Flash Smelting Copper Concentrates Spectral Emission Measurements
Abstract
1. Introduction
2. Spectral Signal Considerations
3. Copper Concentrates Spectral Measurements
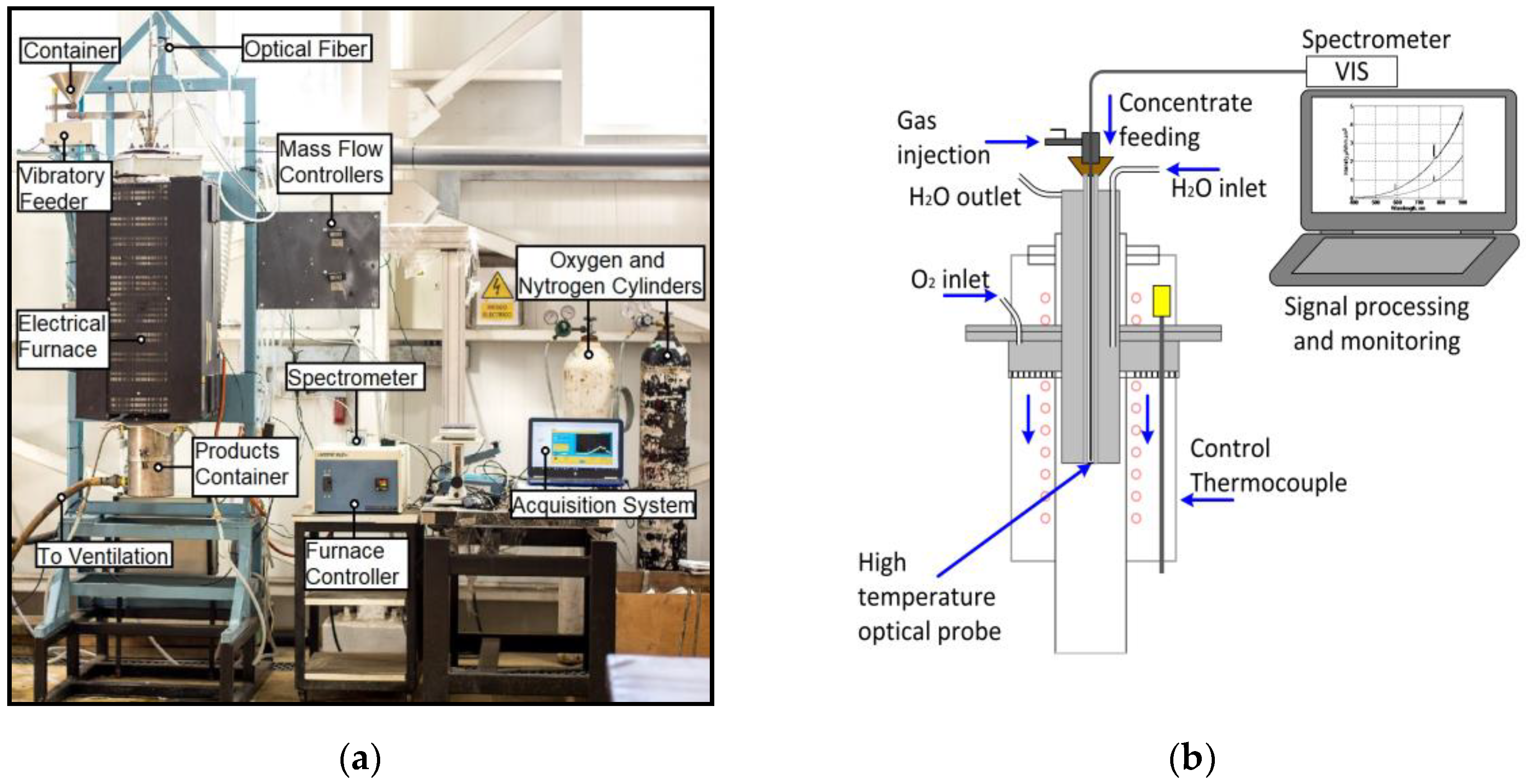
3.1. Experimental Setup
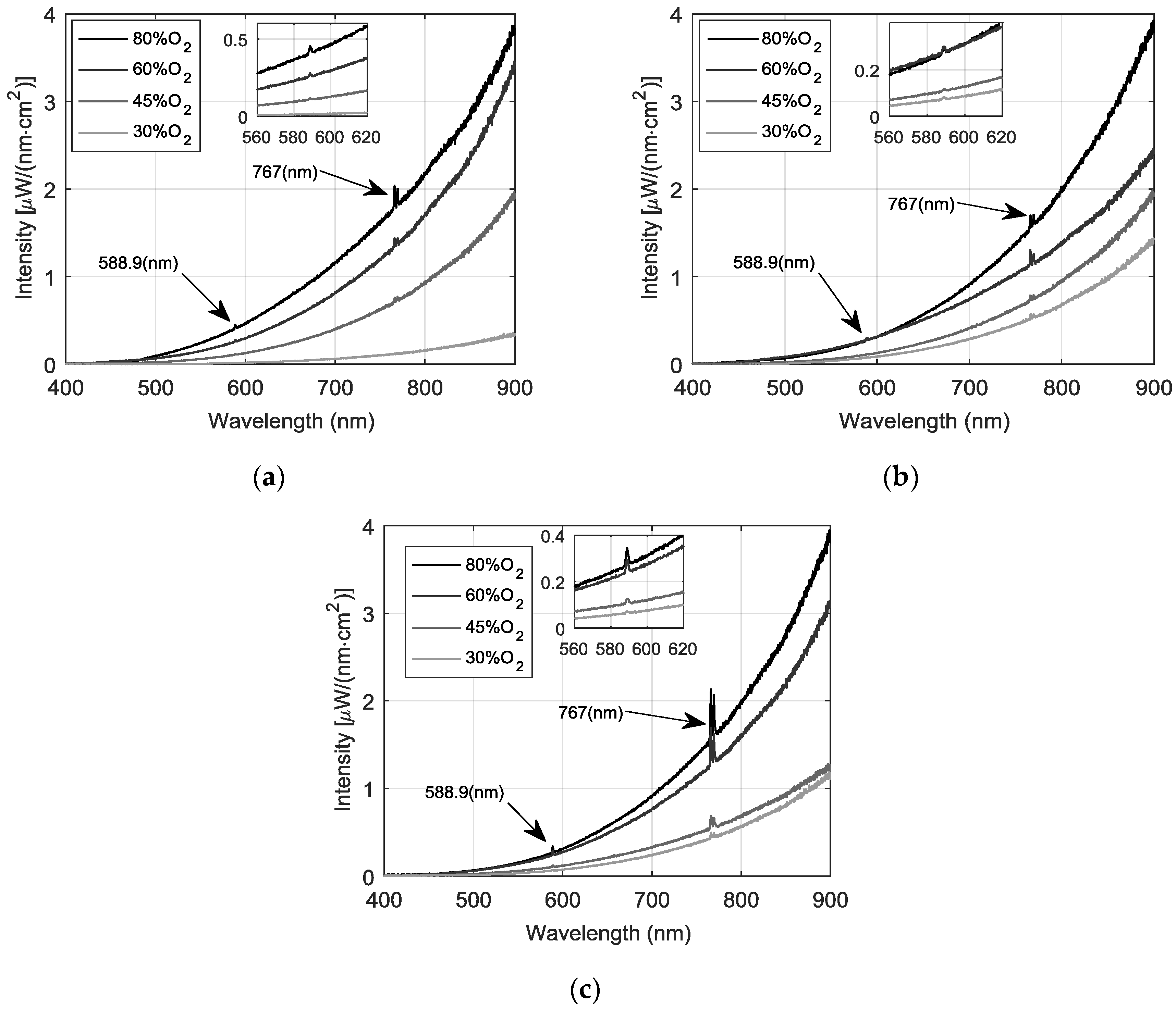
3.2. Spectral Measurements and Analysis
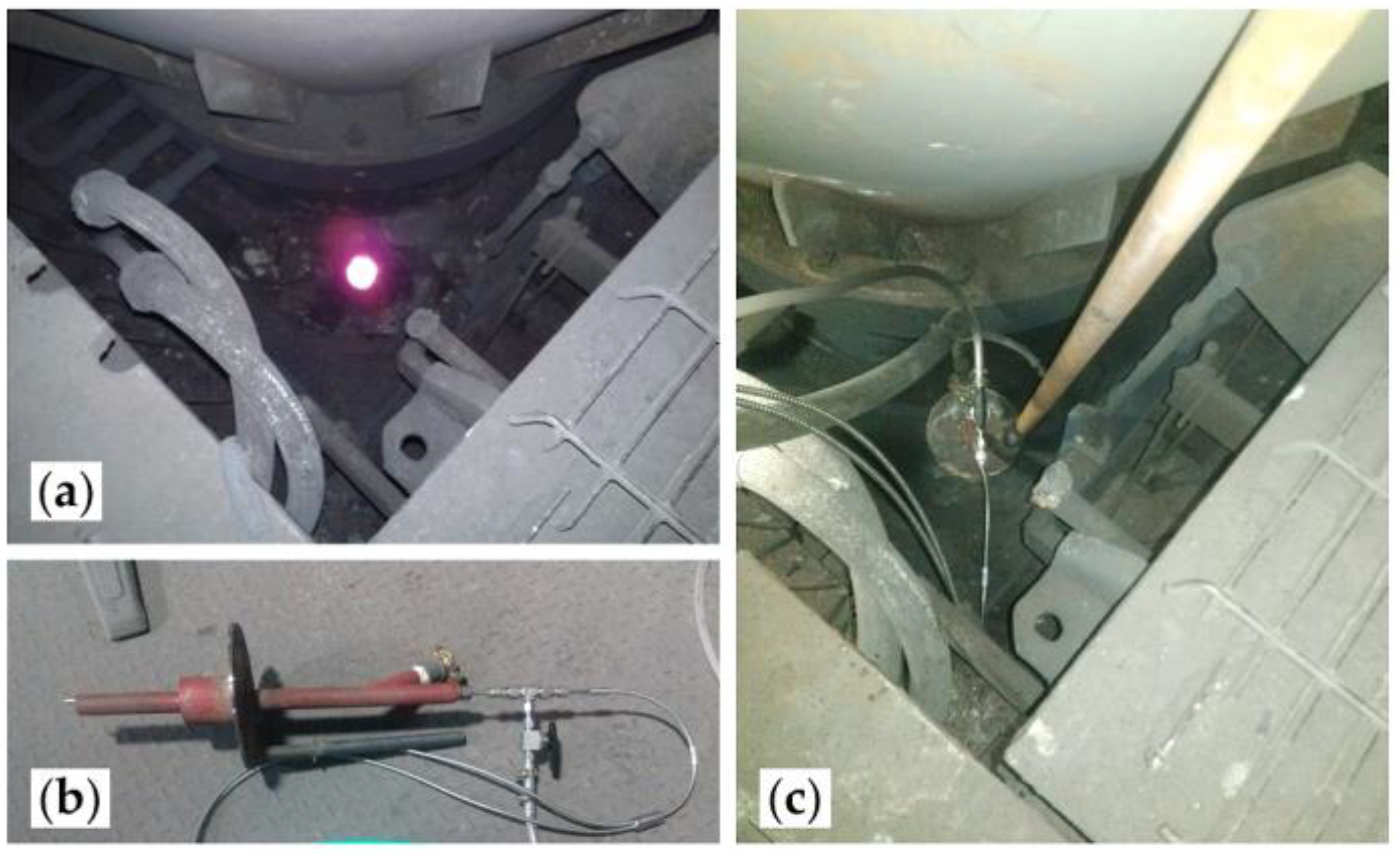
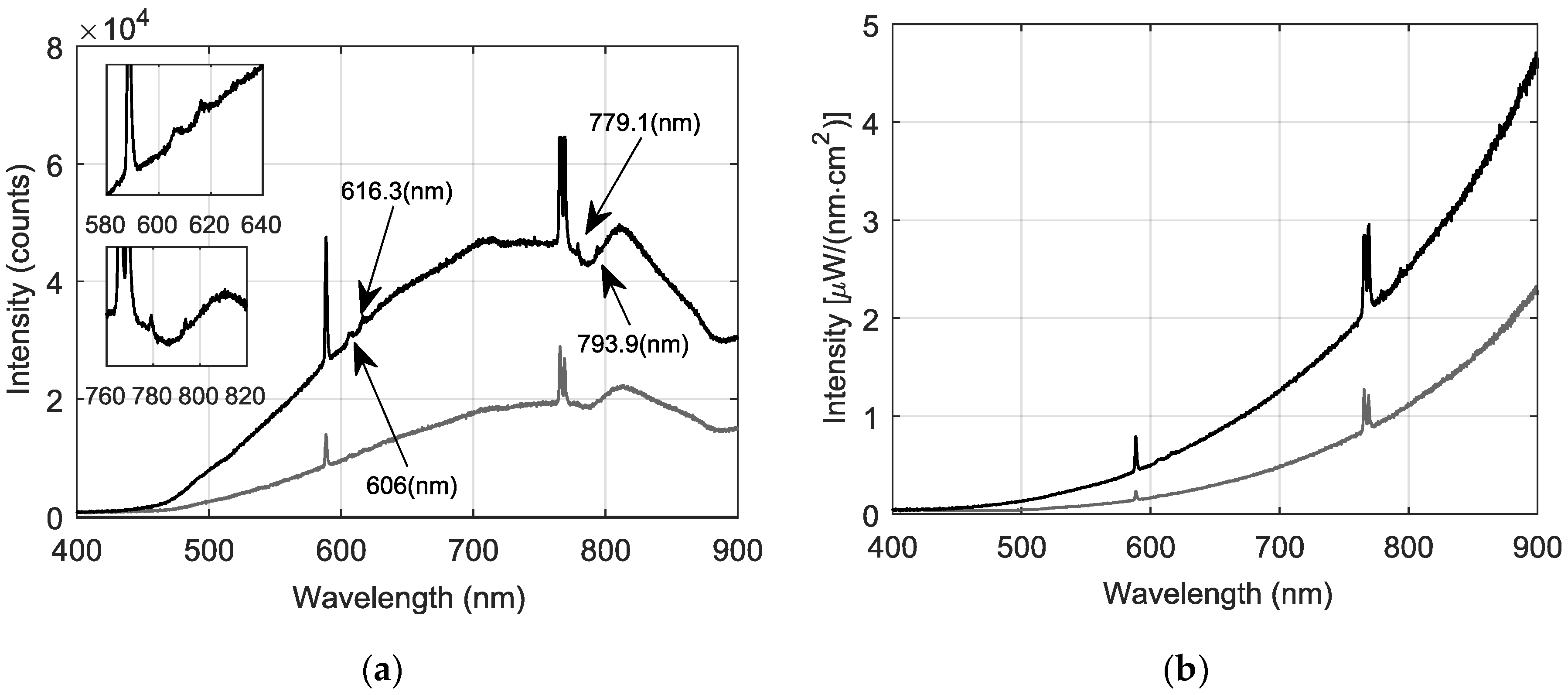
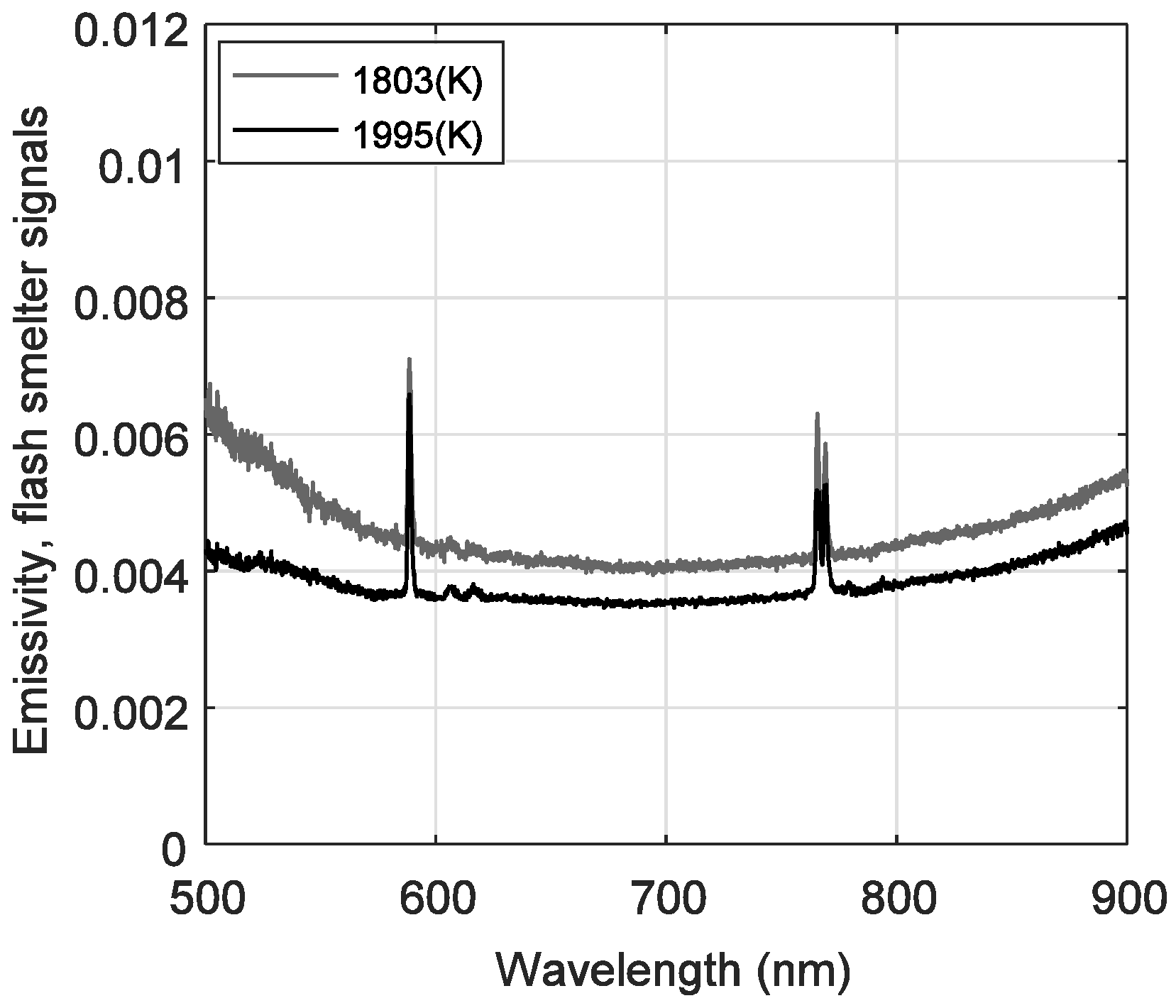
3.3. Spectral Measurements in An Industrial Copper Smelting Plant
4. Temperature and Emissivity Estimation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Docquier, N.; Candel, S. Combustion control and sensors: A review. Prog. Energy Combust. Sci. 2002, 28, 107–150. [Google Scholar] [CrossRef]
- Sbarbaro, D.; Farias, O.; Zawadsky, A. Real-time monitoring and characterization of flames by principal component analysis. Combust. Flame 2003, 132, 591–595. [Google Scholar] [CrossRef]
- Romero, C.; Li, X.; Keyvan, S.; Rossow, R. Spectrometer-based combustion monitoring for flame sotichiometry and temperature control. Appl. Therm. Eng. 2005, 25, 659–676. [Google Scholar] [CrossRef]
- Arias, L.; Torres, S.; Sbarbaro, D.; Farias, O. Photodiode-based sensor for flame sensing and combustion-process monitoring. Appl. Opt. 2008, 47, 66–77. [Google Scholar] [CrossRef]
