Simultaneous Detection of Adenosine Triphosphate and Glucose Based on the Cu-Fenton Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Instruments
2.3. Synthesis of CNDs
2.4. Fluorescence Responses of Cu2+
2.5. Fluorescent H2O2 Assay
2.6. Fluorescence Detection of ATP
2.7. Fluorescence Measurement of Glucose
2.8. Real Sample Detection
2.9. Validation Test of TMB
3. Results and Discussion
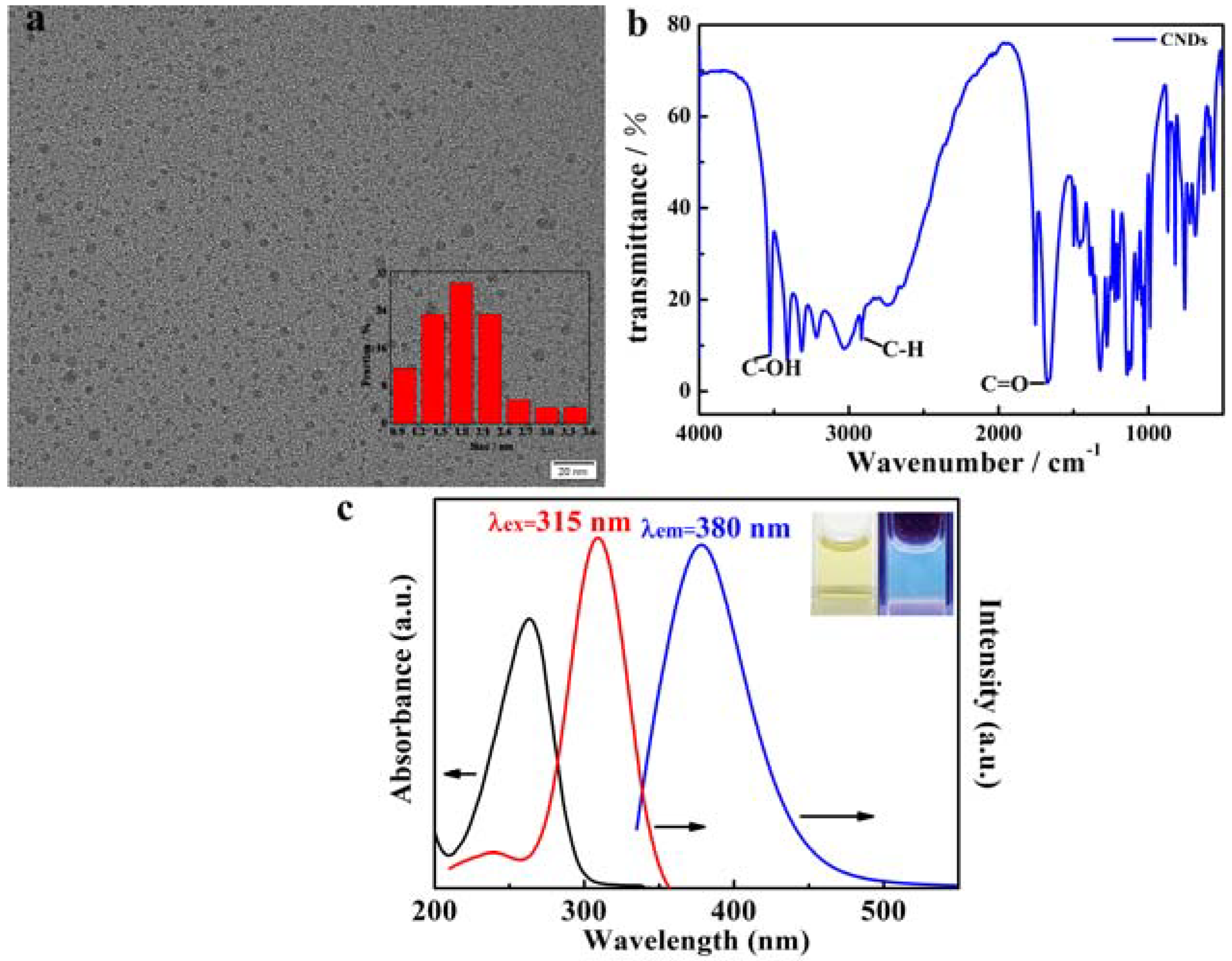
3.1. Characterization of CNDs
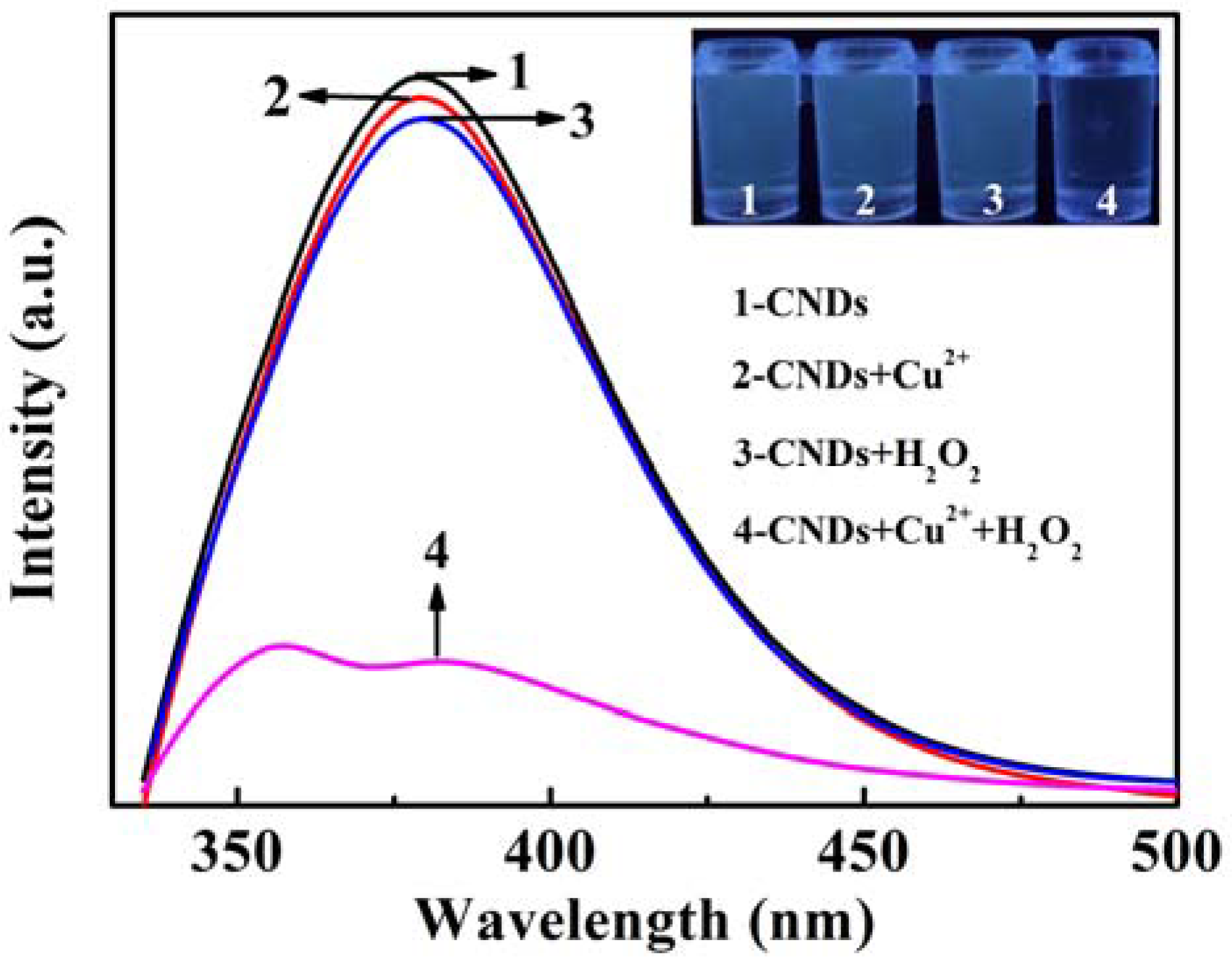
3.2. Fluorescence Quenching of CNDs
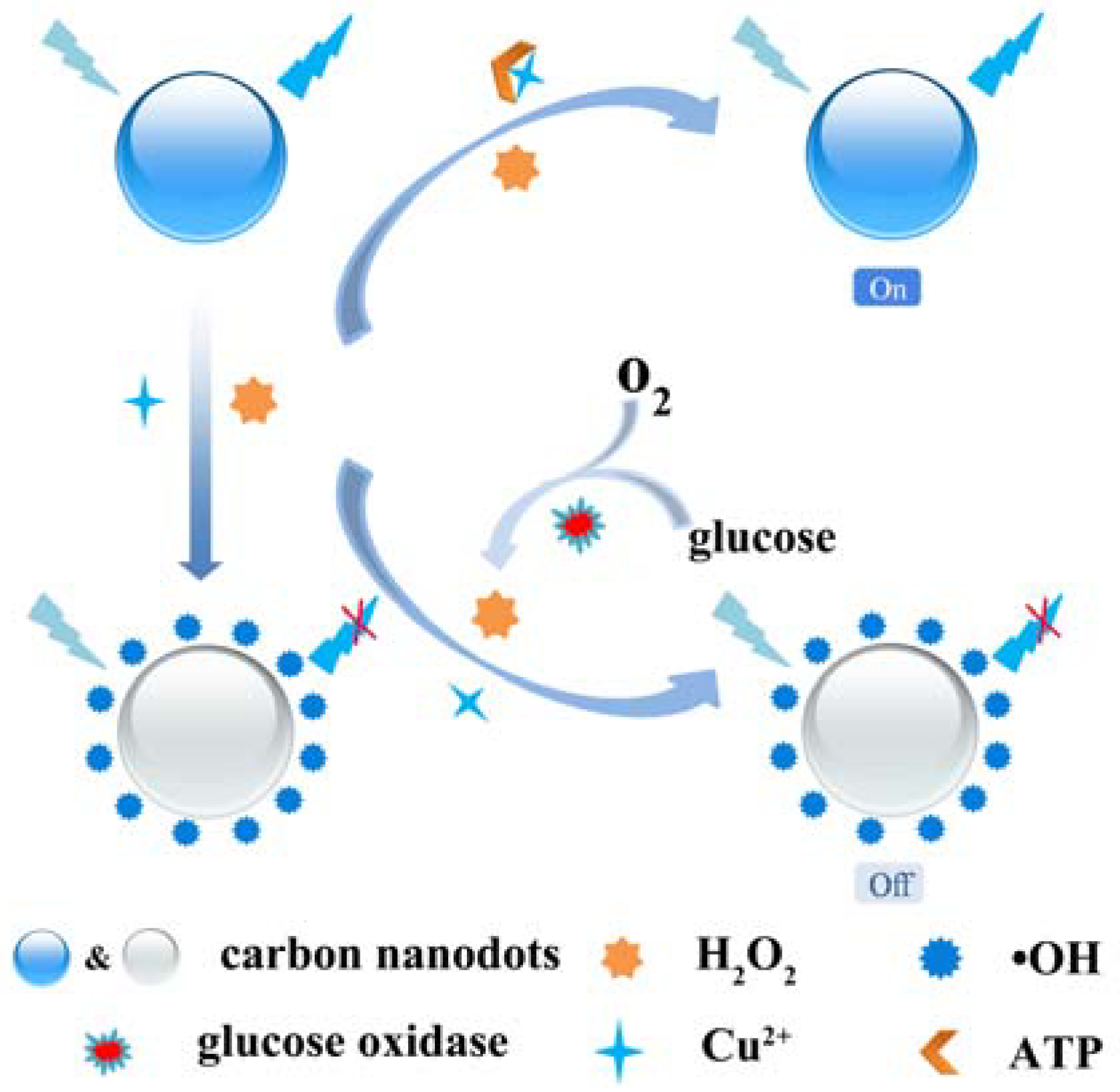
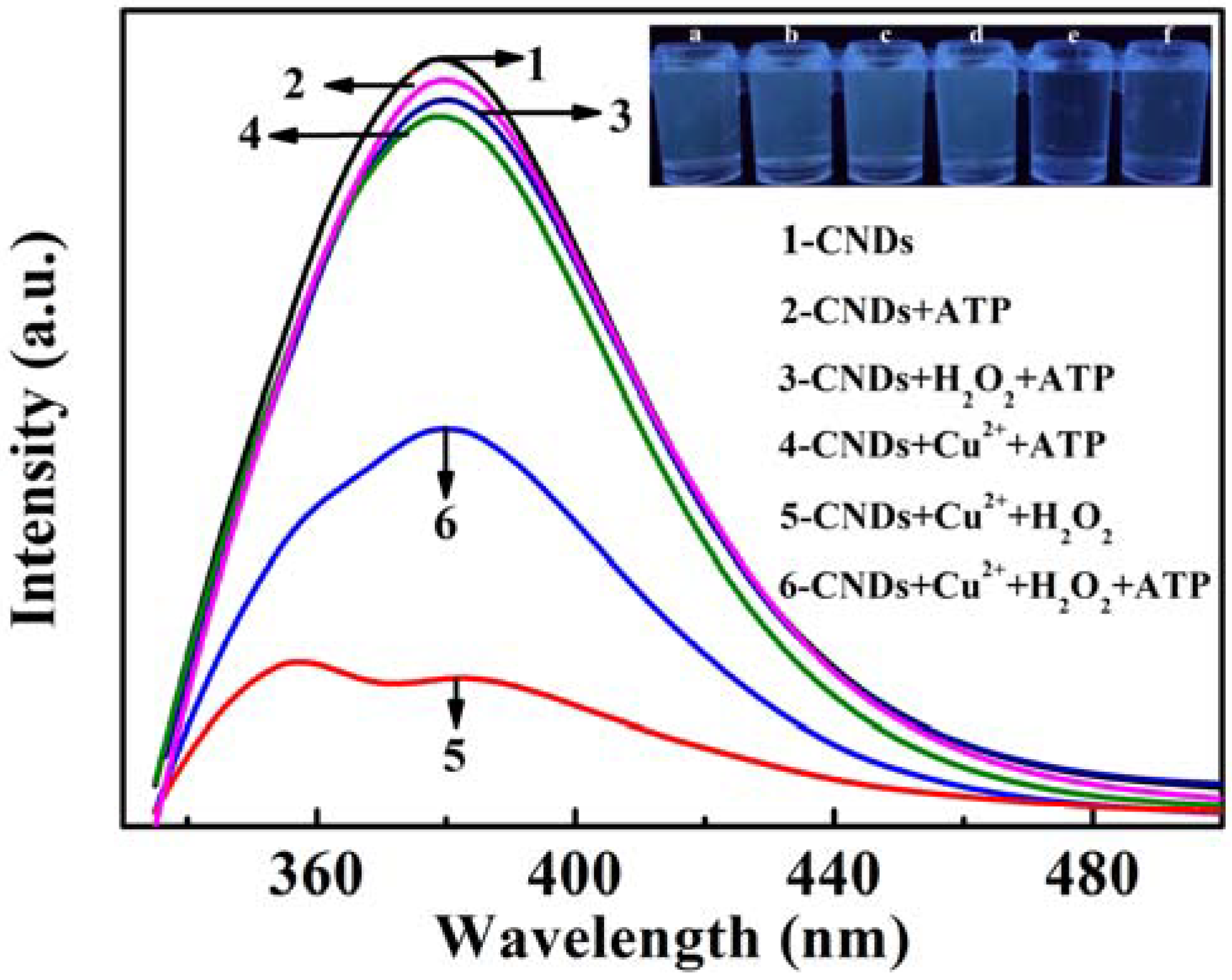
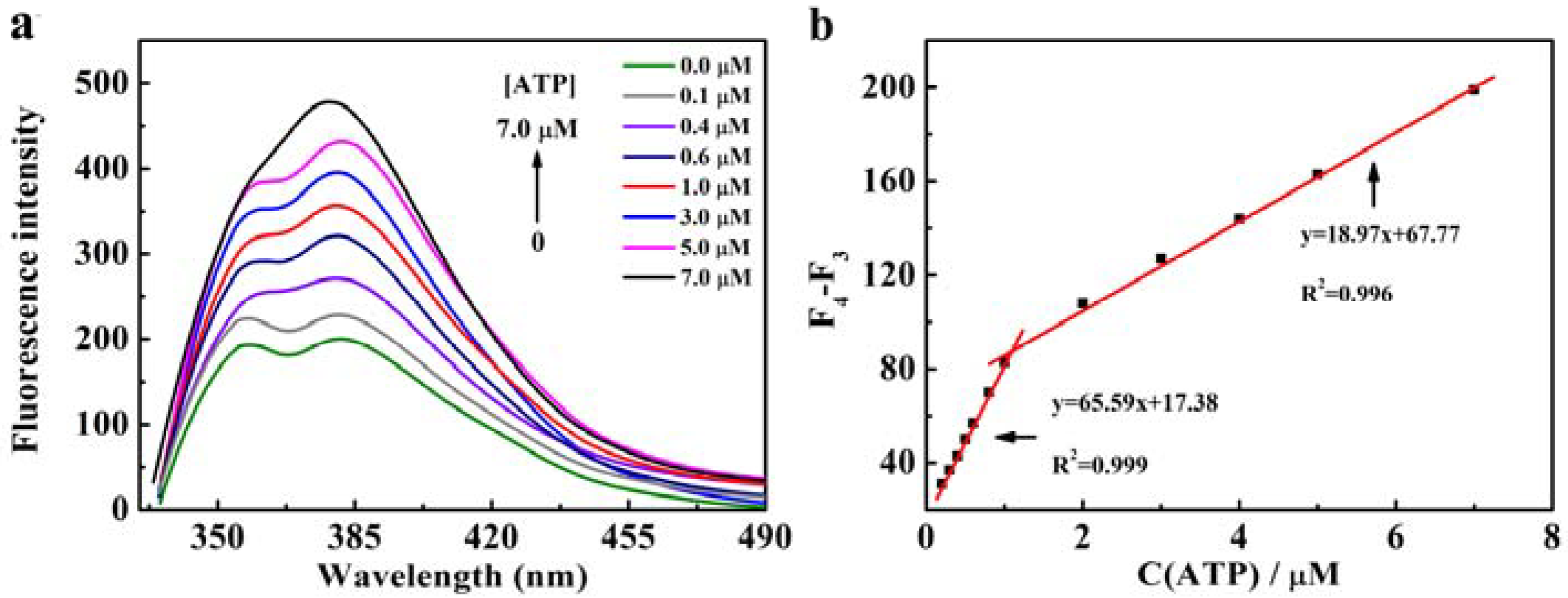
3.3. Fluorescence Recovery of CNDs-Cu2+-H2O2 System by ATP
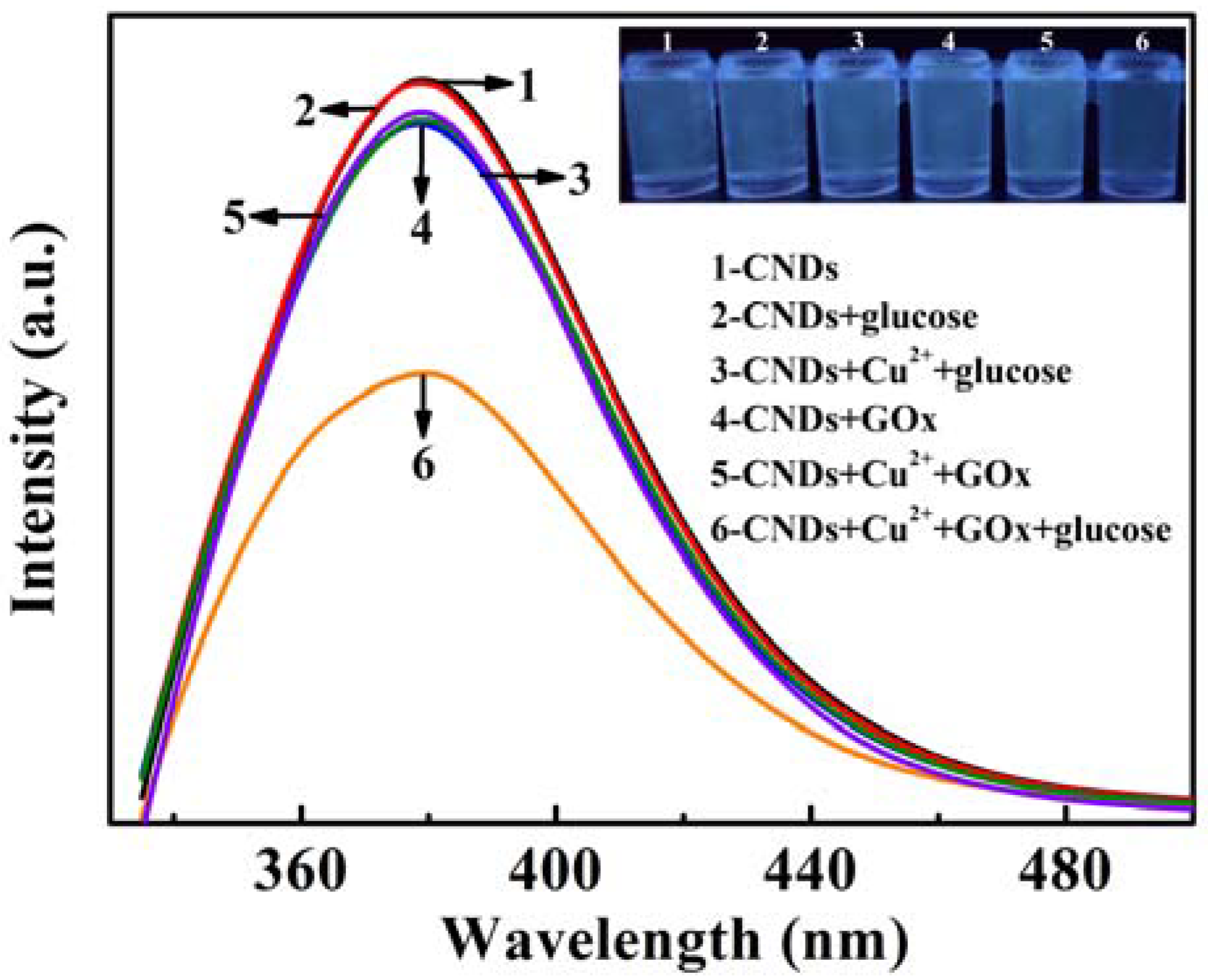
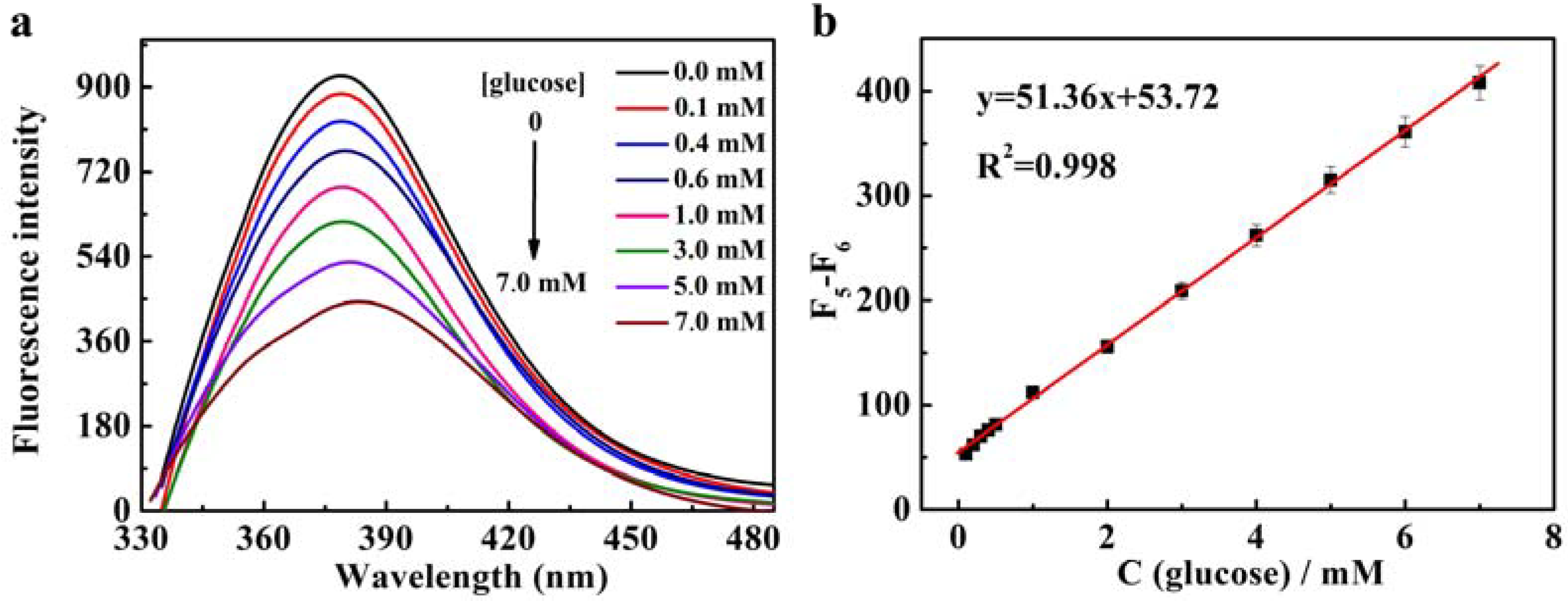
3.4. Detection of Glucose Based on CNDs-Cu2+ System
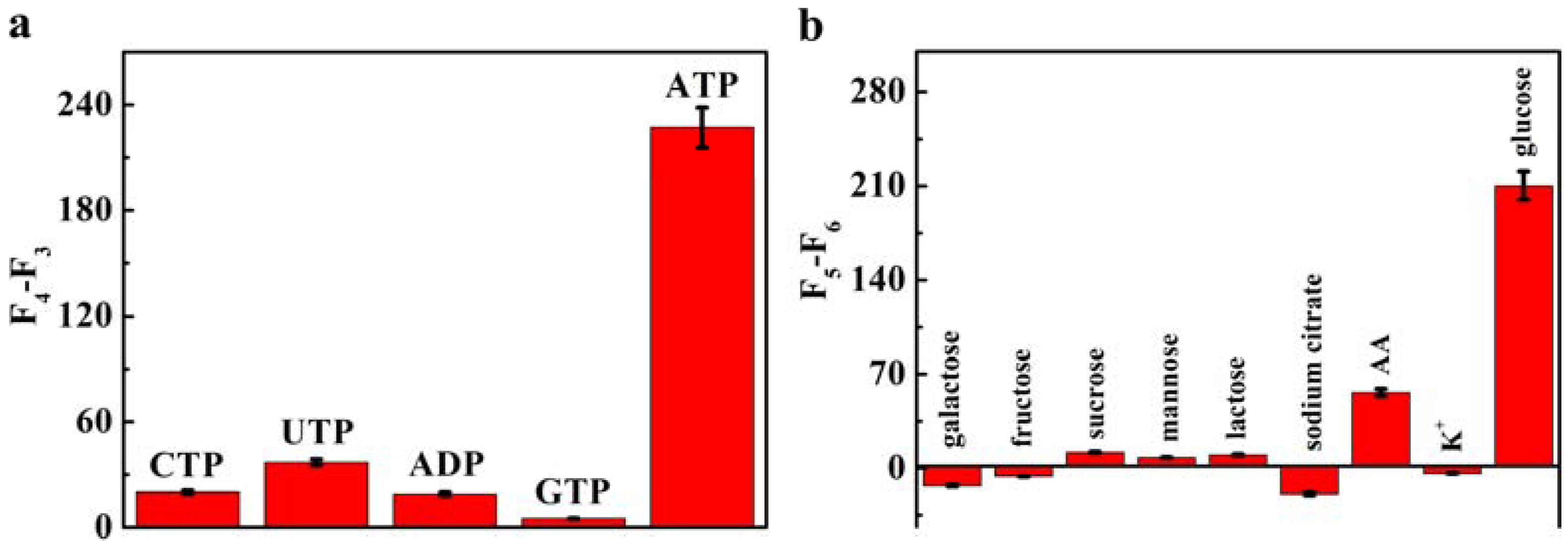
3.5. Selectivity for ATP and Glucose
3.6. Detection of ATP and Glucose in Human Serum Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wei, Y.; Chen, Y.; Li, H.; Shuang, S.; Dong, C.; Wang, G. An exonuclease I-based label-free fluorometric aptasensor for adenosine triphosphate (ATP) detection with a wide concentration range. Biosens. Bioelectron. 2015, 63, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Wang, H.; Li, H.; Wang, S.; Li, X.; Xu, B.; Tian, W. A label-free aptasensor for turn-on fluorescent detection of ATP based on AIE-active probe and water-soluble carbon nanotubes. Sens. Actuators B 2016, 230, 556–558. [Google Scholar] [CrossRef]
