Role of Carboxyl and Amine Termination on a Boron-Doped Diamond Solution Gate Field Effect Transistor (SGFET) for pH Sensing
Abstract
:1. Introduction
2. Materials and Methods
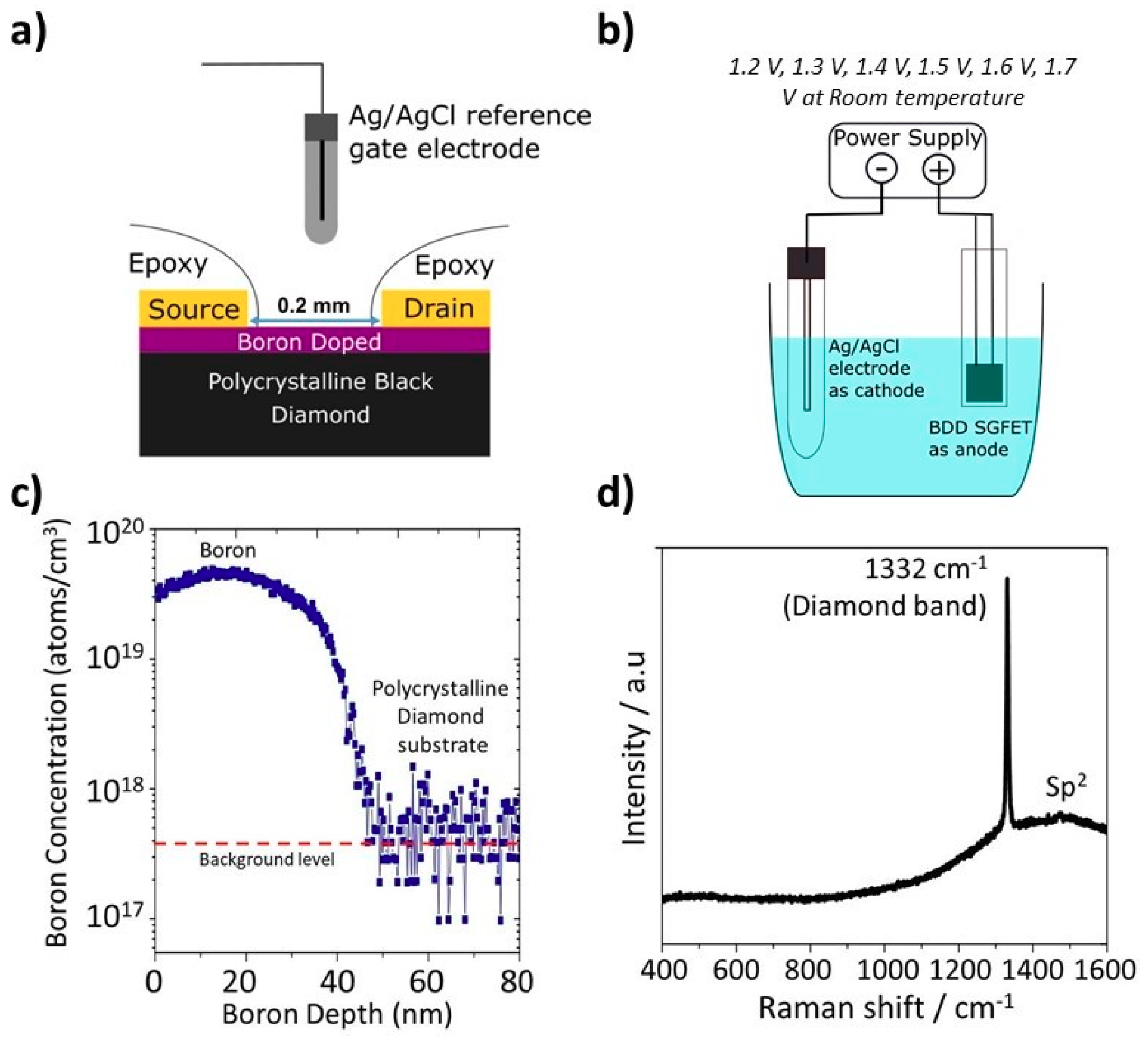
2.1. Thin Film Boron-Doped Layer Deposition
2.2. SGFET Fabrication
2.3. Surface Modifications
2.3.1. Anodic Oxidation
2.3.2. Nitrogen and Amine Termination
2.4. SGFET I-V Characterization
3. Results and Discussion
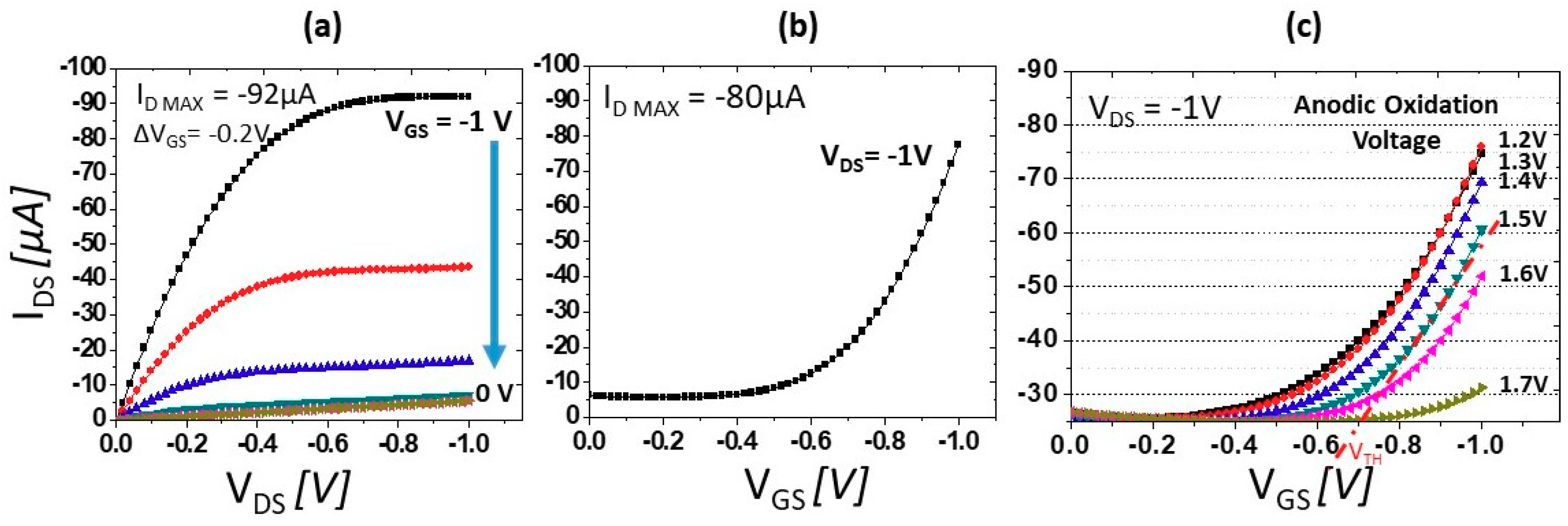
3.1. BDD SGFET Direct-Current Hall Measurement Profile, I-V Characteristics, and Precise VTH Control by Partial Oxidation
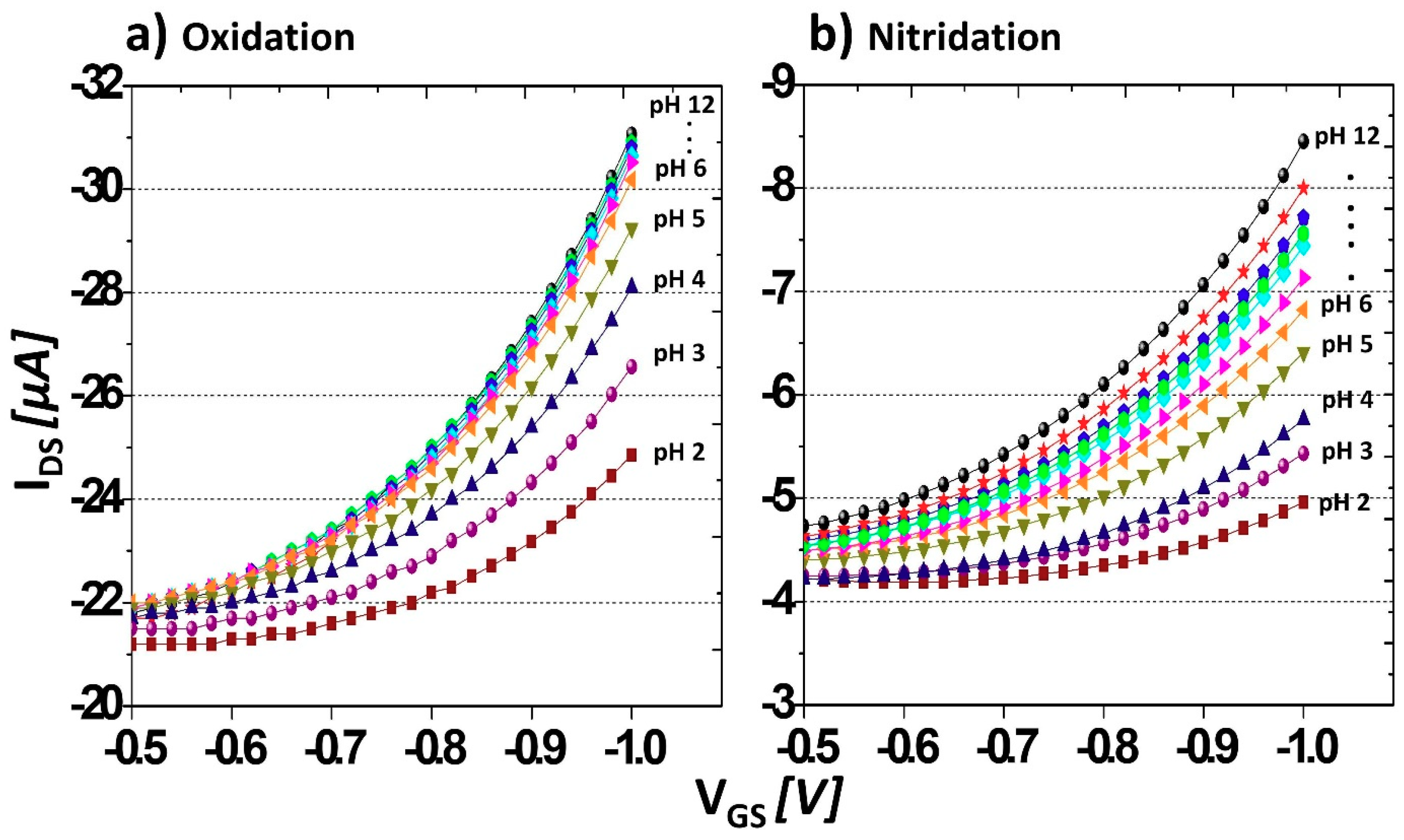
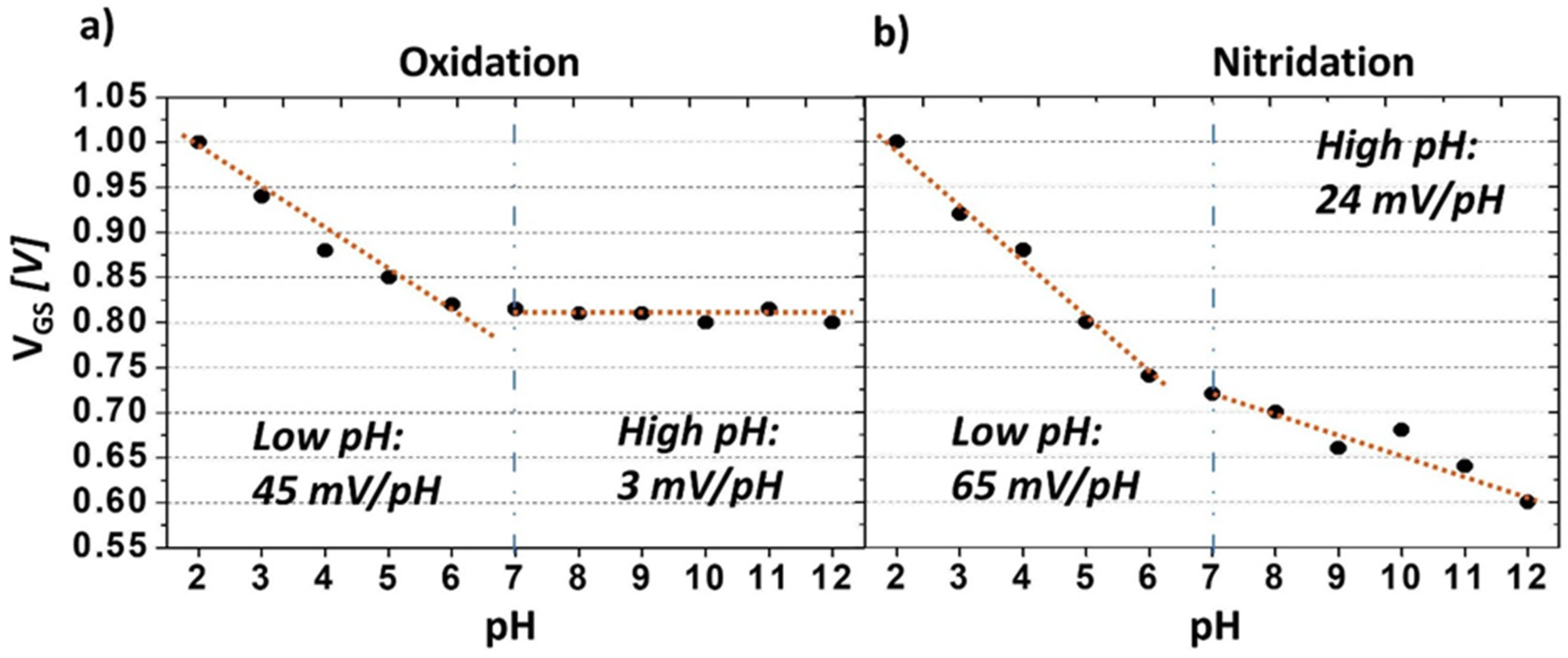
3.2. Sensing Characteristics of Carboxyl- and Amine-Terminated BDD SGFETs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vonau, W.; Guth, U. pH Monitoring: A review. J. Solid State Electrochem. 2006, 10, 746–752. [Google Scholar] [CrossRef]
- Bergveld, P. Development, operation, and application of the ion-sensitive field-effect transistor as a tool for electrophysiology. IEEE Trans. Biomed. Eng. 1972, 19, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Van den Vlekkert, H.; Bousse, L.; de Rooij, N.; Rooij, D.E. The temperature dependence of the surface potential at the Al2O3/electrolyte interface. J. Colloid Interface Sci. 1988, 122, 336–345. [Google Scholar] [CrossRef]
