Using the Transient Response of WO3 Nanoneedles under Pulsed UV Light in the Detection of NH3 and NO2
Abstract
:1. Introduction
2. Materials and Methods
3. Results
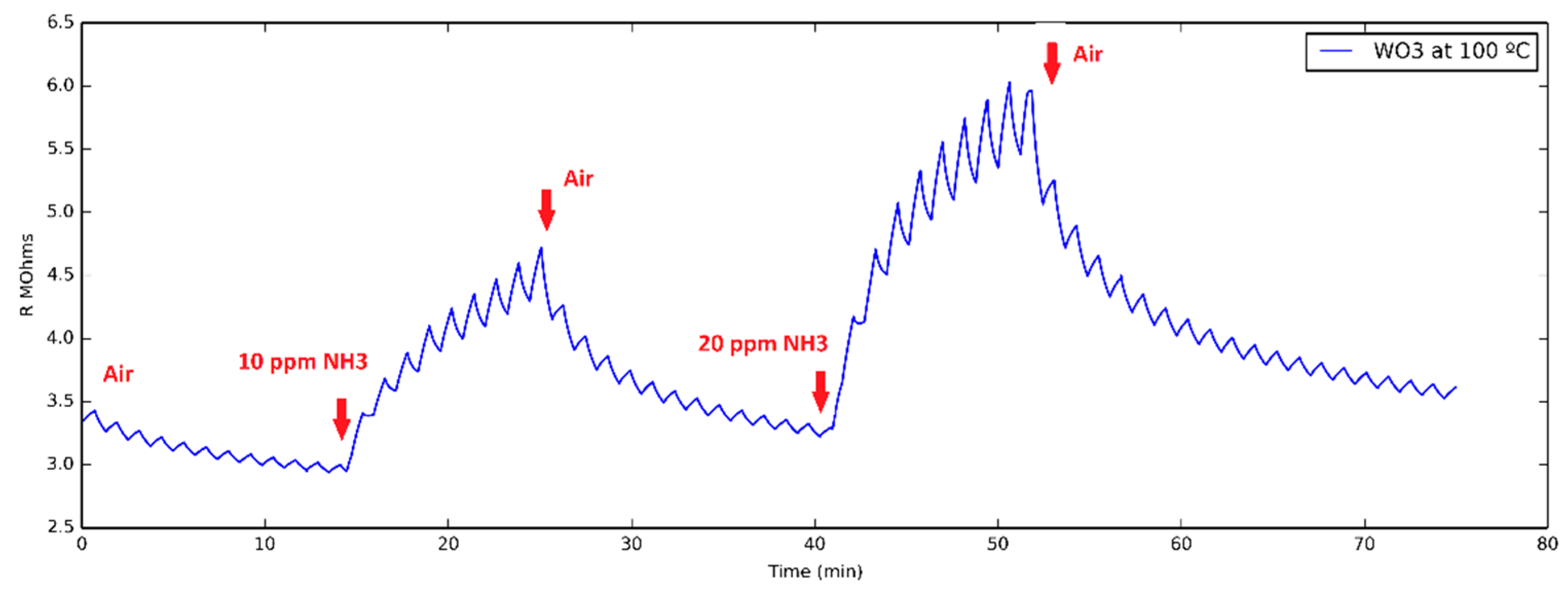
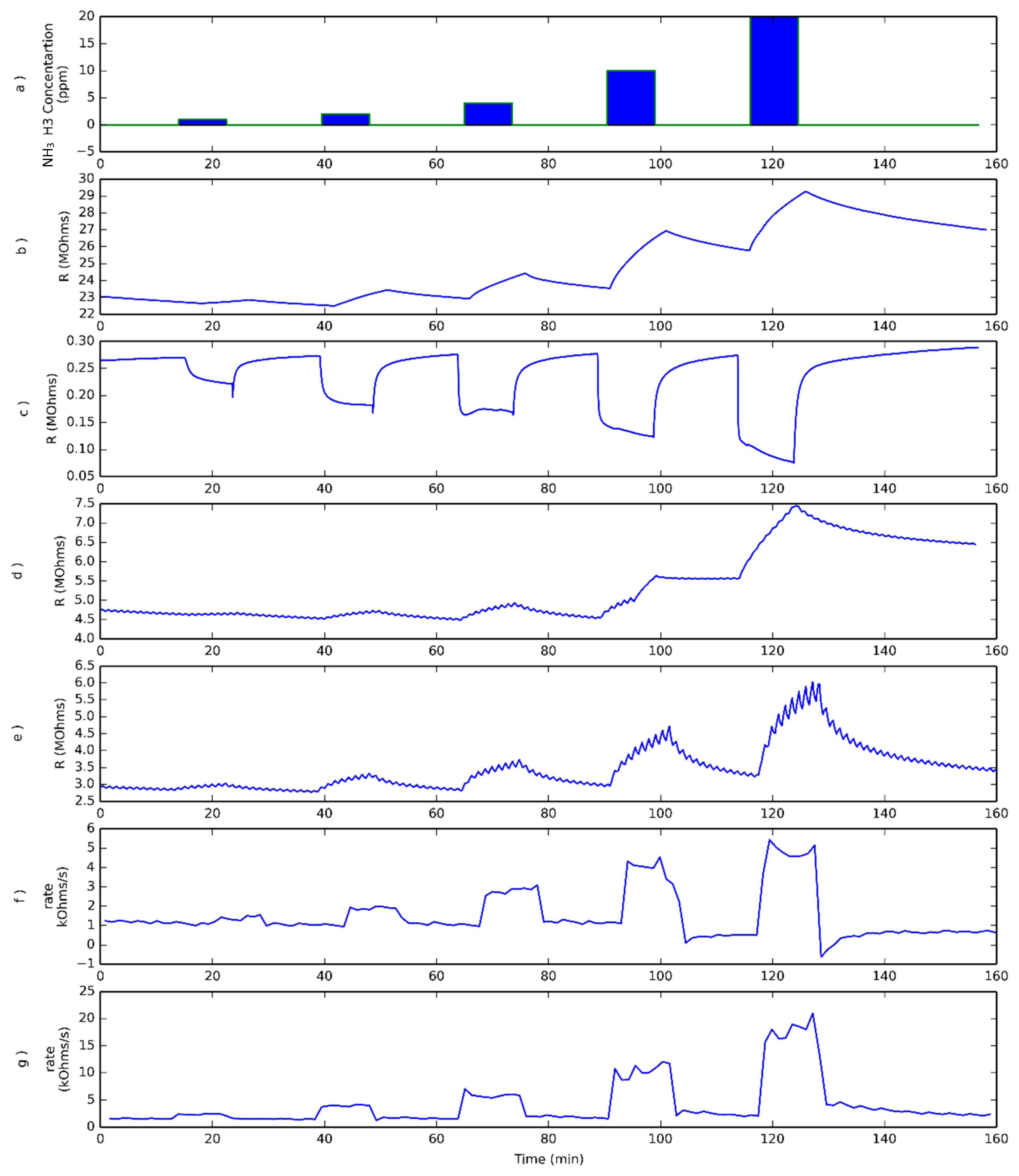
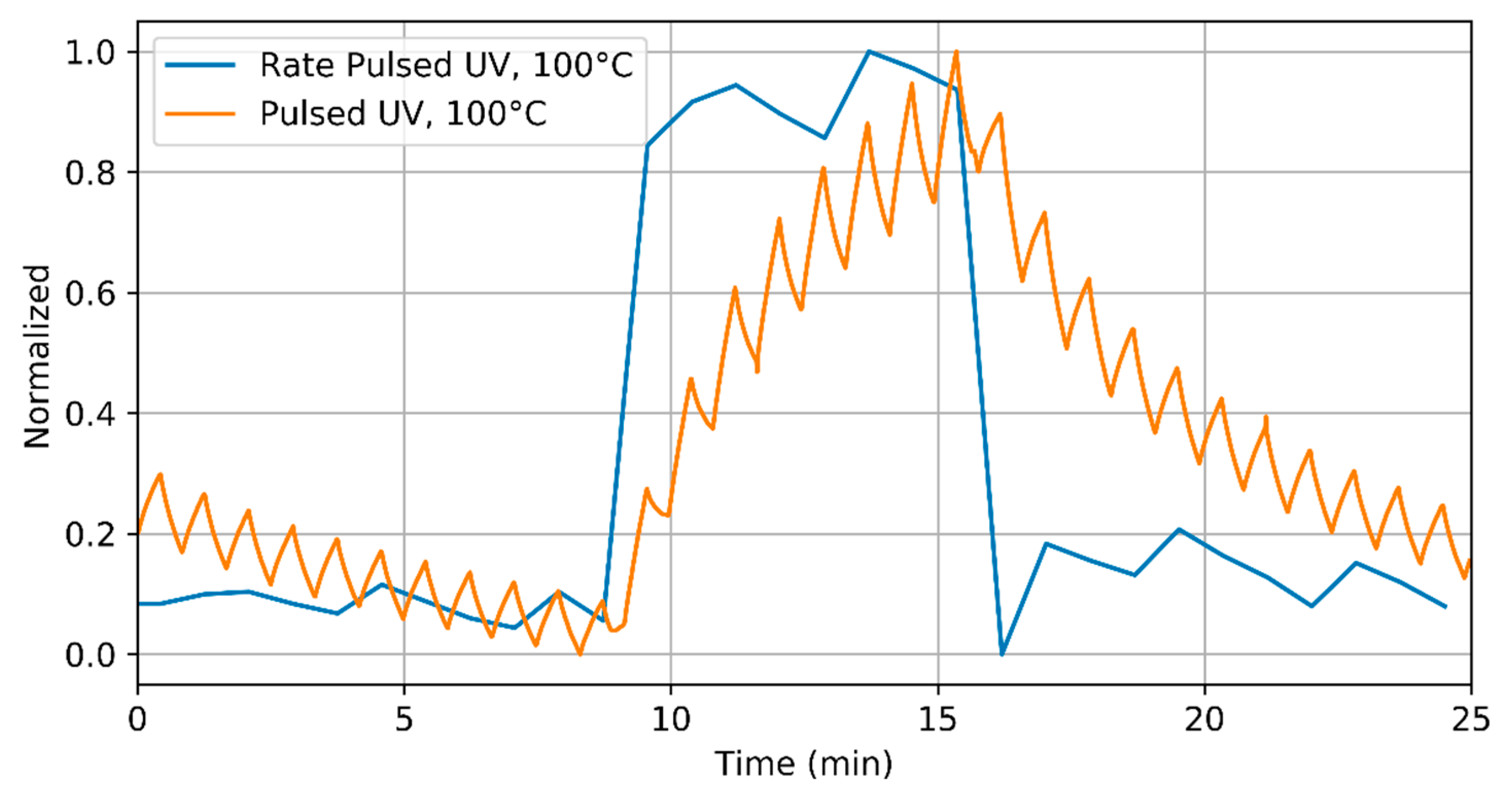
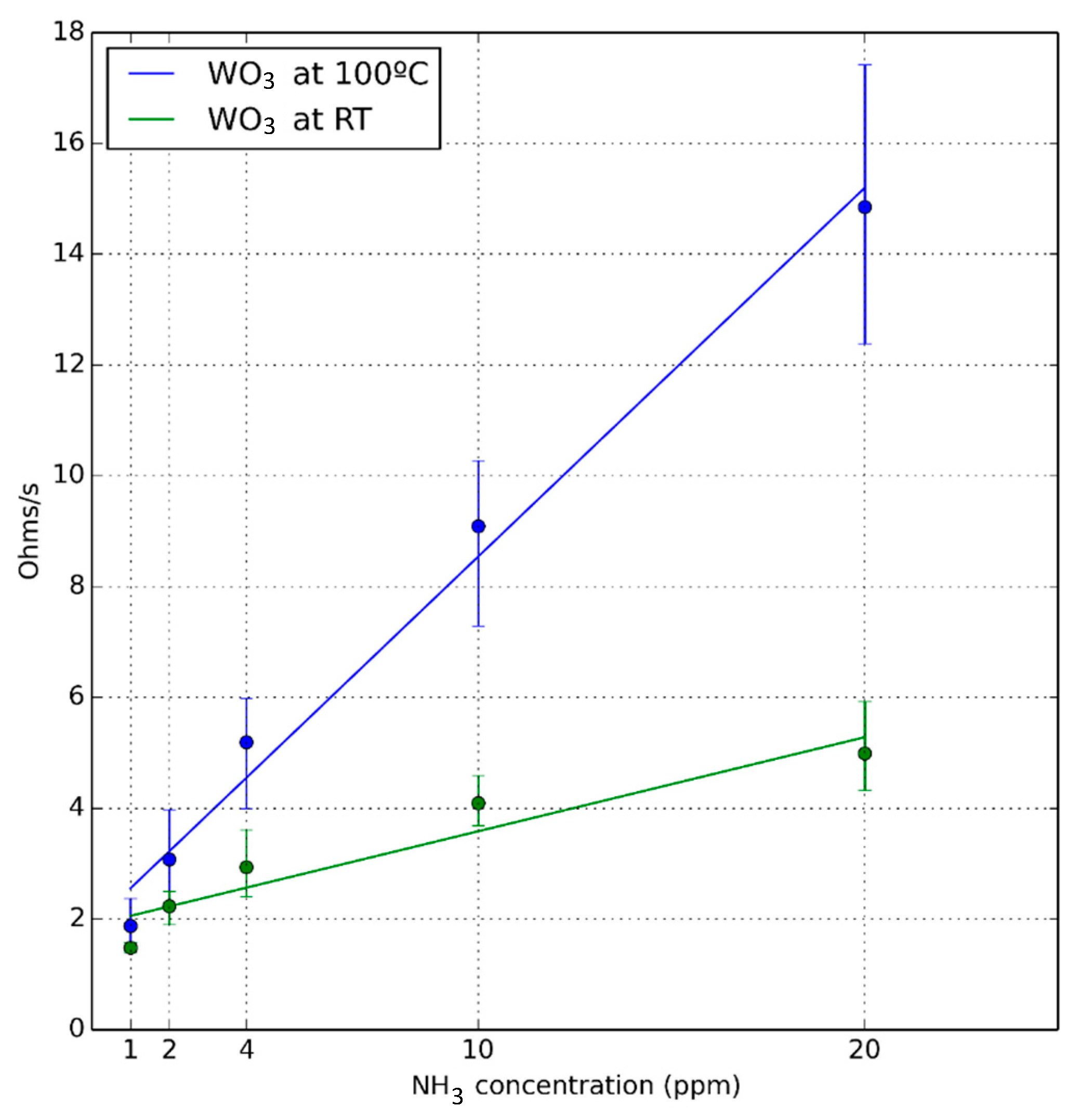
3.1. Ammonia Detection
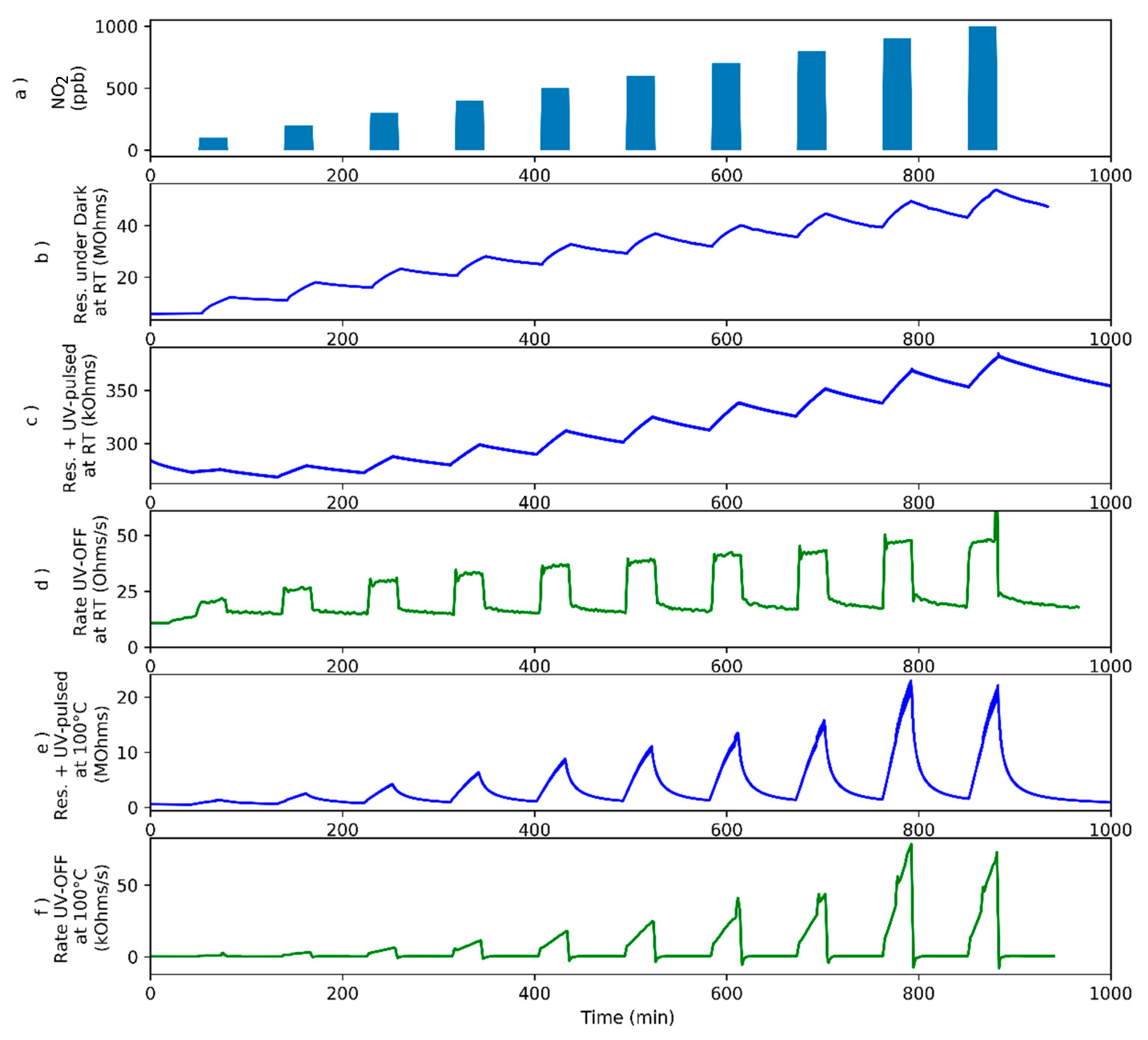
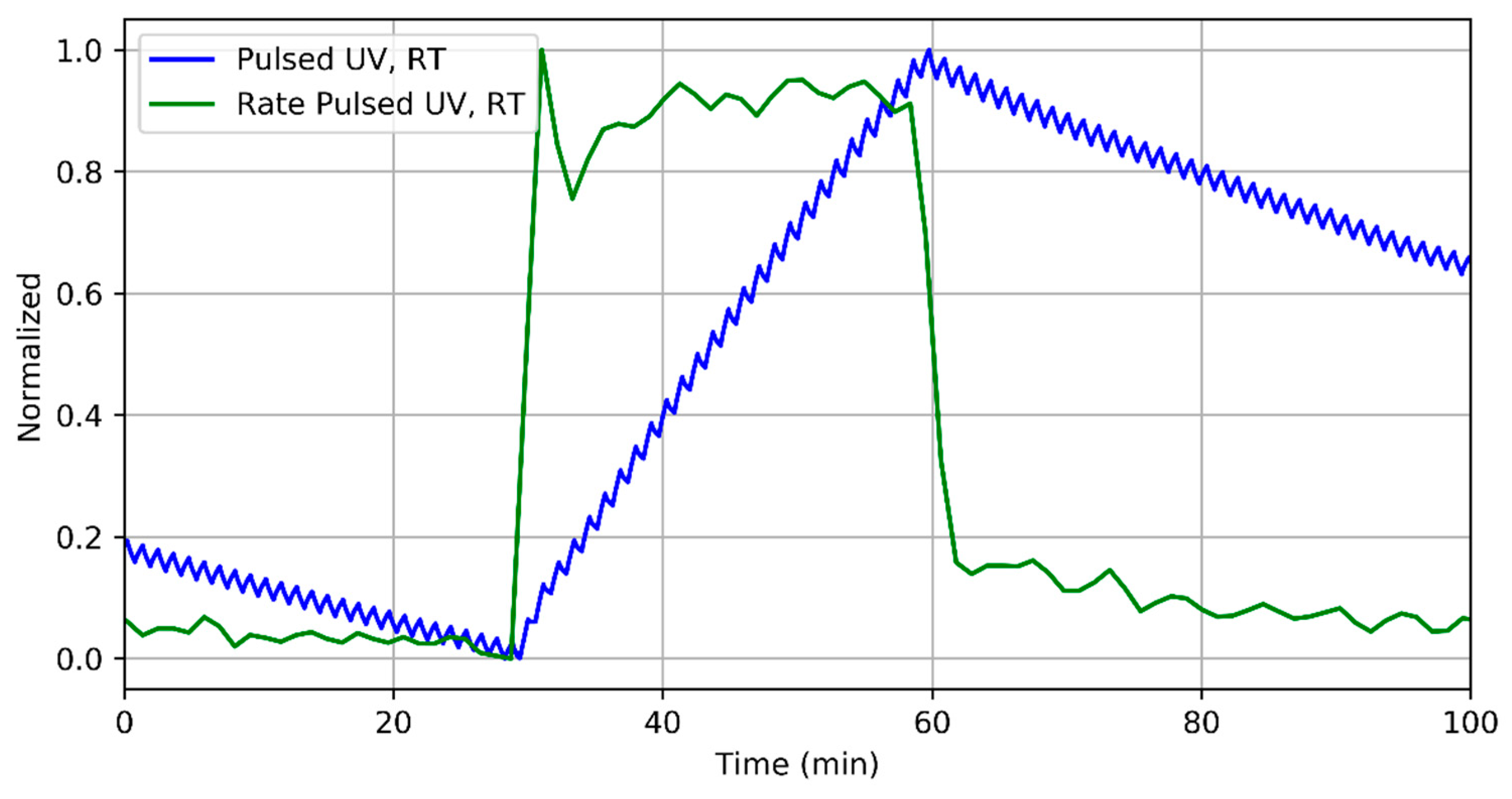
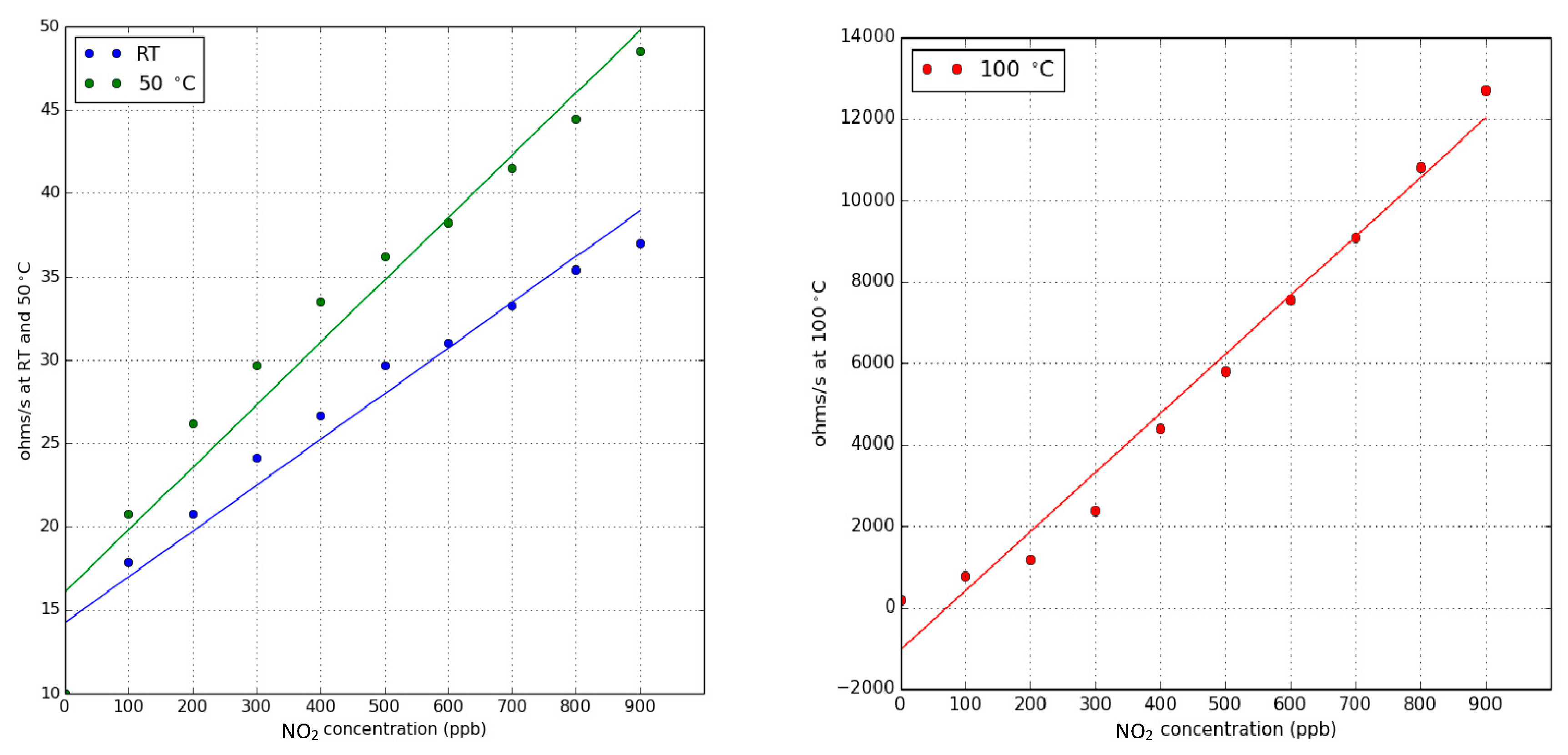
3.2. Nitrogen Dioxide Detection
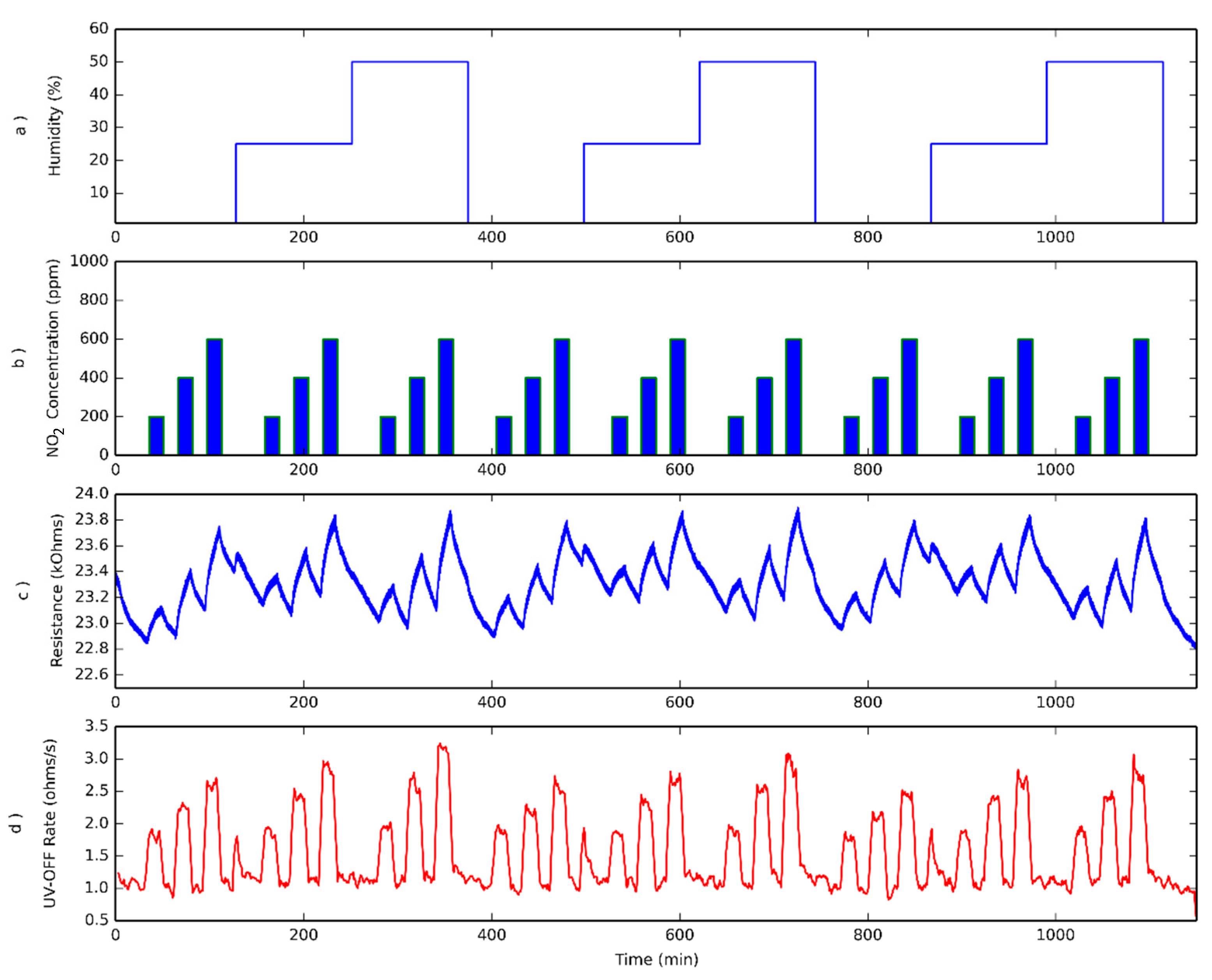
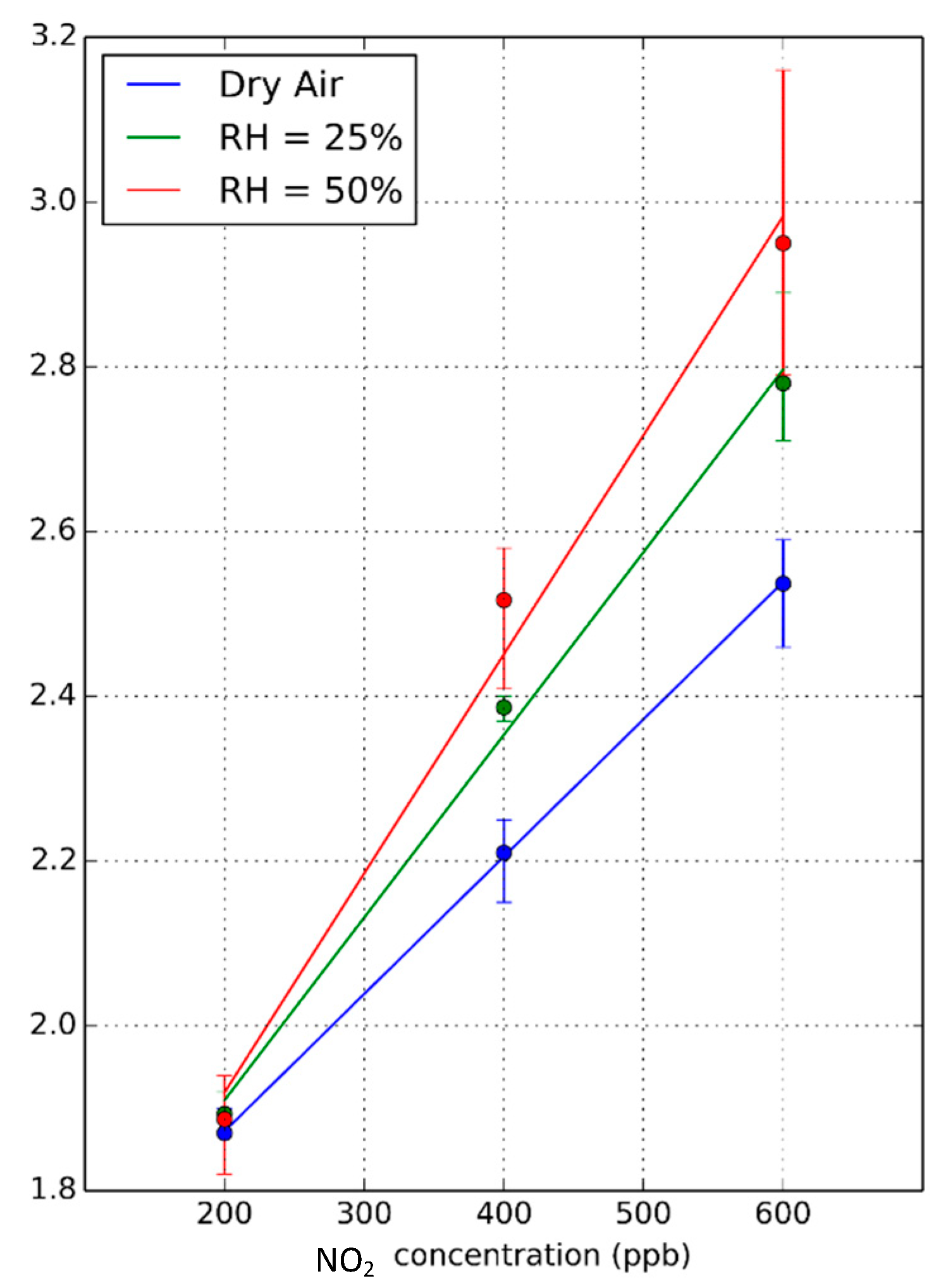
3.3. Humidity Effect
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Llobet, E.; Vilanova, X.; Brezmes, J.; Alcubilla, R.; Calderer, J.; Sueiras, J.E.; Correig, X. Conductance-transient analysis of thick-flint tin oxide gas sensors under successive gas-injection steps. Meas. Sci. Technol. 1997, 8, 1133–1138. [Google Scholar] [CrossRef]
- Llobet, E.; Brezmes, J.; Vilanova, X.; Sueiras, J.E.; Correig, X. Qualitative and quantitative analysis of volatile organic compounds using transient and steady-state responses of a thick-film tin oxide gas sensor array. Sens. Actuators B 1997, 41, 13–21. [Google Scholar] [CrossRef]
- Llobet, E.; Vilanova, X.; Brezmes, J.; Sueiras, J.E.; Alcubilla, R.; Correig, X. Steady-state and transient behavior of thick-film tin oxide sensors in the presence of gas mixtures. J. Electrochem. Soc. 1998, 145, 1772–1779. [Google Scholar] [CrossRef]