- Hao, G.; Wang, D.; Sun, Y.; Yu, J.; Lin, F.; Cao, H. Association of blood glucose and lipid levels with complete blood count indices to establish a regression model. Biomed. Rep. 2017, 6, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yu, C.; Chen, Y.; Shin, J.; Cao, Q.; Kim, J. A self-assembled amphiphilic imidazolium-based ATP probe. Chem. Commun. 2017, 53, 4342–4345. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Ma, V.P.Y.; Leung, K.H.; Chan, D.S.H.; Yang, H.; Cheng, Z.; Leung, C.H.; Ma, D.L. A label-free G-quadruplex-based switch-on fluorescence assay for the selective detection of ATP. Analyst 2012, 137, 1538–1540. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, L.; Karunasagar, D.; Sashidhar, R.B.; Giri, A. Peroxidase-like activity of gum kondagogu reduced/stabilized palladium nanoparticles and its analytical application for colorimetric detection of glucose in biological samples. Sens. Actuators B 2017, 240, 1182–1188. [Google Scholar] [CrossRef]
- Hu, A.; Liu, Y.; Deng, H.; Hong, G.; Liu, A.L.; Lin, X.; Xia, X.; Chen, W. Fluorescent hydrogen peroxide sensor based on cupric oxide nanoparticles and its application for glucose and l-lactate detection. Biosens. Bioelectron. 2014, 61, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Si, J.; Gao, Z.; Zhang, Y.; Lei, J.; Luo, H.; Li, N. Highly selective and sensitive electrochemical biosensor for ATP based on the dual strategy integrating the cofactor-dependent enzymatic ligation reaction with self-cleaving DNAzyme-amplified electrochemical detection. Biosens. Bioelectron. 2015, 63, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Schweinsberg, P.D.; Loo, T.L. Simultaneous analysis of ATP, ADP, AMP, and other purines in human erythrocytes by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1980, 181, 103–107. [Google Scholar] [CrossRef]