- Ding, X.; Tong, Q.; Niu, M. Effect of two types of surface sites on the characteristics of Si3N4-gate pH-ISFET’s. Sens. Actuators B Chem. 1996, 37, 13–17. [Google Scholar] [CrossRef]
- Gimmel, P.; Gompf, B.; Schmeisser, D.; Wiemhöfer, H.D.; Göpel, W.; Klein, M. Ta2O5-gates of pH-sensitive devices: Comparative spectroscopic and electrical studies. Sens. Actuators 1989, 17, 195–202. [Google Scholar] [CrossRef]
- Liao, H.K.; Chou, J.C.; Chung, W.Y.; Sun, T.P.; Hsiung, S.K. Study of amorphous tin oxide thin films for ISFET applications. Sens. Actuators B Chem. 1998, 50, 104–109. [Google Scholar] [CrossRef]
- Bousse, L.; Vlekkert, V.D.; Rooij, D. Hysteresis in A1203 -gate ISFETs. Sens. Actuators B Chem. 1990, 2, 103–110. [Google Scholar] [CrossRef]
- Woias, P.; Meixner, L.; Fröstl, P. Slow pH response effects of silicon nitride ISFET sensors. Sens. Actuators B Chem. 1998, 48, 501–504. [Google Scholar] [CrossRef]
- Xu, J.; Granger, M.C.; Chen, Q.; Strojek, J.W.; Lister, T.E.; Swain, G.M. Peer Reviewed: Boron-Doped Diamond Thin-Film Electrodes. Anal. Chem. 1997, 69, 591A–597A. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhi, J. The application of boron-doped diamond electrodes in amperometric biosensors. Talanta 2009, 79, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Swain, G.M. The Use of Cvd Diamond Thin-Films in Electrochemical Systems. Adv. Mater. 1994, 6, 388–392. [Google Scholar] [CrossRef]
