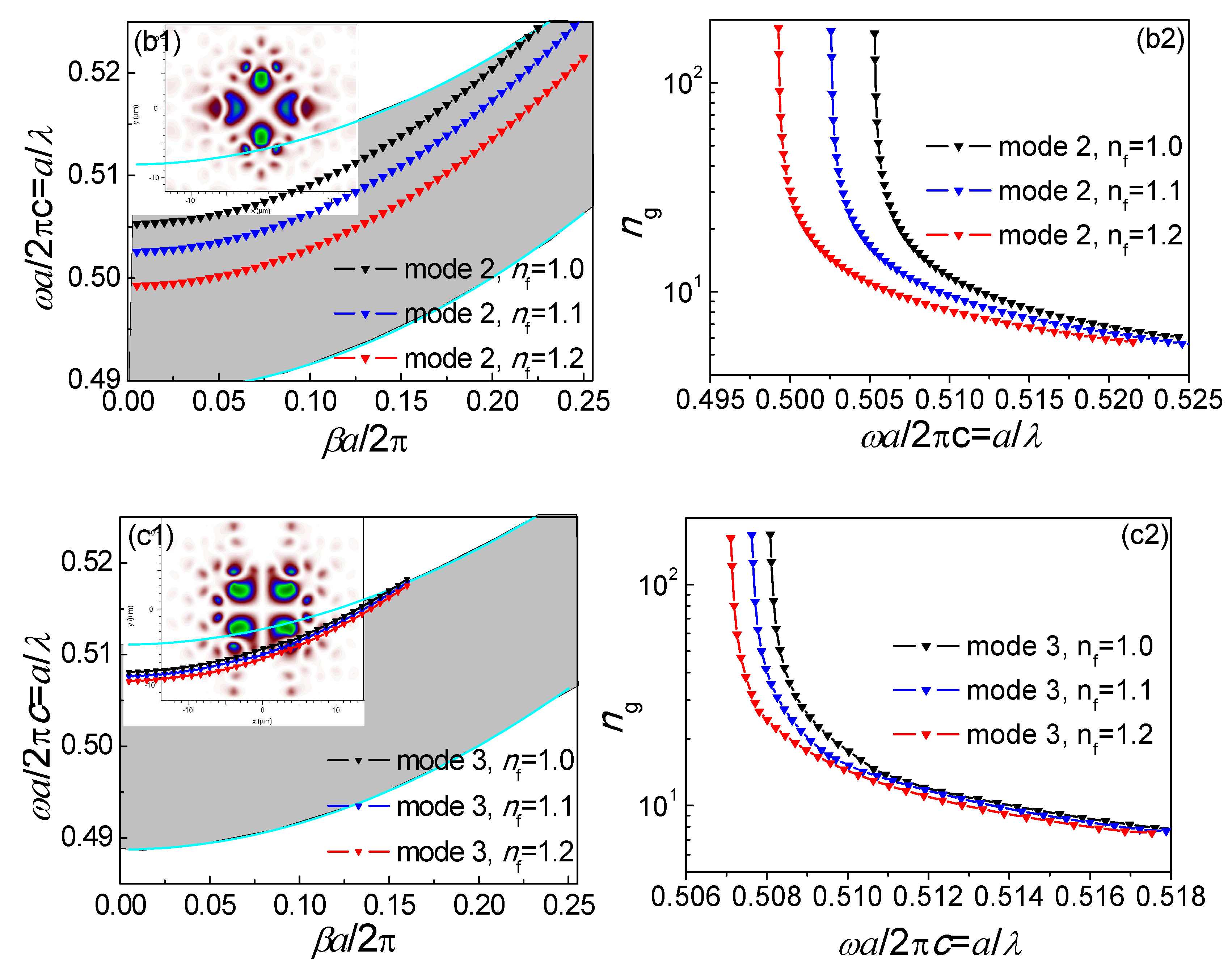
3.2. Infiltrated Hollow-Core Photonic Band-Gap Fiber Mode Analysis
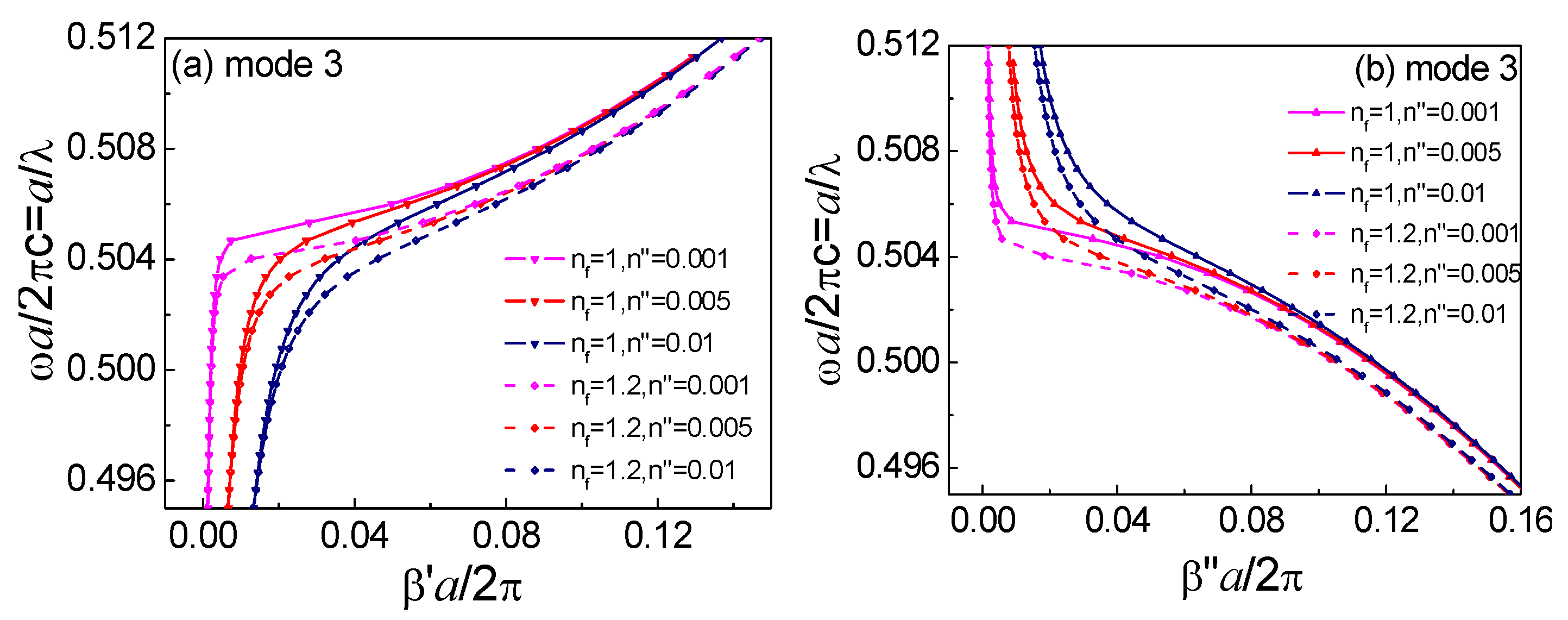
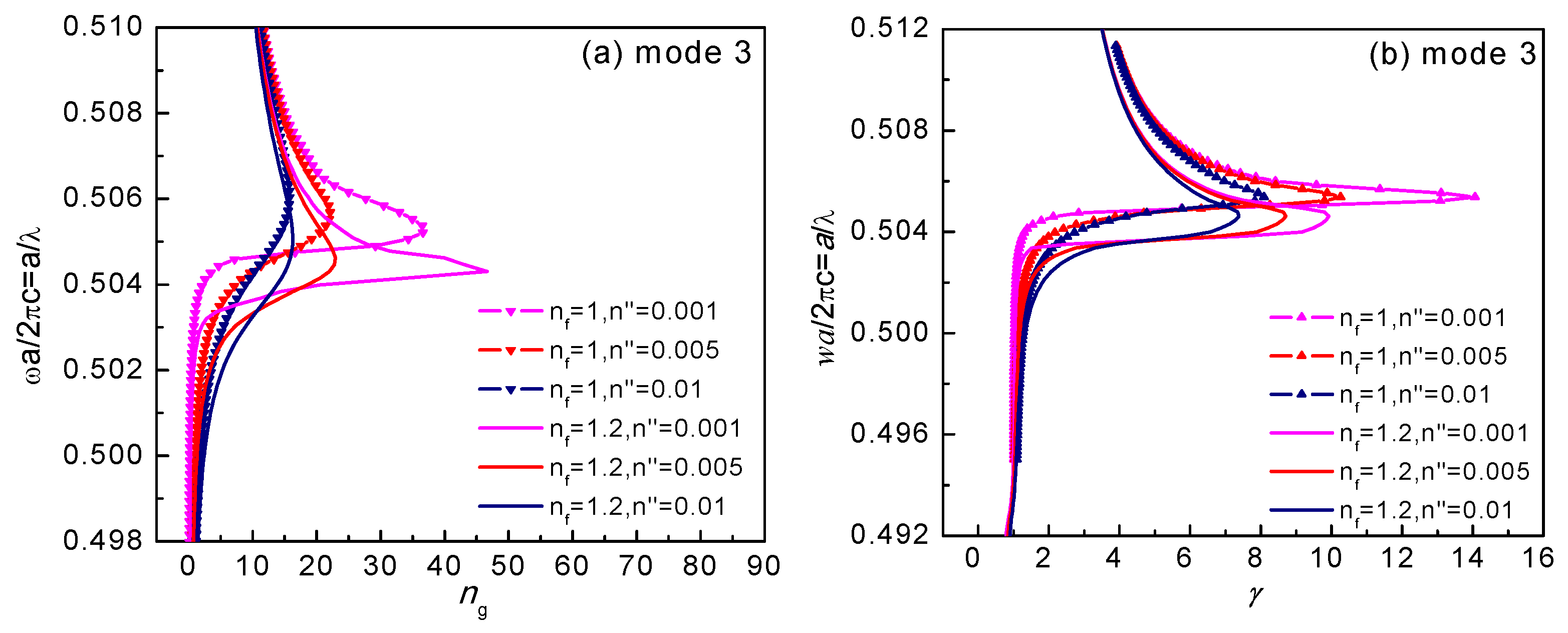
3.3. Slow-Light Effect Analysis
4. Slow-Light Enhanced Gas Sensing Performance Analysis
4.1. Mode Tuning for Near-Infrared Gas Sensing
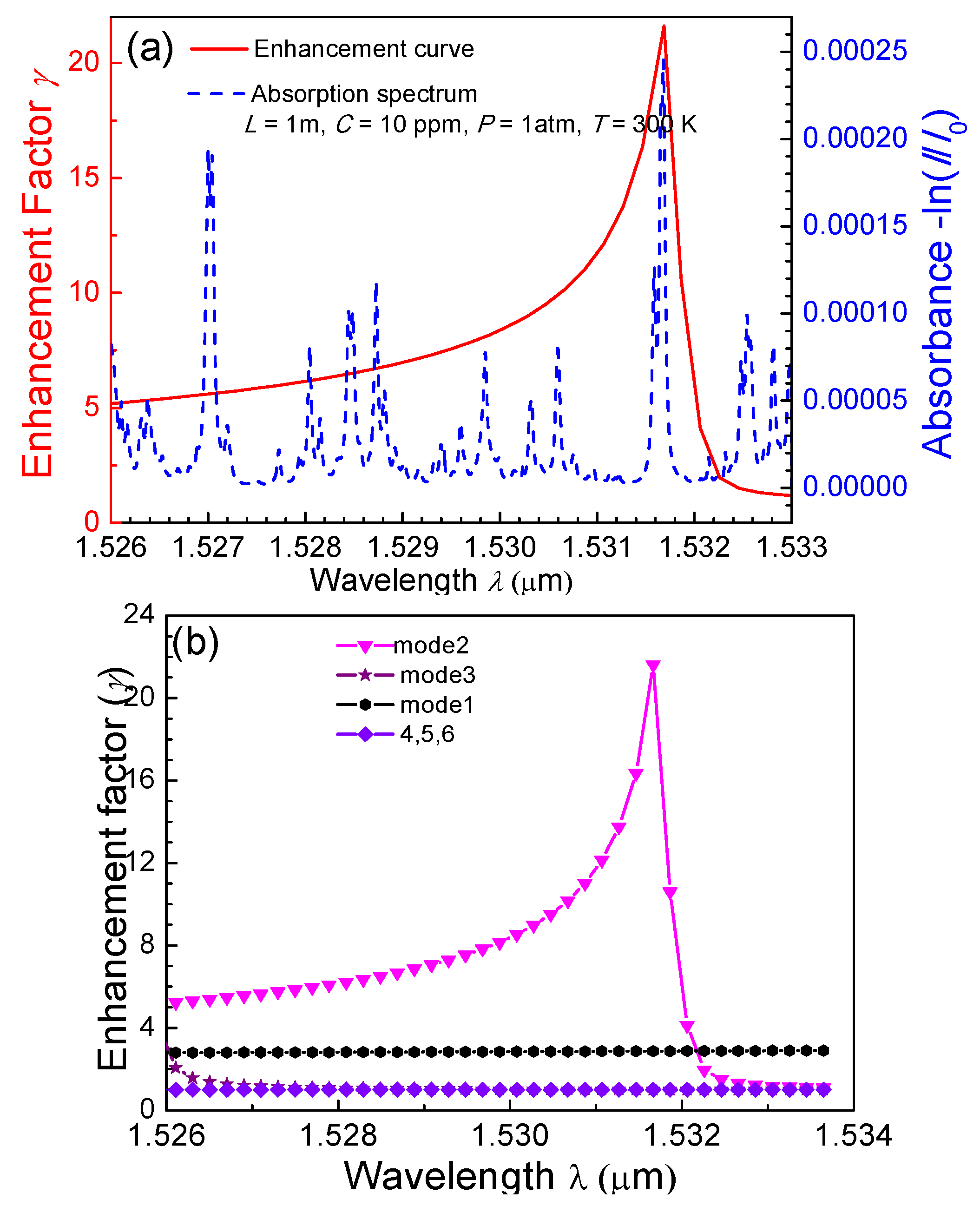
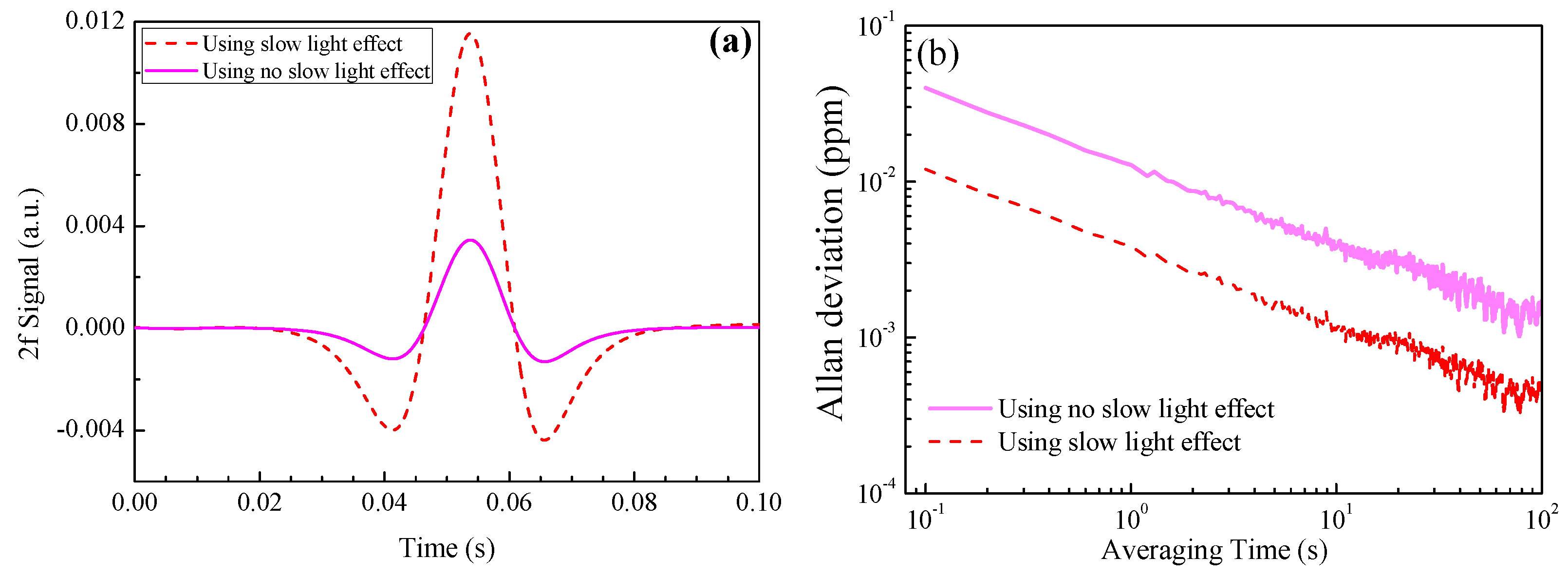
4.2. NH3 Absorption Enhancement Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bamberger, I.; Stieger, J.; Buchmann, N.; Eugster, W. Spatial variability of methane: Attributing atmospheric concentrations to emissions. Environ. Pollut. 2014, 190, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Willer, U.; Saraji, M.; Khorsandi, A.; Geiser, P.; Schade, W. Near-and mid-infrared laser monitoring of industrial processes, environment and security applications. Opt. Lasers Eng. 2006, 44, 699–710. [Google Scholar] [CrossRef]
