A New Method for Immobilization of His-Tagged Proteins with the Application of Low-Frequency AC Electric Field
Abstract
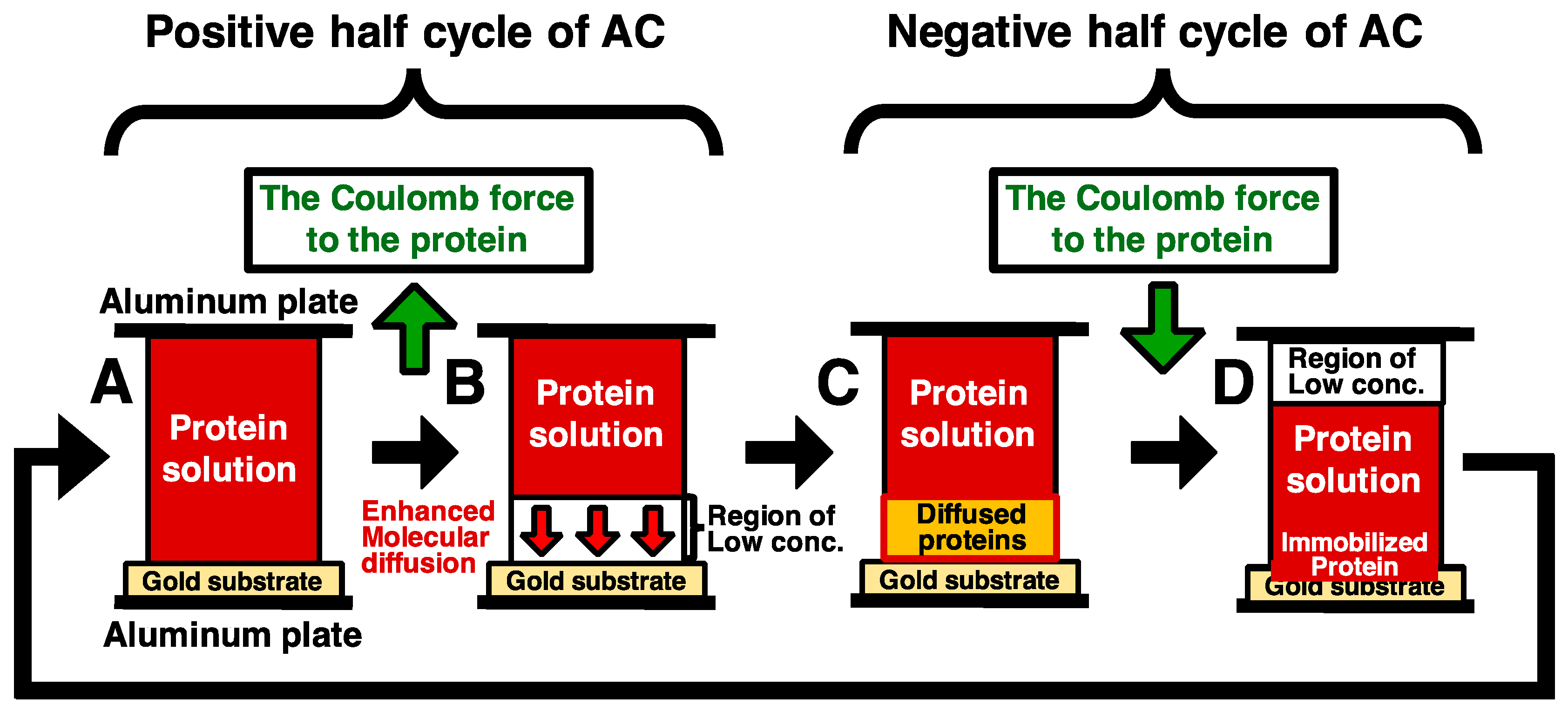
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Construction of His-Tagged DsRed Expression Vector
2.3. Purification of Reconbinant His-Tagged GFP and DsRed
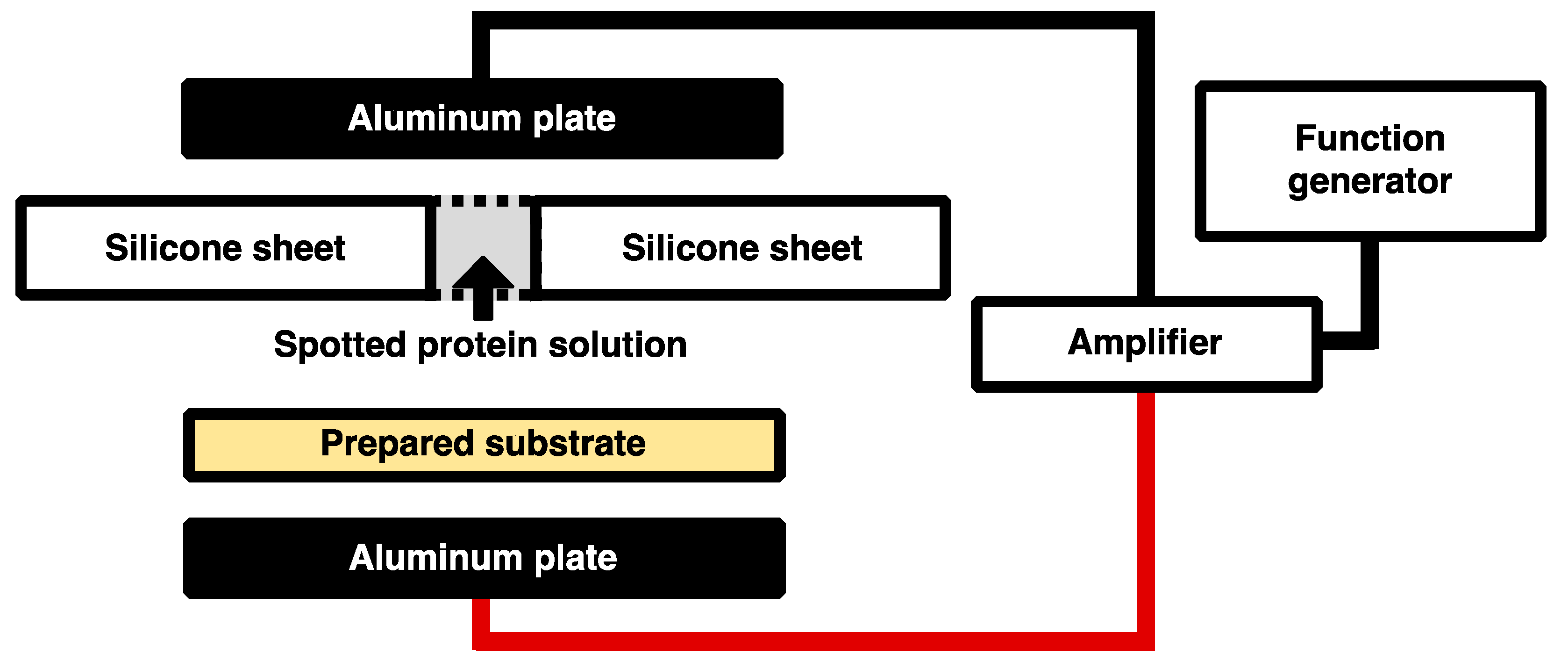
2.4. Preparation of the Modified Gold-Coated Substrate
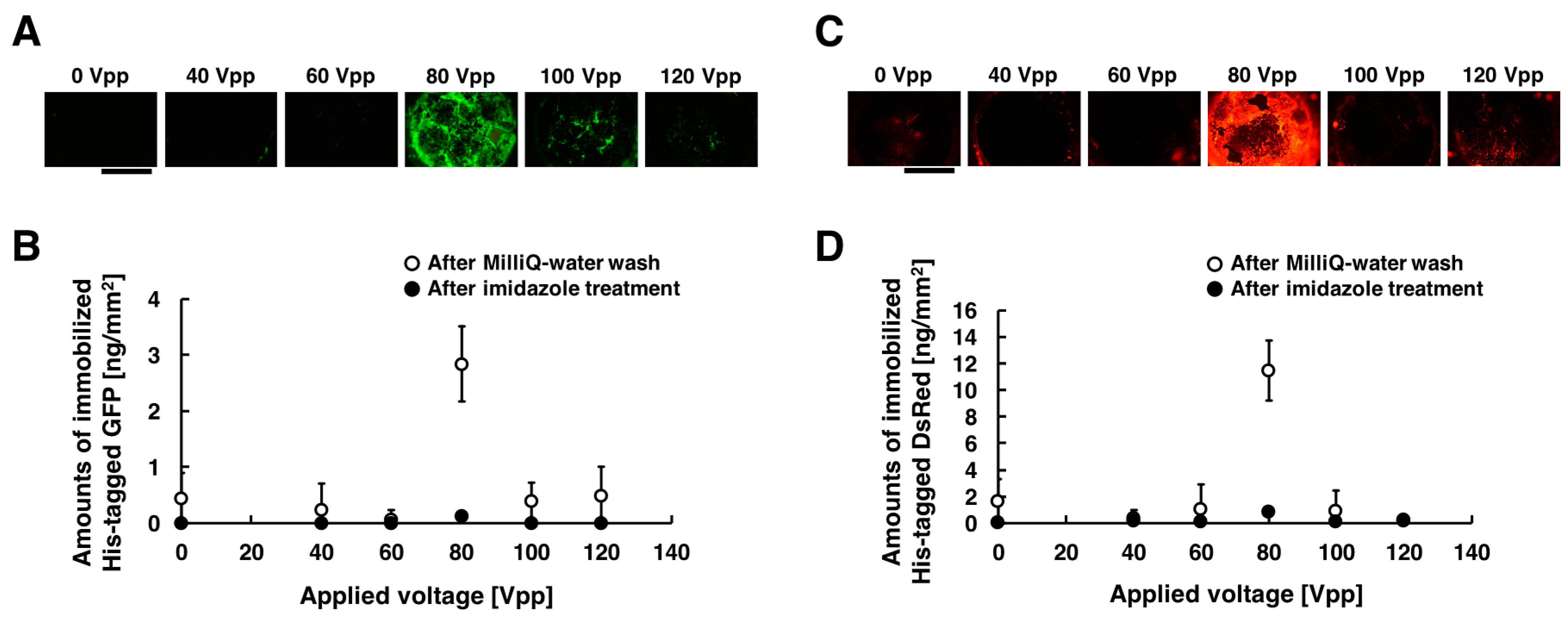
2.5. Immobilization of His-Tagged GFP and DsRed with the Application of Low-Frequency AC Electric Field
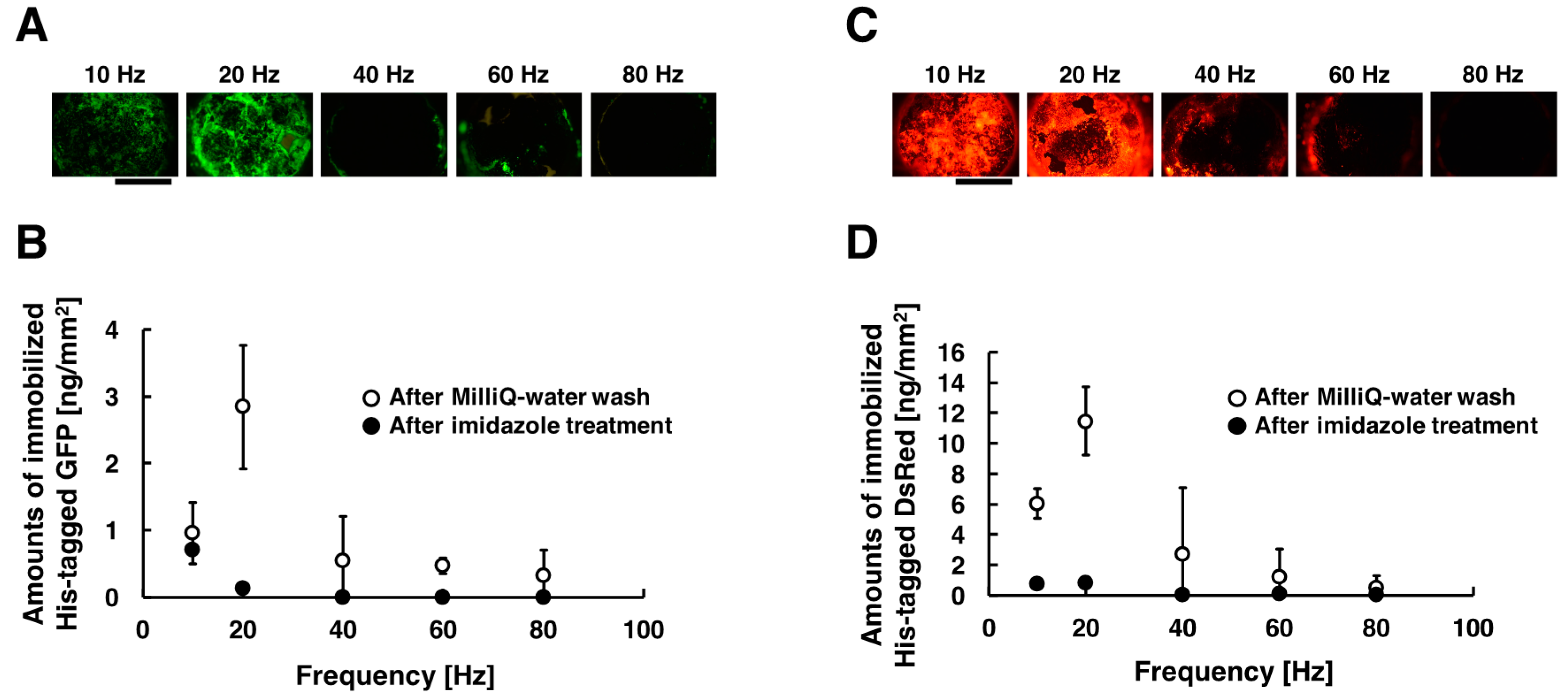
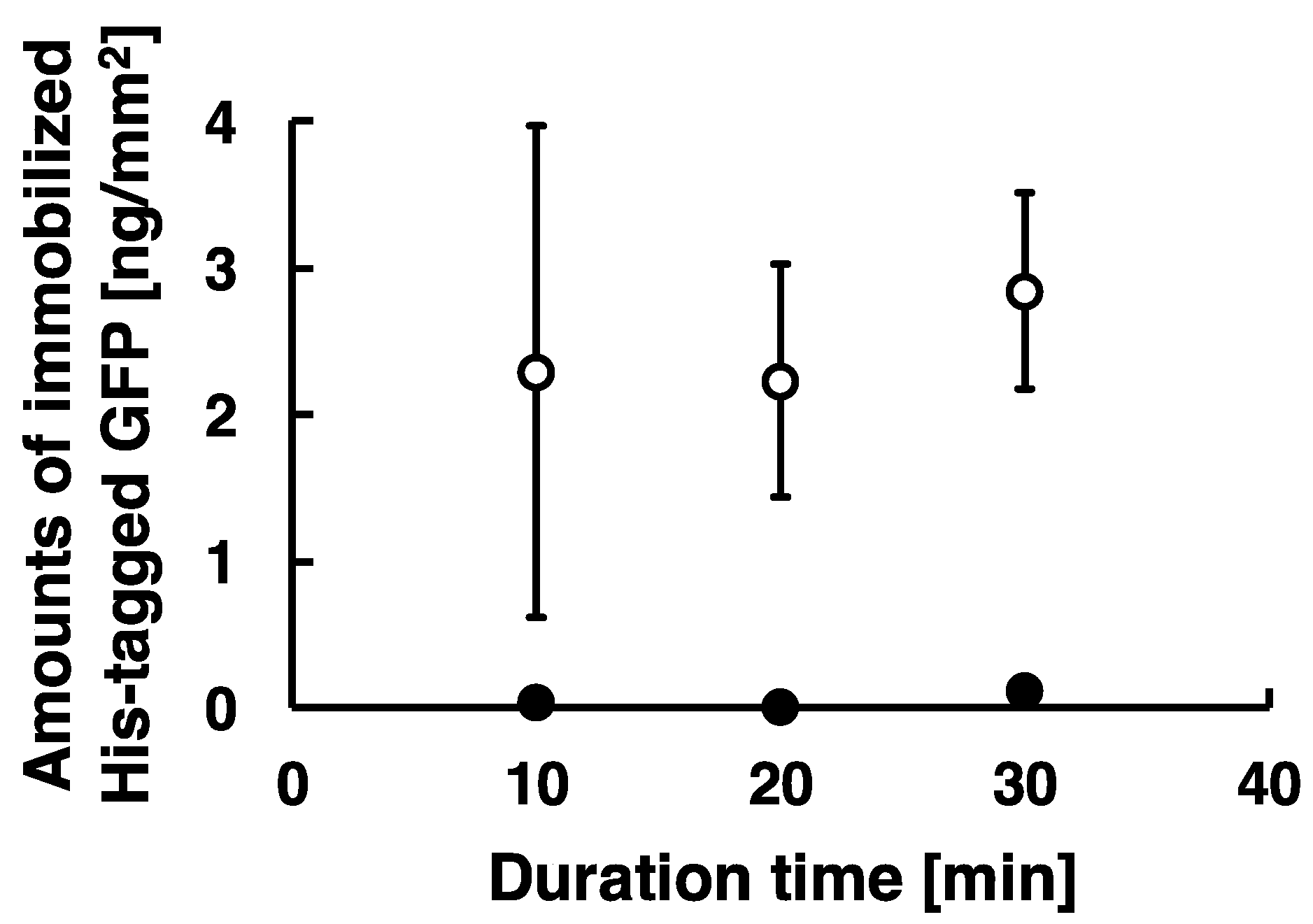
2.6. Immobilization of His-Tagged GFP with Time-Dependent Applied in Low-Frequency AC Electric Field
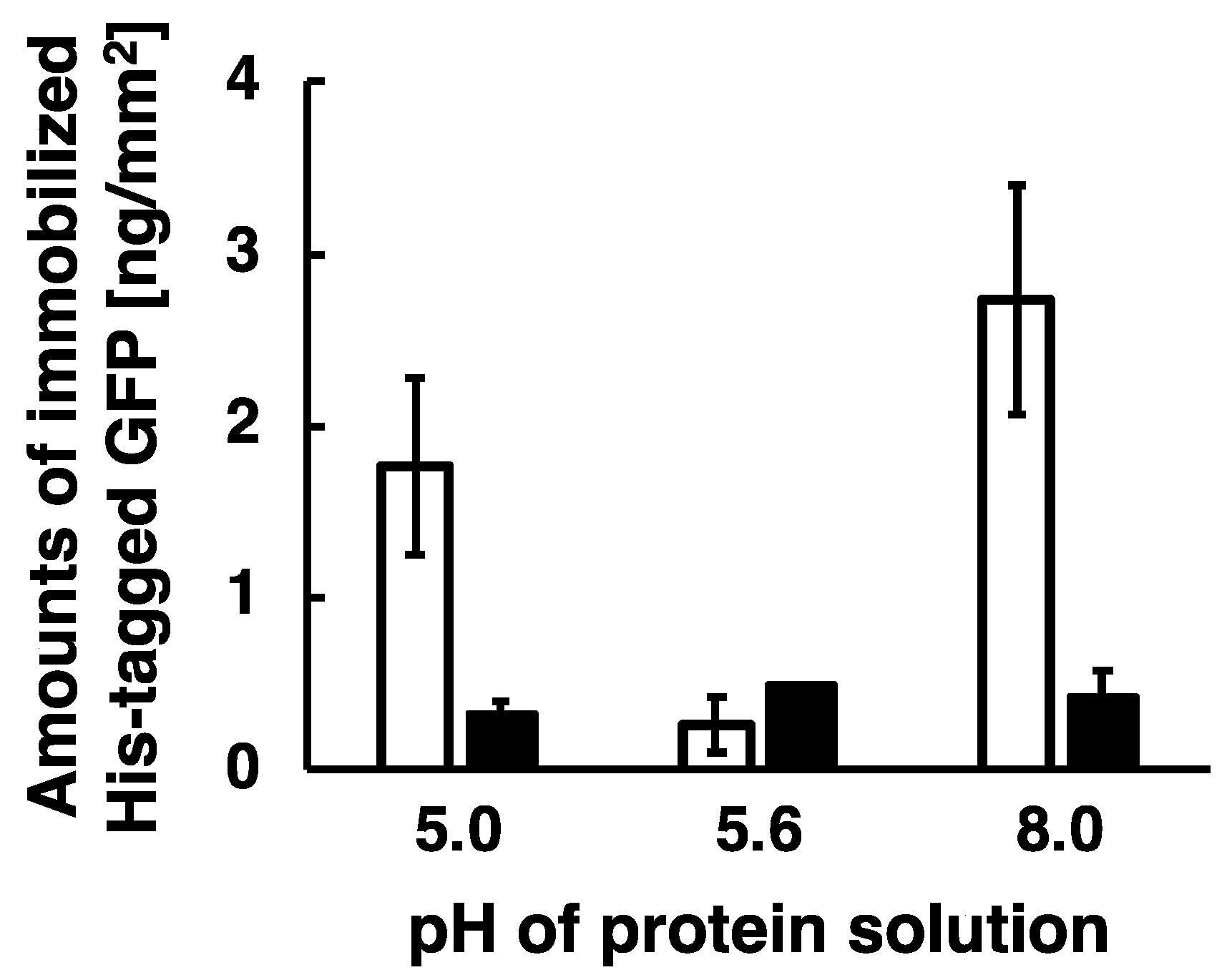
2.7. Immobilization of His-Tagged GFP Under Several pH Conditions
2.8. Observation and Determination of His-Tagged GFP and DsRed Immobilized on the Substrate Surfaces
3. Results and Discussion