- Xu, M.; Gao, Z.; Zhou, Q.; Lin, Y.; Lu, M.; Tang, D. Terbium ion-coordinated carbon dots for fluorescent aptasensing of adenosine 5′-triphosphate with unmodified gold nanoparticles. Biosens. Bioelectron. 2016, 86, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Muthuraj, B.; Chowdhury, S.R.; Mukherjee, S.; Patra, C.R.; Iyer, P.K. Aggregation deaggregation influenced selective and sensitive detection of Cu2+ and ATP by histidine functionalized water-soluble fluorescent perylene diimide under physiological conditions and in living cells. RSC Adv. 2015, 5, 28211–28218. [Google Scholar] [CrossRef]
- Moschou, E.A.; Sharma, B.V.; Deo, S.K.; Daunert, S. Fluorescence glucose detection: Advances toward the ideal in vivo biosensor. J. Fluoresc. 2004, 14, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Guo, X.; Liu, D.; Chen, G.; You, J. Dual-emission carbon nanodots as a ratiometric nanosensor for the detection of glucose and glucose oxidase. Sens. Actuators B 2016, 233, 320–327. [Google Scholar] [CrossRef]
- Liu, S.; Sun, Y. Co-immobilization of glucose oxidase and hexokinase on silicate hybrid sol–gel membrane for glucose and ATP detections. Biosens. Bioelectron. 2007, 22, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Lu, M.; Wang, L.; MacDonald, B.; MacInnis, J.; Mkandawire, M.; Zhang, X.; Oakes, K.D. Chloride accelerated Fenton chemistry for the ultrasensitive and selective colorimetric detection of copper. Chem. Commun. 2016, 52, 2087–2090. [Google Scholar] [CrossRef] [PubMed]