- Llobet, E.; Vilanova, X.; Brezmes, J.; Sueiras, J.E.; Correig, X. Transient response of thick-film tin oxide gas-sensors to multicomponent gas mixtures. Sens. Actuators B Chem. 1998, 47, 104–112. [Google Scholar] [CrossRef]
- Gutierrez-Osuna, R.; Nagle, H.T.; Schiffman, S.S. Transient response analysis of an electronic nose using multi-exponential models. Sens. Actuators B Chem. 1999, 61, 170–182. [Google Scholar] [CrossRef]
- Ngo, K.A.; Lauque, P.; Aguir, K. Identification of toxic gases using steady-state and transient responses of gas sensor array. Sens. Mater. 2006, 18, 251–260. [Google Scholar]
- Clifford, P.K.; Tuma, D.T. Characteristics of semiconductor gas sensors 2. Transient-response to temperature-change. Sens. Actuators 1983, 3, 255–281. [Google Scholar] [CrossRef]
- Kato, Y.; Yoshikawa, K.; Kitora, M. Temperature-dependent dynamic response enables the qualification and quantification of gases by a single sensor. Sens. Actuators B Chem. 1997, 40, 33–37. [Google Scholar] [CrossRef]
- Kohler, H.; Rober, J.; Link, N.; Bouzid, I. New applications of tin oxide gas sensors: I. Molecular identification by cyclic variation of the working temperature and numerical analysis of the signals. Sens. Actuators B Chem. 1999, 61, 163–169. [Google Scholar] [CrossRef]