3.1. Immobilization of His-Tagged GFP and DsRed with the Application of Low-Frequency AC Electric Field
3.2. Immobilization of His-Tagged GFP under Several pH Conditions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Bonroy, K.; Frederix, F.; Reekmans, G.; Dewolf, E.; De Palma, R.; Borghs, G.; Declerck, P.; Goddeeris, B. Comparison of random and oriented immobilisation of antibody fragments on mixed self-assembled monolayers. J. Immunol. Methods 2006, 312, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Anderson, H.; Myrskog, A.; Dunér, G.; Ingemarsson, B.; Aastrup, T. Optimizing immobilization on two-dimensional carboxyl surface: PH dependence of antibody orientation and antigen binding capacity. Anal. Biochem. 2010, 398, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Rusmini, F.; Zhong, Z.; Feijen, J. Protein Immobilization Strategies for Protein Biochips. Biomacromolecules 2007, 8, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.H.; Jung, S.O.; Lee, J.M.; Lee, K.Y.; Lee, Y.; Park, J.S.; Chung, B.H. Oriented immobilization of antibodies with GST-fused multiple Fc-specific B-domains on a gold surface. Anal. Chem. 2007, 79, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Park, H.K.; Jung, Y.; Kim, J.K.; Jung, S.O.; Chung, B.H. Direct immobilization of protein G variants with various numbers of cysteine residues on a gold surface. Anal. Chem. 2007, 79, 2680–2687. [Google Scholar] [CrossRef] [PubMed]
- Pyun, J.C.; Kim, S.D.; Chung, J.W. New immobilization method for immunoaffinity biosensors by using thiolated proteins. Anal. Chem. 2005, 347, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Ge, H. UPA, a Universal Protein Array System for Quantitative Detection of Protein-Protein, Protein-DNA, Protein-RNA and Protein-Ligand Interactions. Nucleic Acid Res. 2000, 28, e3. [Google Scholar] [CrossRef] [PubMed]
- Holt, L.J.; Büssow, K.; Walter, G.; Tomlinson, I.M. By-passing selection: Direct screening for antibody–antigen interactions using protein arrays. Nucleic Acids Res. 2000, 28, e72. [Google Scholar] [CrossRef] [PubMed]
