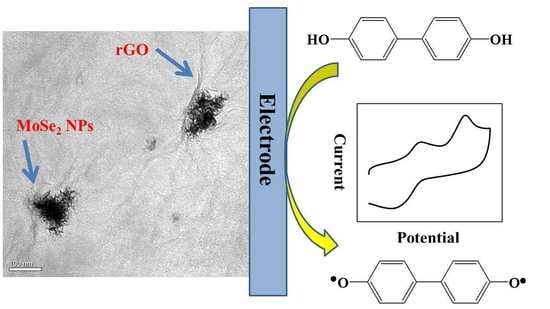
In Situ Determination of Bisphenol A in Beverage Using a Molybdenum Selenide/Reduced Graphene Oxide Nanoparticle Composite Modified Glassy Carbon Electrode
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Instruments
2.3. Preparation of MoSe2/rGO Composite
2.4. Analysis of Real Sample
3. Results and Discussion
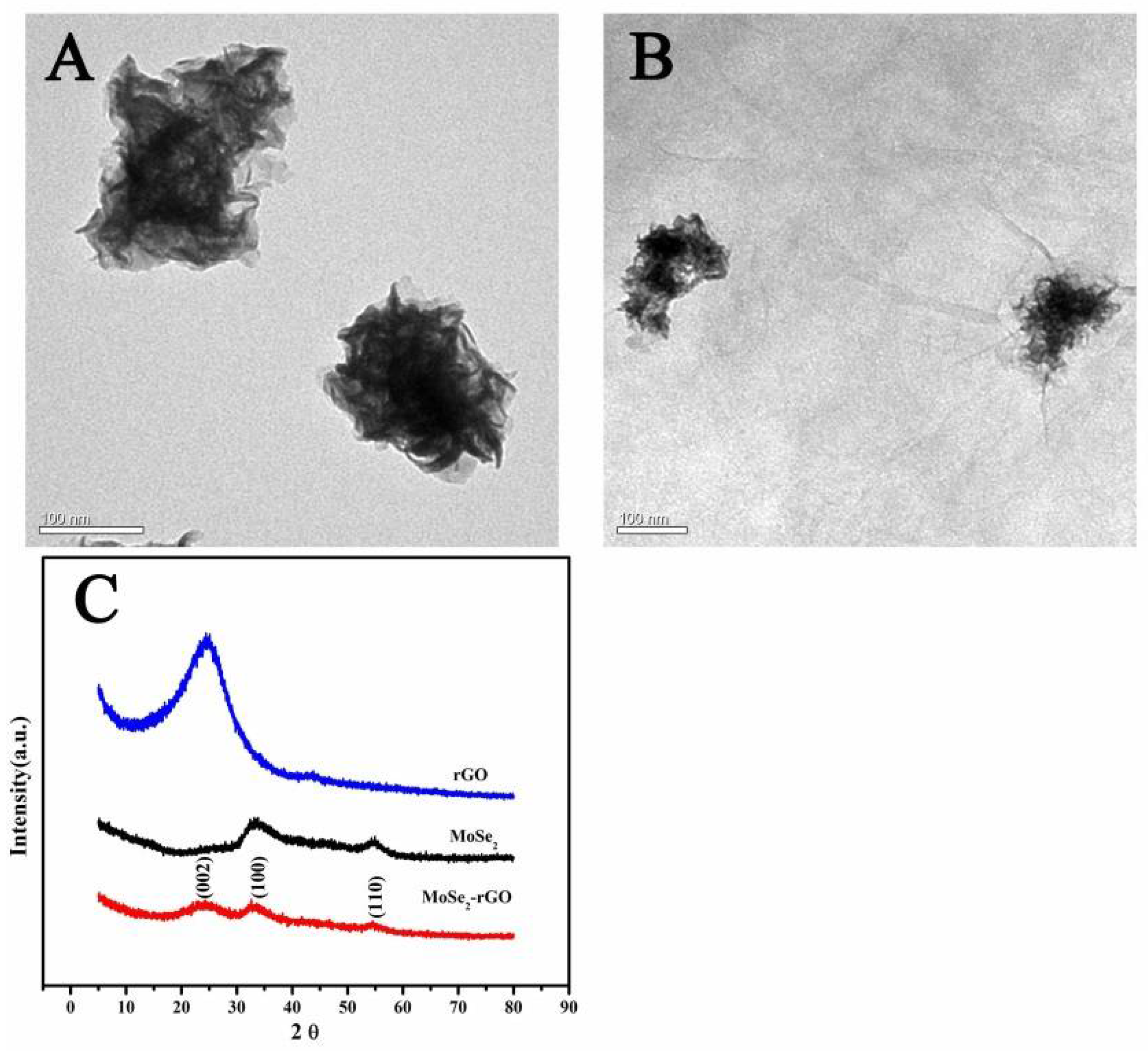
3.1. Characterization of Electrode Materials
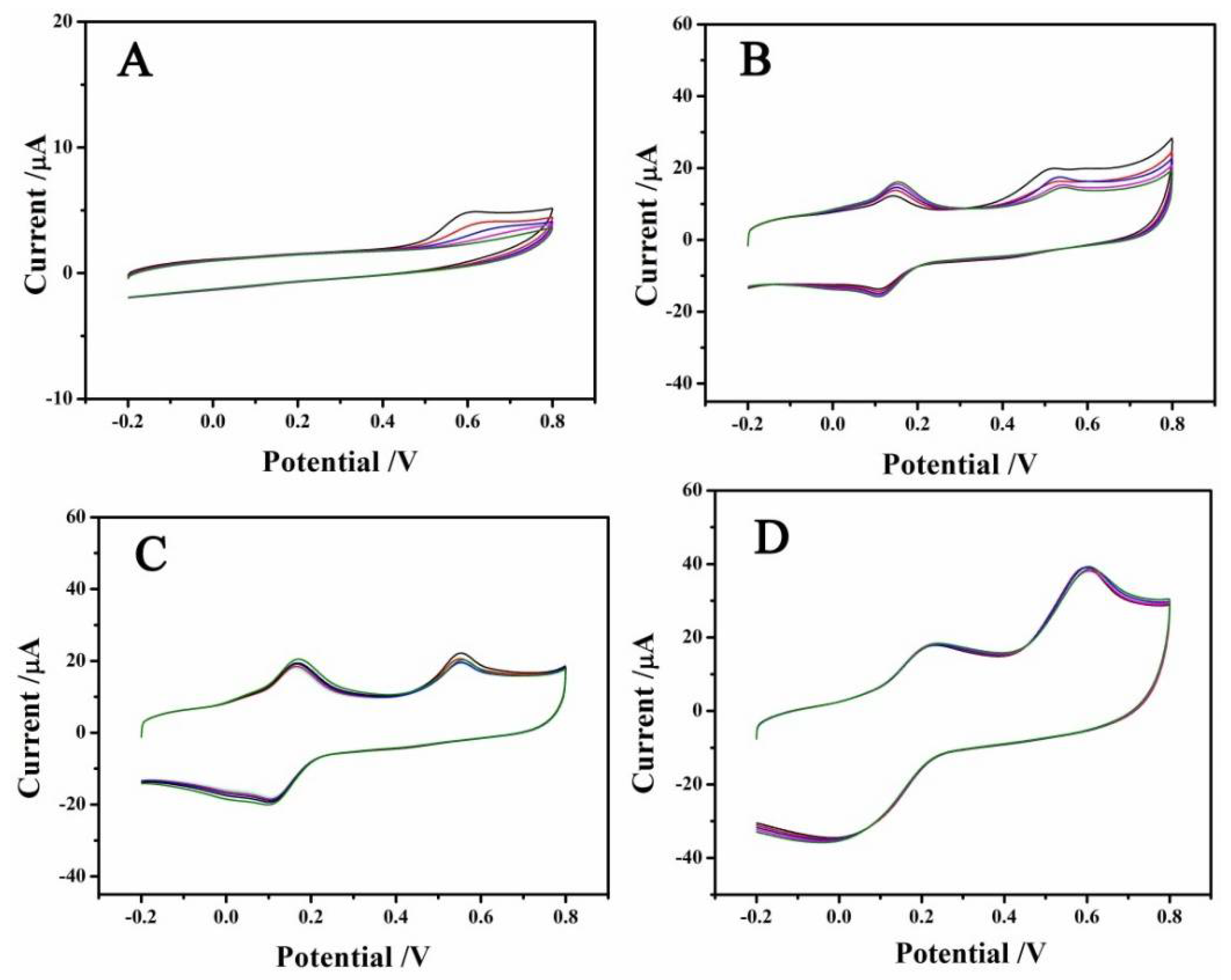
3.2. Enhanced Electrochemical Properties of MoSe2/rGO/GCE
3.3. Reaction Mechanism of BPA on the Surface of MoSe2/rGO/GCE
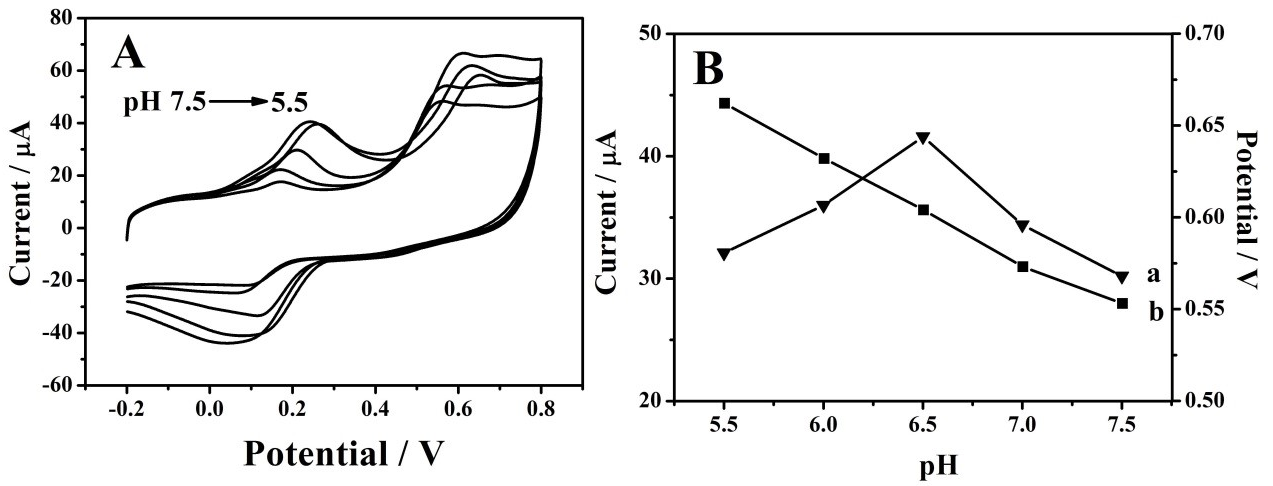
3.4. Interference Analysis
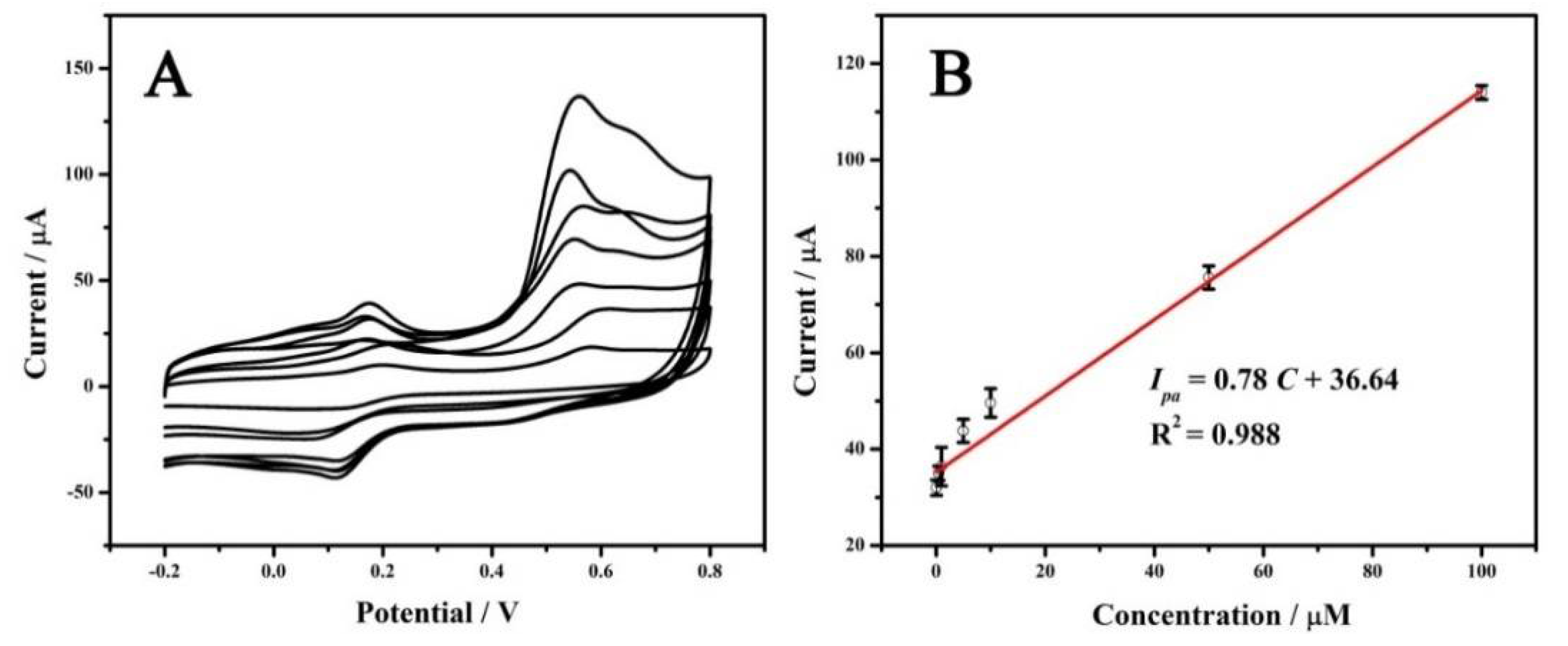
3.5. Analytical Performance
3.6. Samples Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rosenfeld, C.S. Bisphenol A and phthalate endocrine disruption of parental and social behaviors. Front. Neurosci. 2015, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Yin, J.; Xu, Z.; Zhao, C.; Huang, H.; Zhang, H.; Wang, C. Preparation of magnetic molecularly imprinted polymer for rapid determination of bisphenol A in environmental water and milk samples. Anal. Bioanal. Chem. 2009, 395, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Khedr, A. Optimized extraction method for LC–MS determination of bisphenol A, melamine and di(2-ethylhexyl) phthalate in selected soft drinks, syringes, and milk powder. J. Chromatogr. B 2013, 930, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Xu, Z.; Kuang, Y. Electrochemical determination of bisphenol A in plastic bottled drinking water and canned beverages using a molecularly imprinted chitosan–graphene composite film modified electrode. Food Chem. 2014, 157, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Ye, X.; Wong, L.-Y.; Reidy, J.A.; Needham, L.L. Exposure of the U.S. Population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008, 116, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Howdeshell, K.L.; Hotchkiss, A.K.; Thayer, K.A.; Vandenbergh, J.G.; vom Saal, F.S. Environmental toxins: Exposure to bisphenol A advances puberty. Nature 1999, 401, 763–764. [Google Scholar] [PubMed]