- Gutierrez-Osuna, R.; Gutierrez-Galvez, A.; Powar, N. Transient response analysis for temperature-modulated chemoresistors. Sens. Actuators B Chem. 2003, 93, 57–66. [Google Scholar] [CrossRef]
- Yamada, Y.; Ogita, M. Transient response of resistive-type NO2 sensor on temperature change. Sens. Actuators B Chem. 2003, 93, 546–551. [Google Scholar] [CrossRef]
- Parret, F.; Menini, P.; Martinez, A.; Soulantica, K.; Maisonnat, A.; Chaudret, B. Improvement of micromachined SnO2 gas sensors selectivity by optimised dynamic temperature operating mode. Sens. Actuators B Chem. 2006, 118, 276–282. [Google Scholar] [CrossRef]
- Camagni, P.; Faglia, G.; Galinetto, P.; Perego, C.; Samoggia, G.; Sberveglieri, G. Photosensitivity activation of SnO2 thin film gas sensors at room temperature. Sens. Actuators B Chem. 1996, 31, 99–103. [Google Scholar] [CrossRef]
- Comini, E.; Cristalli, A.; Faglia, G.; Sberveglieri, G. Light enhanced gas sensing properties of indium oxide and tin dioxide sensors. Sens. Actuators B Chem. 2000, 65, 260–263. [Google Scholar] [CrossRef]
- Comini, E.; Faglia, G.; Sberveglieri, G. UV light activation of tin oxide thin films for NO2 sensing at low temperatures. Sens. Actuators B Chem. 2001, 78, 73–77. [Google Scholar] [CrossRef]