- Pollak, A.; Blumenfeld, H.; Wax, M.; Baughn, R.L.; Whitesides, G.M. Enzyme immobilization by condensation copolymerization into crosslinked polyacrylamide gels. J. Am. Chem. Soc. 1980, 102, 6324–6336. [Google Scholar] [CrossRef]
- Kim, D.; Karns, K.; Tia, S.Q.; He, M.; Herr, A.E. Electrostatic protein immobilization using charged polyacrylamide gels and cationic detergent microfluidic western blotting. Anal. Chem. 2012, 84, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Kyratzis, I. Covalent immobilization of proteins on carbon nanotubes using the cross-linker 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide—A critical assessment. Bioconjug. Chem. 2008, 19, 1945–1950. [Google Scholar] [CrossRef] [PubMed]
- MacBeath, G.; Schreiber, S.L. Printing proteins as microarrays for high-throughput function determination. Science 2000, 289, 1760–1763. [Google Scholar] [PubMed]
- Camarero, J.A. Recent developments in the site-specific immobilization of proteins onto solid supports. Pept. Sci. 2008, 90, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.A.; Klok, H.A. Peptide/protein–polymer conjugates: Synthetic strategies and design concepts. Chem. Commun. 2008, 2591–2611. [Google Scholar] [CrossRef] [PubMed]
- Frasconi, M.; Mazzei, F.; Ferri, T. Protein immobilization at gold–thiol surfaces and potential for biosensing. Anal. Bioanal. Chem. 2010, 398, 1545–1564. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lee, M.; Choi, S.; Kim, J.-H.; Choi, H.-J.; Kim, S.-H.; Lee, J.; Koh, K. Comparative Study of Protein Immobilization Properties on Calixarene Monolayers. Sensors 2007, 7, 1091–1107. [Google Scholar] [CrossRef]
- Sigal, G.B.; Bamdad, C.; Barberis, A.; Strominger, J.; Whitesides, G.M. A self-assembled monolayer for the binding and study of histidine-tagged proteins by surface plasmon resonance. Anal. Chem. 1996, 68, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Samanta, D.; Sarkar, A. Immobilization of bio-macromolecules on self-assembled monolayers: Methods and sensor applications. Chem. Soc. Rev. 2011, 40, 2567–2592. [Google Scholar] [CrossRef] [PubMed]