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 2008, 300, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Mirmira, P.; Evans-Molina, C. Bisphenol A, obesity, and type 2 diabetes mellitus: Genuine concern or unnecessary preoccupation? Transl. Res. 2014, 164, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Bacle, A.; Thevenot, S.; Grignon, C.; Belmouaz, M.; Bauwens, M.; Teychene, B.; Venisse, N.; Migeot, V.; Dupuis, A. Determination of bisphenol A in water and the medical devices used in hemodialysis treatment. Int. J. Pharm. 2016, 505, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Pouokam, G.B.; Ajaezi, G.C.; Mantovani, A.; Orisakwe, O.E.; Frazzoli, C. Use of bisphenol A-containing baby bottles in cameroon and nigeria and possible risk management and mitigation measures: Community as milestone for prevention. Sci. Total Environ. 2014, 481, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, D.; Huang, C.; Sun, Y.; Lee, Y.-I. Sensitive detection of bisphenol A in complex samples by in-column molecularly imprinted solid-phase extraction coupled with capillary electrophoresis. Microchem. J. 2015, 121, 1–5. [Google Scholar] [CrossRef]
- Li, D.; Park, J.; Oh, J.R. Silyl derivatization of alkylphenols, chlorophenols, and bisphenol A for simultaneous GC/MS determination. Anal. Chem. 2001, 73, 3089–3095. [Google Scholar] [CrossRef] [PubMed]
- Alexiadou, D.K.; Maragou, N.C.; Thomaidis, N.S.; Theodoridis, G.A.; Koupparis, M.A. Molecularly imprinted polymers for bisphenol A for HPLC and SPE from water and milk. J. Sep. Sci. 2008, 31, 2272–2282. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Sedlak, D.L. Analysis of estrogenic hormones in municipal wastewater effluent and surface water using enzyme-linked immunosorbent assay and gas chromatography/tandem mass spectrometry. Environ. Toxicol. Chem. 2001, 20, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Kazane, I.; Gorgy, K.; Gondran, C.; Spinelli, N.; Zazoua, A.; Defrancq, E.; Cosnier, S. Highly sensitive bisphenol-A electrochemical aptasensor based on poly(pyrrole-nitrilotriacetic acid)-aptamer. Anal. Chem. 2016, 88, 7268–7273. [Google Scholar] [CrossRef] [PubMed]