- Lee, H.C.; Hwang, W.S. Enhancing the sensitivity of oxygen sensors through the photocatalytic effect of SnO2/TiO2 film. Mater. Trans. 2005, 46, 1942–1949. [Google Scholar] [CrossRef]
- Manera, M.G.; Taurino, A.; Catalano, M.; Rella, R.; Caricato, A.P.; Buonsanti, R.; Cozzoli, P.D.; Martino, M. Enhancement of the optically activated NO2 gas sensing response of brookite TiO2 nanorods/nanoparticles thin films deposited by matrix-assisted pulsed-laser evaporation. Sens. Actuators B Chem. 2012, 161, 869–879. [Google Scholar] [CrossRef]
- Zhang, S.N.; Lei, T.; Li, D.; Zhang, G.Z.; Xie, C.S. UV light activation of TiO2 for sensing formaldehyde: How to be sensitive, recovering fast, and humidity less sensitive. Sens. Actuators B Chem. 2014, 202, 964–970. [Google Scholar] [CrossRef]
- Ge, C.Q.; Xie, C.S.; Hu, M.L.; Gui, Y.H.; Bai, Z.K.; Zeng, D.W. Structural characteristics and UV-light enhanced gas sensitivity of La-doped ZnO nanoparticles. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2007, 141, 43–48. [Google Scholar] [CrossRef]
- Costello, B.P.J.; Ewen, R.J.; Ratcliffe, N.M.; Richards, M. Highly sensitive room temperature sensors based on the UV-LED activation of zinc oxide nanoparticles. Sens. Actuators B Chem. 2008, 134, 945–952. [Google Scholar] [CrossRef]