- Cappella, P.; Giansanti, V.; Pulici, M.; Gasparri, F. From “Click” to “Fenton” chemistry for 5-bromo-2′-deoxyuridine determination. Cytom. Part A 2013, 83, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Chen, Y.; Huang, K.; Liu, Y. A label-free and ultrasensitive fluorescent sensor for dopamine detection based on double-stranded DNA templated copper nanoparticles. Sens. Actuators B 2015, 220, 146–153. [Google Scholar] [CrossRef]
- Shen, P.; Xia, Y. Synthesis-modification integration: One-step fabrication of boronic acid functionalized carbon dots for fluorescent blood sugar sensing. Anal. Chem. 2014, 86, 5323–5329. [Google Scholar] [CrossRef] [PubMed]
- De, B.; Karak, N. A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana juice. RSC Adv. 2013, 3, 8286–8290. [Google Scholar] [CrossRef]
- Wang, F.; Xie, Z.; Zhang, H.; Liu, C.; Zhang, Y. Highly luminescent organosilane-functionalized carbon dots. Adv. Funct. Mater. 2011, 21, 1027–1031. [Google Scholar] [CrossRef]
- Purbia, R.; Paria, S. A simple turn on fluorescent sensor for the selective detection of thiamine using coconut water derived luminescent carbon dots. Biosens. Bioelectron. 2016, 79, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Lv, K.; Zhong, W.; Wang, H.; Wu, Z.; Yi, P.; Jiang, J. Cyclam-functionalized carbon dots sensor for sensitive and selective detection of copper (II) ion and sulfide anion in aqueous media and its imaging in live cells. Sens. Actuators B 2016, 224, 298–306. [Google Scholar] [CrossRef]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Pham, A.N.; Xing, G.; Miller, C.J.; Waite, T.D. Fenton-like copper redox chemistry revisited: Hydrogen peroxide and superoxide mediation of copper-catalyzed oxidant production. J. Catal. 2013, 301, 54–64. [Google Scholar] [CrossRef]