- Zhang, X.; Li, T.; Ma, P.; Wang, B. Spray Combustion Characteristics and Soot Emission Reduction of Hydrous Ethanol Diesel Emulsion Fuel Using Color-Ratio Pyrometry. Energies 2017, 10, 2062. [Google Scholar] [CrossRef]
- Rego-Barcena, S.; Mani, R.; Yang, F.; Saari, R.; Thomson, M.J. Real-Time, Optical Measurement of Gas Temperature and Particle Emissivity in a Full-Scale Steelmaking Furnace. Metall. Mater. Trans. B 2009, 40, 158–168. [Google Scholar] [CrossRef]
- Kojo, I.; Jokilaakso, A.; Hanniala, P. Flash smelting and converting furnaces: A 50 year retrospect. JOM Comb. Pyrometall. 2000, 52, 57–61. [Google Scholar] [CrossRef]
- Moskalyka, R.; Alfantazib, A. Review of copper pyrometallurgical practice: Today and tomorrow. J. Min. Engine. 2003, 16, 893–919. [Google Scholar] [CrossRef]
- Memarya, R.; Giurcoa, D.; Muddb, G.; Masona, L. Life cycle assessment: A time-series analysis of copper. J. Cleaner Prod. 2012, 33, 97–108. [Google Scholar] [CrossRef]
- Jorgensen, F.; Koh, P. Combustion in flash smelting furnaces. JOM Comb. Pyrometall. 2001, 53, 16–20. [Google Scholar] [CrossRef]
- Morgan, G.; Brimacombe, J. Kinetics of the flash converting of MK (chalcocite) concentrate. Metall. Mater. Trans. B 1996, 27, 163–175. [Google Scholar] [CrossRef]