- Peng, L.; Zhao, Q.D.; Wang, D.J.; Zhai, J.L.; Wang, P.; Pang, S.; Xie, T.F. Ultraviolet-assisted gas sensing: A potential formaldehyde detection approach at room temperature based on zinc oxide nanorods. Sens. Actuators B Chem. 2009, 136, 80–85. [Google Scholar] [CrossRef]
- Fan, S.W.; Srivastava, A.K.; Dravid, V.P. UV-activated room-temperature gas sensing mechanism of polycrystalline ZnO. Appl. Phys. Lett. 2009, 95, 142106. [Google Scholar] [CrossRef]
- Peng, L.A.; Zhai, J.L.; Wang, D.J.; Zhang, Y.; Wang, P.; Zhao, Q.D.; Xie, T.F. Size- and photoelectric characteristics-dependent formaldehyde sensitivity of ZnO irradiated with UV light. Sens. Actuators B Chem. 2010, 148, 66–73. [Google Scholar] [CrossRef]
- Luo, L.; Sosnowchik, B.D.; Lin, L.W. Local vapor transport synthesis of zinc oxide nanowires for ultraviolet-enhanced gas sensing. Nanotechnology 2010, 21, 495502. [Google Scholar] [CrossRef] [PubMed]
- Trawka, M.P.; Smulko, J.M.; Hasse, L.Z.; Granqvist, C.G.; Ionescu, R.; Llobet, E.; Annanouch, F.E.; Kish, L.B. UV-Light-Induced Fluctuation Enhanced Sensing by WO3-Based Gas Sensors. IEEE Sens. J. 2016, 16, 5152–5159. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Grimes, C.A. A self-cleaning, room-temperature titania-nanotube hydrogen gas sensor. Sens. Lett. 2003, 1, 42–46. [Google Scholar] [CrossRef]