- Wannapob, R.; Thavarungkul, P.; Dawan, S.; Numnuam, A.; Limbut, W.; Kanatharana, P. A simple and highly stable porous gold-based electrochemical sensor for bisphenol A detection. Electroanalysis 2017, 29, 472–480. [Google Scholar] [CrossRef]
- Li, X.; Chu, S.; Fu, S.; Ma, L.; Liu, X.; Xu, X. Off-line concentration of bisphenol A and three alkylphenols by SPE then on-line concentration and rapid separation by reverse-migration micellar electrokinetic chromatography. Chromatographia 2005, 61, 161–166. [Google Scholar] [CrossRef]
- Vilchez, J.L.; Zafra, A.; Gonzalez-Casado, A.; Hontorio, E.; del Olmo, M. Determination of trace amounts of bisphenol f, bisphenol A and their diglycidyl ethers in wastewater by gas chromatography-mass spectrometry. Anal. Chim. Acta 2001, 431, 31–40. [Google Scholar] [CrossRef]
- Sungur, S.; Koroglu, M.; Ozkan, A. Determination of bisphenol A migrating from canned food and beverages in markets. Food Chem. 2014, 142, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Li, Y.; Zhao, L.; Lin, J.-M. Determination of estrogens and bisphenol A in bovine milk by automated on-line C30 solid-phase extraction coupled with high-performance liquid chromatography–mass spectrometry. J. Chromatogr. A 2009, 1216, 7539–7545. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hashi, Y.; Pan, F.; Yao, J.; Song, G.; Lin, J.-M. Automated on-line liquid chromatography–photodiode array–mass spectrometry method with dilution line for the determination of bisphenol A and 4-octylphenol in serum. J. Chromatogr. A 2006, 1133, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Messaoud, N.B.; Ghica, M.E.; Dridi, C.; Ali, M.B.; Brett, C.M. Electrochemical sensor based on multiwalled carbon nanotube and gold nanoparticle modified electrode for the sensitive detection of bisphenol A. Sens. Actuators B Chem. 2017, 253, 513–522. [Google Scholar] [CrossRef]