- Moffett, J.W.; Zika, R.G. Reaction kinetics of hydrogen peroxide with copper and iron in seawater. Environ. Sci. Technol. 1987, 21, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Millero, F.J.; Sharma, V.K.; Karn, B. The rate of reduction of copper (II) with hydrogen peroxide in seawater. Mar. Chem. 1991, 36, 71–83. [Google Scholar] [CrossRef]
- Gray, R.D. Kinetics of oxidation of copper (I) by molecular oxygen in perchloric acid-acetonitrile solutions. J. Am. Chem. Soc. 1969, 91, 56–62. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Y.; Zhou, C.; Fang, X. Aptamer-based ATP assay using a luminescent light switching complex. Anal. Chem. 2005, 77, 3542–3546. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hu, X.; Shi, S.; Gao, R.; Huang, H.; Zhu, Y.; Lv, X.; Yao, T. Ultrasensitive and universal fluorescent aptasensor for the detection of biomolecules (ATP, adenosine and thrombin) based on DNA/Ag nanoclusters fluorescence light-up system. Biosens. Bioelectron. 2016, 79, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Liu, X.; Liang, Z.; Song, S.; Li, W.; Li, G.; Fan, C. A Gold Nanoparticle-Based Aptamer Target Binding Readout for ATP Assay. Adv. Mater. 2007, 19, 3943–3946. [Google Scholar] [CrossRef]