- Szunerits, S.; Jama, C.; Coffinier, Y.; Marcus, B.; Delabouglise, D.; Boukherroub, R. Direct amination of hydrogen-terminated boron doped diamond surfaces. Electrochem. Commun. 2006, 8, 1185–1190. [Google Scholar] [CrossRef]
- Kawarada, H.; Araki, Y.; Sakai, T.; Ogawa, T. Electrolyte-Solution-Gate FETs Using Diamond Surface for Biocompatible Ion Sensors. Phys. Status Solidi A 2001, 83, 79–83. [Google Scholar] [CrossRef]
- Sasaki, Y.; Kawarada, H. Low drift and small hysteresis characteristics of diamond electrolyte-solution-gate FET. J. Phys. D. Appl. Phys. 2010, 43, 374020. [Google Scholar] [CrossRef]
- Hauf, M.V.; Hess, L.H.; Howgate, J.; Dankerl, M.; Stutzmann, M.; Garrido, J.A. Low-frequency noise in diamond solution-gated field effect transistors. Appl. Phys. Lett. 2010, 97. [Google Scholar] [CrossRef]
- Garrido, J.A.; Härtl, A.; Kuch, S.; Stutzmann, M.; Williams, O.A.; Jackmann, R.B. PH sensors based on hydrogenated diamond surfaces. Appl. Phys. Lett. 2005, 86, 1–3. [Google Scholar] [CrossRef]
- Denisenko, A.; Jamornmarn, G.; El-Hajj, H.; Kohn, E. pH sensor on O-terminated diamond using boron-doped channel. Diam. Relat. Mater. 2007, 16, 905–910. [Google Scholar] [CrossRef]
- Shintani, Y.; Kobayashi, M.; Kawarada, H. An All-Solid-State pH Sensor Employing Fluorine-Terminated Polycrystalline Boron-Doped Diamond as a pH-Insensitive Solution-Gate Field-Effect Transistor. Sensors 2017, 17, 1040. [Google Scholar] [CrossRef] [PubMed]