- Chen, H.; Mei, C.; Xie, K.; Li, X.; Zhuo, J.; Wang, X.; Ge, Z. Operation optimization of concentrate burner in cooper flash smelting process. Trans. Nonferr. Met. Soc. China 2004, 14, 631–636. [Google Scholar]
- Vaarno, J.; Jarvi, J.; Ahokainen, T.; Laurila, T.; Taskinen, P. Development of a mathematical model of flash smelting and converting process. In Proceedings of the Third International Conference on CFD in the Minerals and Process Industries, Melbourne, Australia, 10–12 December 2003; pp. 147–154. [Google Scholar]
- Arias, L.; Torres, S.; Sbarbaro, D.; Ngendakumana, P. On the spectral band measurements for combustion monitoring. Comb. Flame 2011, 158, 423–433. [Google Scholar] [CrossRef]
- Juang, Y. Consistent Spectral Irradiance Measurements with Optical Fibers. Available online: https://oceanoptics.com/consistent-spectral-irradiance-measurements-optical-fibers/ (accessed on 18 January 2017).
- Jenniskens, P.; Lacey, M.; Allan, B.J.; Self, D.E.; Plane, J.M.C. FeO “orange arc” emission detected in optical spectrum of Leonid persistent train. Earth Moon Planets 2000, 82, 429–438. [Google Scholar]