- Tan, F.; Cong, L.; Li, X.; Zhao, Q.; Zhao, H.; Quan, X.; Chen, J. An electrochemical sensor based on molecularly imprinted polypyrrole/graphene quantum dots composite for detection of bisphenol A in water samples. Sens. Actuators B Chem. 2016, 233, 599–606. [Google Scholar] [CrossRef]
- Dadkhah, S.; Ziaei, E.; Mehdinia, A.; Kayyal, T.B.; Jabbari, A. A glassy carbon electrode modified with amino-functionalized graphene oxide and molecularly imprinted polymer for electrochemical sensing of bisphenol A. Microchim. Acta 2016, 183, 1933–1941. [Google Scholar] [CrossRef]
- Liao, Y.; Zhao, Z. Effects of phosphoric acid and ageing time on solvent extraction behavior of phosphotungstic acid. Hydrometallurgy 2017, 169, 515–519. [Google Scholar] [CrossRef]
- Gan, T.; Hu, C.; Chen, Z.; Hu, S. Fabrication and application of a novel plant hormone sensor for the determination of methyl jasmonate based on self-assembling of phosphotungstic acid-graphene oxide nanohybrid on graphite electrode. Sens. Actuators B Chem. 2010, 151, 8–14. [Google Scholar] [CrossRef]
- Xia, X.; Shen, X.; Du, Y.; Ye, W.; Wang, C. Study on glutathione’s inhibition to dopamine polymerization and its application in dopamine determination in alkaline environment based on silver selenide/molybdenum selenide/glassy carbon electrode. Sens. Actuators B Chem. 2016, 237, 685–692. [Google Scholar] [CrossRef]
- Huang, K.-J.; Zhang, J.-Z.; Cai, J.-L. Preparation of porous layered molybdenum selenide-graphene composites on Ni foam for high-performance supercapacitor and electrochemical sensing. Electrochim. Acta 2015, 180, 770–777. [Google Scholar] [CrossRef]