- Werle, P.; Slemr, F.; Maurer, K.; Kormann, R.; Mucke, R. Near-and mid-infrared laser-optical sensors for gas analysis. Opt. Lasers Eng. 2002, 37, 101–114. [Google Scholar] [CrossRef]
- Foltynowicz, A.; Maslowski, P.; Fleisher, A.J.; Bjork, B.J.; Ye, J. Cavity-enhanced optical frequency comb spectroscopy in the mid-infrared application to trace detection of hydrogen peroxide. Appl. Phys. B 2013, 110, 163–175. [Google Scholar] [CrossRef]
- Zheng, C.T.; Ye, W.L.; Huang, J.Q.; Cao, T.S.; Lv, M.; Dang, J.M.; Wang, Y.D. Performance improvement of a near-infrared CH4 detection device using wavelet-denoising-assisted wavelength modulation technique. Sens. Actuators B Chem. 2014, 190, 249–258. [Google Scholar] [CrossRef]
- Momeni, B.; Askari, M.; Shah Hosseini, E.; Atabaki, A.; Adibi, A. An on-chip silicon grating spectrometer using a photonic crystal reflector. J. Opt. 2010, 12, 220–221. [Google Scholar] [CrossRef]
- Lai, W.C.; Chakravarty, S.; Wang, X.; Lin, C.; Chen, R.T. On-chip methane sensing by near-IR absorption signatures in a photonic crystal slot waveguide. Opt. Lett. 2011, 36, 984–986. [Google Scholar] [CrossRef] [PubMed]
- Deotare, P.; Kogos, L.; Quan, Q.; Llic, R. On-chip integrated spectrometer using nanobeam photonic crystal cavities. In Proceedings of the Conference on Lasers and Electro Optics (CLEO 2012), San Jose Convention Center, San Jose, CA, USA, 6–11 May 2012; pp. 1–2. [Google Scholar]
- Hirsch, M.; Majchrowicz, D.; Wierzba, P.; Weber, M.; Bechelany, M.; Jędrzejewska-Szczerska, M. Low-Coherence Interferometric Fiber-Optic Sensors with Potential Applications as Biosensors. Sensors 2017, 17, 261. [Google Scholar] [CrossRef] [PubMed]