- Lynch, P.; Krier, H.; Glumac, N. Emissivity of aluminum-oxide particle clouds: application to pyrometry of explosive fireballs. J. Thermophys. Heat Transf. 2010, 24, 301–308. [Google Scholar] [CrossRef]
- Gole, J.L. Oxidation of small metal and metalloid molecules. In Gas-phase Metal Reactions, 1st ed.; Fontjin, A., Ed.; Elsevier: New York, NY, USA, 1992; pp. 596–597. [Google Scholar]
- Maxwell, K.L.; Hudson, M.K. Spectral study of metallic molecular bands in hybrid rocket plumes. J. Pyrotech. 2005, 21, 59–69. [Google Scholar]
- National Institute of Standards and Technology: Atomic Spectra Database. Available online: https://www.nist.gov/pml/atomic-spectra-database (accessed on 22 January 2018).
- Demtröder, W. Absorption and Emission of Light. In Laser Spectroscopy: Basic Concepts and Instrumention, 3rd ed.; Springer: Berlin, Germany, 2002; pp. 12–28. [Google Scholar]
- Hottel, H.C.; Broughton, F.P. Determination of the true temperature and total radiation from luminous gas flames. Ind. Eng. Chem. Anal. Ed. 1932, 4, 166–175. [Google Scholar] [CrossRef]
- Magunov, A.N. Spectral pyrometry (review). Instrum. Exp. Tech. 2009, 52, 451–472. [Google Scholar] [CrossRef]