- Tedsana, W.; Tuntulani, T.; Ngeontae, W. A highly selective turn-on ATP fluorescence sensor based on unmodified cysteamine capped CdS quantum dots. Anal. Chim. Acta 2013, 783, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, G.; Zhang, L.; Jiang, J.; Li, Z.; Peng, Z.; Deng, L. A new method for the detection of ATP using a quantum-dot-tagged aptamer. Anal. Bioanal. Chem. 2008, 392, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Song, S.; Zhang, J.; Pan, D.; Wang, L.; Fan, C. A target-responsive electrochemical aptamer switch (TREAS) for reagentless detection of nanomolar ATP. J. Am. Chem. Soc. 2007, 129, 1042–1043. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Numata, M.; Takeuchi, M.; Shinkai, S. A sensitive colorimetric and fluorescent probe based on a polythiophene derivative for the detection of ATP. Angew. Chem. Int. Ed. 2005, 44, 6371–6374. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhong, L.; Guo, L.; Fu, F.; Chen, G. Seeing diabetes: Visual detection of glucose based on the intrinsic peroxidase-like activity of MoS2 nanosheets. Nanoscale 2014, 6, 11856–11862. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhao, H.; Lin, Y.; Zhu, N.; Ma, Y.; Mao, L. Colorimetric detection of glucose in rat brain using gold nanoparticles. Angew. Chem. 2010, 122, 4910–4914. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, P.; Xu, Z.; Chen, M.; Ding, Y.; Yue, K.; Xu, J. A facile strategy to prepare porphyrin functionalized ZnS nanoparticles and their peroxidase-like catalytic activity for colorimetric sensor of hydrogen peroxide and glucose. Sens. Actuators B 2017, 251, 339–348. [Google Scholar] [CrossRef]