- Shi, R.; Liang, J.; Zhao, Z.; Liu, A.; Tian, Y. An electrochemical bisphenol A sensor based on one step electrochemical reduction of cuprous oxide wrapped graphene oxide nanoparticles modified electrode. Talanta 2017, 169, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Kang, T.; Lu, L.; Shen, F.; Cheng, S. A novel electrochemical sensor based on gold nanoparticles and molecularly imprinted polymer with binary functional monomers for sensitive detection of bisphenol A. J. Electroanal. Chem. 2017, 786, 102–111. [Google Scholar] [CrossRef]
- Toh, S.Y.; Loh, K.S.; Kamarudin, S.K.; Daud, W.R.W. Graphene production via electrochemical reduction of graphene oxide: Synthesis and characterisation. Chem. Eng. J. 2014, 251, 422–434. [Google Scholar] [CrossRef]
- Hossain, M.M.; Aldous, L. Polyoxometalates as solution-phase electrocatalytic mediators for reduced electrode fouling and the improved oxidative response of phenols. Electrochem. Commun. 2016, 69, 32–35. [Google Scholar] [CrossRef]
- Najafi, M.; Khalilzadeh, M.A.; Karimi-Maleh, H. A new strategy for determination of bisphenol A in the presence of Sudan I using a ZNO/CNTs/ionic liquid paste electrode in food samples. Food Chem. 2014, 158, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Cao, Y.; Cao, G. Electrochemical sensor based on magnetic molecularly imprinted nanoparticles at surfactant modified magnetic electrode for determination of bisphenol A. Biosens. Bioelectron. 2014, 54, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, Y.; Chang, L.; Zhou, L.; Tang, F.; Wu, X. Β-cyclodextrin non-covalently modified ionic liquid-based carbon paste electrode as a novel voltammetric sensor for specific detection of bisphenol A. Sens. Actuators B Chem. 2013, 186, 648–656. [Google Scholar] [CrossRef]
- Zheng, Z.; Du, Y.; Wang, Z.; Feng, Q.; Wang, C. Pt/graphene–CNTs nanocomposite based electrochemical sensors for the determination of endocrine disruptor bisphenol A in thermal printing papers. Analyst 2013, 138, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Yang, W.; Wang, G.; Ren, J.; Guo, H.; Gao, J. A novel electrochemical sensor of bisphenol A based on stacked graphene nanofibers/gold nanoparticles composite modified glassy carbon electrode. Electrochim. Acta 2013, 98, 167–175. [Google Scholar] [CrossRef]