- Song, K.S.; Nakamura, Y.; Sasaki, Y.; Degawa, M.; Yang, J.H.; Kawarada, H. pH-sensitive diamond field-effect transistors (FETs) with directly aminated channel surface. Anal. Chim. Acta 2006, 573–574, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Pleskov, Y.; Tameev, A.; Varnin, V.; Teremetskaya, I. Comparison of the hole mobility in undoped and boron-doped polycrystalline CVD diamond films. J. Solid State Electrochem. 1998, 3, 25–30. [Google Scholar] [CrossRef]
- Watanabe, T.; Honda, Y.; Kanda, K.; Einaga, Y. Tailored design of boron-doped diamond electrodes for various electrochemical applications with boron-doping level and sp2-bonded carbon impurities. Phys. Status Solidi Appl. Mater. Sci. 2014, 211, 2709–2717. [Google Scholar] [CrossRef]
- Sakai, T.; Song, K.S.; Kanazawa, H.; Nakamura, Y.; Umezawa, H.; Tachiki, M.; Kawarada, H. Ozone-treated channel diamond field effect transistor. Diam. Relat. Mater. 2003, 12, 1971–1975. [Google Scholar] [CrossRef]
- Chaplin, B.P.; Hubler, D.K.; Farrell, J. Understanding anodic wear at boron doped diamond film electrodes. Electrochim. Acta 2013, 89, 122–131. [Google Scholar] [CrossRef]
- Shintani, Y.; Ibori, S.; Igarashi, K.; Naramura, T.; Inaba, M.; Kawarada, H. Polycrystalline boron-doped diamond with an oxygen-terminated surface channel as an electrolyte-solution-gate field-effect transistor for pH sensing. Electrochim. Acta 2016, 212, 10–15. [Google Scholar] [CrossRef]