- Ling, Y.; Zhang, N.; Qu, F.; Wen, T.; Gao, Z.; Li, N.; Luo, H. Fluorescent detection of hydrogen peroxide and glucose with polyethyleneimine-templated Cu nanoclusters. Spectrochim. Acta Part A 2014, 118, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, D.; Du, J. Prussian blue nanoparticles as peroxidase mimetics for sensitive colorimetric detection of hydrogen peroxide and glucose. Talanta 2014, 120, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tian, J.; Wang, L.; Luo, Y.; Sun, X. A general strategy for the production of photoluminescent carbon nitride dots from organic amines and their application as novel peroxidase-like catalysts for colorimetric detection of H2O2 and glucose. RSC Adv. 2012, 2, 411–413. [Google Scholar] [CrossRef]
| Method | Probe | Linear Range | LOD | Ref. |
|---|---|---|---|---|
| Fluorescence | Ru complex | 0–0.1 µM | 20 nM | 29 |
| Fluorescence | DNA template Ag nanoclusters (AgNCs) | 0.5–8.0 µM | 91.6 nM | 30 |
| Colorimetric detection | Gold nanoparticles | 4.4–132.7 µM | 0.6 µM | 31 |
| Fluorescence | Cysteamine capped CdS quantum dots (QDs) | 20–80 µM | 17 µM | 32 |
| Fluorescence | DNA binding dye berberine | 0.5–50 µM | 140 nM | 1 |
| Fluorescence | QD-tagged aptamer | 0.1–1 mM | 24 µM | 33 |
| Electrochemical | labeled anti-ATP aptamer | 10 nM–1 mM | - | 34 |
| Fluorescence and Colorimetric detection | cationic polythiophene derivative | 10−8–10−4 M | - | 35 |
| Fluorescence | CNDs | 0.1–7 µM | 30.2 nM | This work |
| Method | Probe | Linear Range | LOD | Ref. |
|---|---|---|---|---|
| Colorimetric detection | MoS2 nanosheets | 5–150 µM | 1.2 µM | 36 |
| Fluorescence | boronic acid modified carbon dots | 9–900 µM | 1.5 µM | 19 |
| Colorimetric detection | Gold nanoparticles | 0–5 mM | - | 37 |
| Colorimetric detection | ZnS nanoparticles | 0.05–0.5 mM | 36 µM | 38 |