- Toro, C.; Arias, L.; Torres, S.; Sbarbaro, D. Flame spectra-temperature estimation based on a color imaging camera and a spectral reconstruction technique. Appl. Opt. 2014, 53, 6351–6361. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lou, C.; Zhou, H. A simple judgment method of gray property of flames based on spectral analysis and the two-color method for measurements of temperatures and emissivity. Proc. Comb. Inst. 2011, 33, 735–741. [Google Scholar] [CrossRef]
- Tuffrey, N.E.; Richards, G.G.; Brimacombe, J.K. Two-wavelength pyrometry study of the combustion of sulfide minerals: Part I. Apparatus and general observations. Metall. Mater. Trans. B 1995, 26, 929–942. [Google Scholar] [CrossRef]
- Davenport, W.G.; Partelpoeg, E.H. Flash Smelting: Analysis, Control and Optimization, 1st ed.; Pergamon Press: New York, NY, USA, 1987. [Google Scholar]
- Parada, R. Combustion of Copper Concentrate in Flash Smelting Furnaces. Ph.D. Thesis, University of Oviedo, Oviedo, Spain, 2015. [Google Scholar]
- Laurila, T.; Oikari, R.; Joutsenoja, T.; Mikkola, P.; Ranki-Kilpinen, T.; Taskinen, P.; Hernberg, R. Pyrometric temperature and size measurements of chalcopyrite particles during flash oxidation in a laminar flow reactor. Metall. Mater. Trans. B 2005, 36, 201–208. [Google Scholar] [CrossRef]