- Edgington, R.; Ruslinda, A.R.; Sato, S.; Ishiyama, Y.; Tsuge, K.; Ono, T.; Kawarada, H.; Jackman, R.B. Boron δ-doped (111) diamond solution gate field effect transistors. Biosens. Bioelectron. 2012, 33, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Horton, H.R.; Moran, L.A.; Ochs, R.S.; Rawn, J.D.; Scrimgeour, K.G. Principle of Biochemistry, 4th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2007. [Google Scholar]
- Suaebah, E.; Seshimo, Y.; Shibata, M.; Kono, S.; Hasegawa, M.; Kawarada, H. Aptamer strategy for ATP detection on nanocrystalline diamond functionalized by a nitrogen and hydrogen radical beam system. J. Appl. Phys. 2017, 121. [Google Scholar] [CrossRef]
- Zhu, D.; Bandy, J.A.; Li, S.; Hamers, R.J. Amino-terminated diamond surfaces: Photoelectron emission and photocatalytic properties. Surf. Sci. 2015, 650, 295–301. [Google Scholar] [CrossRef]
- Parizi, K.B.; Xu, X.; Pal, A.; Hu, X.; Philip Wong, H.S. ISFET pH Sensitivity: Counter-Ions Play a Key Role. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.L.; Chou, J.C.; Chen, Y.C.; Liau, G.S.; Cheng, C.C. Drift and Hysteresis Effects on AlN/SiO2 Gate pH Ion-Sensitive Field-Effect Transistor. Jpn. J. Appl. Phys. Part 1 2003, 42, 4973–4977. [Google Scholar] [CrossRef]