- Trocino, S.; Frontera, P.; Donato, A.; Busacca, C.; Scarpino, L.A.; Antonucci, P.; Neri, G. Gas sensing properties under UV radiation of In2O3 nanostructures processed by electrospinning. Mater. Chem. Phys. 2014, 147, 35–41. [Google Scholar] [CrossRef]
- Gonzalez, O.; Roso, S.; Calavia, R.; Vilanova, X.; Llobet, E. NO2 sensing properties of thermally or UV activated In2O3 nano-octahedra. Procedia Eng. 2015, 120, 773–776. [Google Scholar] [CrossRef]
- Gonzalez, O.; Roso, S.; Vilanova, X.; Llobet, E. Enhanced detection of nitrogen dioxide via combined heating and pulsed UV operation of indium oxide nano-octahedra. Beilstein J. Nanotechnol. 2016, 7, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, O.; Welearegay, T.; Llobet, E.; Vilanova, X. Pulsed UV light activated gas sensing in tungsten oxide nanowires. Procedia Eng. 2016, 168, 351–354. [Google Scholar] [CrossRef]
- Vallejos, S.; Umek, P.; Stoycheva, T.; Annanouch, F.; Llobet, E.; Correig, X.; de Marco, P.; Bittencourt, C.; Blackman, C. Single-Step Deposition of Au- and Pt-Nanoparticle-Functionalized Tungsten Oxide Nanoneedles Synthesized Via Aerosol-Assisted CVD, and Used for Fabrication of Selective Gas Microsensor Arrays. Adv. Funct. Mater. 2013, 10, 1313–1322. [Google Scholar] [CrossRef]
- SETI Inc. UV Diodes Datasheets. 2017. Available online: http://web.sensor-ic.com:8000/zlxiazai/s-et/320nm.pdf (accessed on 22 November 2017).