| Fluorescence | Copper nanoclusters | 10–100 µM | 8 µM | 39 |
| Colorimetric detection | Prussian blue nanoparticles | 0.1–50 µM | 0.03 µM | 40 |
| Fluorescence | carbon nitride dots | 1–100 µM | 0.4 µM | 41 |
| Fluorescence | CNDs | 0.1–7 mM | 39.8 µM | This work |
| Sample | Standard Added µM | Found Value µM | Recovery (%) | RSD (n = 3, %) |
|---|---|---|---|---|
| 1 | 0.0 | 0.433 * | - | 1.43 |
| 2 | 0.1 | 0.537 | 104.0 | 1.68 |
| 3 | 1.0 | 1.497 | 106.4 | 1.16 |
| 4 | 5.0 | 5.553 | 102.4 | 1.61 |
| Sample | Standard Added mM | Found Value mM | Recovery (%) | RSD (n = 3, %) |
|---|---|---|---|---|
| 1 | 0 | 0.341 * | - | 1.38 |
| 2 | 0.1 | 0.439 | 98.0 | 1.02 |
| 3 | 1.0 | 1.389 | 104.8 | 1.52 |
| 4 | 3.0 | 3.284 | 98.1 | 1.83 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, F.; Li, J.; Han, W.; Xia, L.; You, J. Simultaneous Detection of Adenosine Triphosphate and Glucose Based on the Cu-Fenton Reaction. Sensors 2018, 18, 2151. https://doi.org/10.3390/s18072151
Qu F, Li J, Han W, Xia L, You J. Simultaneous Detection of Adenosine Triphosphate and Glucose Based on the Cu-Fenton Reaction. Sensors. 2018; 18(7):2151. https://doi.org/10.3390/s18072151
Chicago/Turabian StyleQu, Fei, Jingwen Li, Wenli Han, Lian Xia, and Jinmao You. 2018. "Simultaneous Detection of Adenosine Triphosphate and Glucose Based on the Cu-Fenton Reaction" Sensors 18, no. 7: 2151. https://doi.org/10.3390/s18072151
APA StyleQu, F., Li, J., Han, W., Xia, L., & You, J. (2018). Simultaneous Detection of Adenosine Triphosphate and Glucose Based on the Cu-Fenton Reaction. Sensors, 18(7), 2151. https://doi.org/10.3390/s18072151