- Mierczynska, A.; Michelmore, A.; Tripathi, A.; Goreham, R.V.; Sedev, R.; Vasilev, K. PH-tunable gradients of wettability and surface potential. Soft Matter 2012, 8, 8399–8404. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falina, S.; Kawai, S.; Oi, N.; Yamano, H.; Kageura, T.; Suaebah, E.; Inaba, M.; Shintani, Y.; Syamsul, M.; Kawarada, H. Role of Carboxyl and Amine Termination on a Boron-Doped Diamond Solution Gate Field Effect Transistor (SGFET) for pH Sensing. Sensors 2018, 18, 2178. https://doi.org/10.3390/s18072178
Falina S, Kawai S, Oi N, Yamano H, Kageura T, Suaebah E, Inaba M, Shintani Y, Syamsul M, Kawarada H. Role of Carboxyl and Amine Termination on a Boron-Doped Diamond Solution Gate Field Effect Transistor (SGFET) for pH Sensing. Sensors. 2018; 18(7):2178. https://doi.org/10.3390/s18072178
Chicago/Turabian StyleFalina, Shaili, Sora Kawai, Nobutaka Oi, Hayate Yamano, Taisuke Kageura, Evi Suaebah, Masafumi Inaba, Yukihiro Shintani, Mohd Syamsul, and Hiroshi Kawarada. 2018. "Role of Carboxyl and Amine Termination on a Boron-Doped Diamond Solution Gate Field Effect Transistor (SGFET) for pH Sensing" Sensors 18, no. 7: 2178. https://doi.org/10.3390/s18072178
APA StyleFalina, S., Kawai, S., Oi, N., Yamano, H., Kageura, T., Suaebah, E., Inaba, M., Shintani, Y., Syamsul, M., & Kawarada, H. (2018). Role of Carboxyl and Amine Termination on a Boron-Doped Diamond Solution Gate Field Effect Transistor (SGFET) for pH Sensing. Sensors, 18(7), 2178. https://doi.org/10.3390/s18072178