- Majchrowicz, D.; Hirsch, M.; Wierzba, P.; Bechelany, M.; Viter, R.; Jędrzejewska-Szczerska, M. Application of Thin ZnO ALD Layers in Fiber-Optic Fabry-Pérot Sensing Interferometers. Sensors 2016, 16, 416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Zhao, Y.; Wu, D.; Wang, Q. Theoretical research on high sensitivity gas sensor due to slow light in slotted photonic crystal waveguide. Sens. Actuators B Chem. 2012, 173, 505–509. [Google Scholar] [CrossRef]
- Wang, Q.; Han, B.; Wu, D.; Zhao, Y. Novel Fiber Optic Gas Sensor Based on Photonic Crystal Slow-Light Waveguide. Microw. Opt. Technol. Lett. 2013, 55, 1796–1800. [Google Scholar] [CrossRef]
- Huang, Y.; Min, C.; Dastmalchi, P.; Veronis, G. Slow-light enhanced subwavelength plasmonic waveguide refractive index sensors. Opt. Express 2015, 23, 14922. [Google Scholar] [CrossRef] [PubMed]
- Mortenseen, N.A.; Xiao, S. Slow-light enhancement of Beer-Lambert-Bouguer absorption. Appl. Phys. Lett. 2007, 90, 374. [Google Scholar] [CrossRef]