- Giberti, A.; Malagù, C.; Guidi, V. WO3 sensing properties enhanced by UV illumination: An evidence of surface effect. Sens. Actuators B Chem. 2012, 165, 59–61. [Google Scholar] [CrossRef]
- Dao, D.V.; Shibuya, K.; Bui, T.T.; Sugiyama, S. Micromachined NH3 gas sensor with ppb-level sensitivity based on WO3 nanoparticles Thinfilm. Procedia Eng. 2011, 25, 1149. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Dang, D.V.; Nguyen, D.C. Hydrothermal synthesis and NH3 gas sensing property of WO3 nanorods at low temperature. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 035006. [Google Scholar] [CrossRef]
- Li, H.; Xie, W.; Ye, T.; Liu, B.; Xiao, S.; Wang, C.; Wang, Y.; Li, Q.; Wang, T. Temperature-Dependent Abnormal and Tunable p-n Response of Tungsten Oxide–Tin Oxide Based Gas Sensors. ACS Appl. Mater. Interfaces 2015, 7, 24887–24894. [Google Scholar] [CrossRef] [PubMed]
- Annanouch, F.E.; Haddi, Z.; Vallejos, S.; Umek, P.; Guttmann, P.; Bittencourt, C.; Llobet, E. Aerosol-Assisted CVD-Grown WO3 Nanoneedles Decorated with Copper Oxide Nanoparticles for the Selective and Humidity-Resilient Detection of H2S. ACS Appl. Mater. Interfaces 2015, 7, 6842–6851. [Google Scholar] [CrossRef] [PubMed]