- Moinul, M.; Lu, G.; Sun, D.; Yan, Y. Three-dimensional reconstruction of flame temperature and emissivity distribution using optical tomographic and two-colour pyrometric techniques. Meas. Sci. Technol. 2013, 24, 1–10. [Google Scholar]


| Concentrate | A (wt.%) | B (wt.%) | C (wt.%) |
|---|---|---|---|
| CuFeS2 | 89.17 | 67.44 | 61.23 |
| Cu5FeS4 | 0.89 | 2.13 | 1.24 |
| FeS2 | 5.08 | 17.71 | 31.23 |
| CuS | 2.00 | 7.02 | 3.26 |
| FeO | 0.46 | 0.44 | 0.61 |
| Cu2O | 0.02 | 0.06 | 0.05 |
| MoS2 | 0.28 | 0.41 | 0.05 |
| SiO2 | 1.90 | 2.05 | 2.03 |
| Concentrate | S (wt.%) | Cu (wt.%) | Fe (wt.%) | S/Cu Ratio |
|---|---|---|---|---|
| A | 32.25 | 30.27 | 27.55 | 1.07 |
| B | 34.86 | 27.47 | 26.51 | 1.27 |
| C | 38.74 | 20.89 | 32.28 | 1.85 |
| Reaction | (kJ/mol) | |
|---|---|---|
| 1 | CuFeS2 = 0.5Cu2S + FeS + 0.25S2(g) | 81.10 |
| 2 | Cu5FeS4 = 2.5Cu2S + FeS + 0.25S2(g) | 112.06 |
| 3 | FeS2 = FeS + 0.5S2(g) | 134.13 |
| 4 | FeS + 0.5O2(g) = FeO + 0.5S2(g) | −101.30 |
| 5 | CuS + 0.5O2(g) = CuO + 0.5S2(g) | −35.50 |
| 6 | Cu2S + O2(g) = 2CuO + 0.5S2(g) | −167.80 |
| 7 | Cu2S + 0.4FeS + 0.1S2(g) = 0.4Cu5FeS4 | −44.83 |
| 8 | CuO + FeO = CuFeO2 | −89.89 |
| 9 | 2FeO + SiO2 = 2FeO·SiO2 | −33.72 |
| 10 | S2(g) + 2O2(g) = 2SO2(g) | −722.23 |
| Concentrate | Temperature (K) and (Estimation Error in %) | |||