- Grgic, J.; Xiao, S.; Mork, J.; Jauho, A.P.; Mortensen, N.A. Slow-light enhanced absorption in a hollow-core fiber. Opt. Express 2010, 18, 14270–14279. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, Y.; Yariv, A. Fabrication of functional microstructured optical fibers through a selective-filling technique. Appl. Phys. Lett. 2004, 85, 5182–5184. [Google Scholar] [CrossRef]
- Xiao, L.; Jin, W.; Demokan, M.S.; Ho, H.L.; Hoo, Y.L.; Zhao, C. Fabrication of selective injection microstructured optical fbers with a concentional fusion splicer. Opt. Express 2005, 13, 9014–9022. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.-F.; Zheng, C.-T.; Liu, Z.-W.; Yao, D.; Zheng, W.-X.; Wang, Y.-D.; Wang, F.; Zhang, D.-M. Investigation of A Slow-Light Enhanced Near-Infrared Absorption Spectroscopic Gas Sensor, Based on Hollow-Core Photonic Band-Gap Fiber. Sensors 2018, 18, 2192. https://doi.org/10.3390/s18072192
Wu Z-F, Zheng C-T, Liu Z-W, Yao D, Zheng W-X, Wang Y-D, Wang F, Zhang D-M. Investigation of A Slow-Light Enhanced Near-Infrared Absorption Spectroscopic Gas Sensor, Based on Hollow-Core Photonic Band-Gap Fiber. Sensors. 2018; 18(7):2192. https://doi.org/10.3390/s18072192
Chicago/Turabian StyleWu, Zhi-Fa, Chuan-Tao Zheng, Zhi-Wei Liu, Dan Yao, Wen-Xue Zheng, Yi-Ding Wang, Fei Wang, and Da-Ming Zhang. 2018. "Investigation of A Slow-Light Enhanced Near-Infrared Absorption Spectroscopic Gas Sensor, Based on Hollow-Core Photonic Band-Gap Fiber" Sensors 18, no. 7: 2192. https://doi.org/10.3390/s18072192
APA StyleWu, Z.-F., Zheng, C.-T., Liu, Z.-W., Yao, D., Zheng, W.-X., Wang, Y.-D., Wang, F., & Zhang, D.-M. (2018). Investigation of A Slow-Light Enhanced Near-Infrared Absorption Spectroscopic Gas Sensor, Based on Hollow-Core Photonic Band-Gap Fiber. Sensors, 18(7), 2192. https://doi.org/10.3390/s18072192