- Annanouch, F.E.; Haddi, Z.; Ling, M.; di Maggio, F.; Vallejos, S.; Vilic, T.; Zhu, Y.; Shujah, T.; Umek, P.; Bittencourt, C.; et al. Aerosol-Assisted CVD-Grown PdO Nanoparticle-Decorated Tungsten Oxide Nanoneedles Extremely Sensitive and Selective to Hydrogen. ACS Appl. Mater. Interfaces 2016, 8, 10413–10421. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, X.; Dutta, P.K.; Wang, J. Room temperature impedance spectroscopy-based sensing of formaldehyde with porous TiO2 under UV illumination. Sens. Actuators B Chem. 2013, 185, 1–9. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez, O.; Welearegay, T.G.; Vilanova, X.; Llobet, E. Using the Transient Response of WO3 Nanoneedles under Pulsed UV Light in the Detection of NH3 and NO2. Sensors 2018, 18, 1346. https://doi.org/10.3390/s18051346
Gonzalez O, Welearegay TG, Vilanova X, Llobet E. Using the Transient Response of WO3 Nanoneedles under Pulsed UV Light in the Detection of NH3 and NO2. Sensors. 2018; 18(5):1346. https://doi.org/10.3390/s18051346
Chicago/Turabian StyleGonzalez, Oriol, Tesfalem G. Welearegay, Xavier Vilanova, and Eduard Llobet. 2018. "Using the Transient Response of WO3 Nanoneedles under Pulsed UV Light in the Detection of NH3 and NO2" Sensors 18, no. 5: 1346. https://doi.org/10.3390/s18051346
APA StyleGonzalez, O., Welearegay, T. G., Vilanova, X., & Llobet, E. (2018). Using the Transient Response of WO3 Nanoneedles under Pulsed UV Light in the Detection of NH3 and NO2. Sensors, 18(5), 1346. https://doi.org/10.3390/s18051346