|---|---|---|---|---|
| O2 = 30% | O2 = 45% | O2 = 60% | O2 = 80% | |
| A | 1655 (7.9) | 1762 (2.6) | 1902 (3.0) | 2045 (3.8) |
| B | 1732 (4.3) | 1761 (2.2) | 2090 (3.8) | 1833 (1.9) |
| C | 1754 (1.9) | 1892 (0.8) | 1902 (2.4) | 1850 (1.9) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias, L.; Torres, S.; Toro, C.; Balladares, E.; Parra, R.; Loeza, C.; Villagrán, C.; Coelho, P. Flash Smelting Copper Concentrates Spectral Emission Measurements. Sensors 2018, 18, 2009. https://doi.org/10.3390/s18072009
Arias L, Torres S, Toro C, Balladares E, Parra R, Loeza C, Villagrán C, Coelho P. Flash Smelting Copper Concentrates Spectral Emission Measurements. Sensors. 2018; 18(7):2009. https://doi.org/10.3390/s18072009
Chicago/Turabian StyleArias, Luis, Sergio Torres, Carlos Toro, Eduardo Balladares, Roberto Parra, Claudia Loeza, Camilo Villagrán, and Pablo Coelho. 2018. "Flash Smelting Copper Concentrates Spectral Emission Measurements" Sensors 18, no. 7: 2009. https://doi.org/10.3390/s18072009
APA StyleArias, L., Torres, S., Toro, C., Balladares, E., Parra, R., Loeza, C., Villagrán, C., & Coelho, P. (2018). Flash Smelting Copper Concentrates Spectral Emission Measurements. Sensors, 18(7), 2009. https://doi.org/10.3390/s18072009