- Cosio, M.S.; Pellicanò, A.; Brunetti, B.; Fuenmayor, C.A. A simple hydroxylated multi-walled carbon nanotubes modified glassy carbon electrode for rapid amperometric detection of bisphenol A. Sens. Actuators B Chem. 2017, 246, 673–679. [Google Scholar] [CrossRef]
- Zhan, T.; Song, Y.; Tan, Z.; Hou, W. Electrochemical bisphenol A sensor based on exfoliated Ni2Al-layered double hydroxide nanosheets modified electrode. Sens. Actuators B Chem. 2017, 238, 962–971. [Google Scholar] [CrossRef]
- Huang, K.-J.; Liu, Y.-J.; Liu, Y.-M.; Wang, L.-L. Molybdenum disulfide nanoflower-chitosan-Au nanoparticles composites based electrochemical sensing platform for bisphenol A determination. J. Hazard. Mater. 2014, 276, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Gallart-Ayala, H.; Moyano, E.; Galceran, M.T. Liquid chromatography/multi-stage mass spectrometry of bisphenol A and its halogenated derivatives. Rapid Commun. Mass Spectrom. 2007, 21, 4039–4048. [Google Scholar] [CrossRef] [PubMed]

| Modified Electrode | Linear Range (µM) | Detection Limit (µM) | Repeatability (RSD) | Reproducibility (RSD) | Medium | Reference |
|---|---|---|---|---|---|---|
| Magnetic non-imprinted NPs/CPE | 0.6–100 | 0.1 | n = 7, 1.4% | 4.3% | water | [34] |
| polypyrrole/graphene quantum dots/GCE | 0.1-50 | 0.04 | 2.2% | - | water | [23] |
| AuNPs/MoS2/GCE | 0.05–100 | 0.005 | n = 10, 2.35% | 4.72% | rubber, water | [40] |
| β-cyclodextrin (β-CD)-ionic liquid/CPE | 0.1–11 | 0.083 | n = 6, 2.35% | 5.09% | water, food package | [35] |
| Pt/graphene-CNTs | 0.06–10, 10–80 | 0.042 | n = 5, 5.3% | - | thermal printing papers | [36] |
| AuNPs/tacked graphene nanofibers/GCE | 0.08–250 | 0.035 | n = 10, 2.55% | 4.46% | bottles | [37] |
| Ni2Al layered double hydroxide/GCE | 0.02–1.51 | 0.0068 | n = 5, 4.3% | - | milk | [39] |
| Hydroxylated-MWCNT/GCE | 1–24 | 0.81 | n = 11, 1.81% | - | water, bottles | [38] |
| MoSe2/rGO | 0.1–100 | 0.012 | n = 5, 0.429% | 2.2% | beverages | This work |
| Sample | Added (µM) | Found (μM, n = 5) | RSD (%, n = 5) | Recovery (%) |
|---|---|---|---|---|
| Liquid milk | 0 | <LOD | ||
| 10 | 10.08 ± 0.243 | 2.40 | 100.8 | |
| 15 | 15.09 ± 0.067 | 0.45 | 100.6 | |
| 20 | 20.69 ± 0.361 | 1.18 | 103.5 | |
| Orange juice | 0 | 1.7 | ||
| 10 | 10.16 ± 0.102 | 1.00 | 101.6 | |
| 15 | 15.66 ± 0.523 | 3.34 | 104.4 | |
| 20 | 20.73 ± 0.264 | 1.27 | 103.7 | |
| Coffee | 0 | <LOD | ||
| 10 | 10.30 ± 0.282 | 2.75 | 103.0 | |
| 15 | 15.21 ± 0.212 | 1.39 | 101.4 | |
| 20 | 20.98 ± 0.552 | 2.62 | 104.9 | |
| Soybean milk | 0 | <LOD | ||
| 10 | 9.88 ± 0.145 | 1.47 | 98.8 | |
| 15 | 15.41 ± 0.260 | 1.68 | 102.7 | |
| 20 | 20.47 ± 0.250 | 1.25 | 102.4 | |
| Prepared juice | 0 | <LOD | ||
| 10 | 10.64 ± 0.306 | 2.87 | 106.4 | |
| 15 | 14.95 ± 0.342 | 2.29 | 99.8 | |
| 20 | 20.57 ± 0.291 | 1.42 | 102.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, R.; Liang, J.; Zhao, Z.; Liu, Y.; Liu, A. In Situ Determination of Bisphenol A in Beverage Using a Molybdenum Selenide/Reduced Graphene Oxide Nanoparticle Composite Modified Glassy Carbon Electrode. Sensors 2018, 18, 1660. https://doi.org/10.3390/s18051660
Shi R, Liang J, Zhao Z, Liu Y, Liu A. In Situ Determination of Bisphenol A in Beverage Using a Molybdenum Selenide/Reduced Graphene Oxide Nanoparticle Composite Modified Glassy Carbon Electrode. Sensors. 2018; 18(5):1660. https://doi.org/10.3390/s18051660
Chicago/Turabian StyleShi, Rongguang, Jing Liang, Zongshan Zhao, Yi Liu, and Aifeng Liu. 2018. "In Situ Determination of Bisphenol A in Beverage Using a Molybdenum Selenide/Reduced Graphene Oxide Nanoparticle Composite Modified Glassy Carbon Electrode" Sensors 18, no. 5: 1660. https://doi.org/10.3390/s18051660
APA StyleShi, R., Liang, J., Zhao, Z., Liu, Y., & Liu, A. (2018). In Situ Determination of Bisphenol A in Beverage Using a Molybdenum Selenide/Reduced Graphene Oxide Nanoparticle Composite Modified Glassy Carbon Electrode. Sensors, 18(5), 1660. https://doi.org/10.3390/s18051660