- Ertekin, Ö.; Öztürk, S.; Öztürk, Z.Z. Label Free QCM Immunobiosensor for AFB1 Detection Using Monoclonal IgA Antibody as Recognition Element. Sensors 2016, 16, 1274. [Google Scholar] [CrossRef] [PubMed]
- Miyao, H.; Ikeda, Y.; Shiraishi, A.; Kawakami, Y.; Sueda, S. Immobilization of immunoglobulin-G-binding domain of protein A on a gold surface modified with biotin ligase. Anal. Biochem. 2015, 484, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, A.; Blomstergren, A.; Nord, O.; Lukacs, M.; Lundeberg, J.; Uhlén, M. The biotin-streptavidin interaction can be reversibly broken using water at elevated temperatures. Electrophoresis 2005, 26, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Willard, F.S.; Siderovski, D.P. Covalent immobilization of histidine-tagged proteins for surface plasmon resonance. Anal. Chem. 2006, 353, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; He, M.; Taussig, M.J. Double-hexahistidine tag with high-affinity binding for protein immobilization, purification, and detection on Ni−nitrilotriacetic acid surfaces. Anal. Chem. 2006, 78, 3072–3079. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, S.; Pei, Y.; Dong, H.; Aastrup, T.; Pei, Z. Oriented and reversible immobilization of His-tagged proteins on two-and three-dimensional surfaces for study of protein–protein interactions by a QCM biosensor. Sens. Actuators B Chem. 2016, 224, 814–822. [Google Scholar] [CrossRef]
- Lesaicherre, M.L.; Lue, R.Y.; Chen, G.Y.; Zhu, Q.; Yao, S.Q. Intein-mediated biotinylation of proteins and its application in a protein microarray. J. Am. Chem. Soc. 2002, 124, 8768–8769. [Google Scholar] [CrossRef] [PubMed]
- Girish, A.; Sun, H.; Yeo, D.S.; Chen, G.Y.; Chua, T.K.; Yao, S.Q. Site-specific immobilization of proteins in a microarray using intein-mediated protein splicing. Bioorg. Med. Chem. Lett. 2005, 15, 2447–2451. [Google Scholar] [CrossRef] [PubMed]
- Oshige, M.; Yumoto, K.; Miyata, H.; Takahashi, S.; Nakada, M.; Ito, K.; Tamegai, M.; Kawaura, H.; Katsura, S. Immobilization of His-tagged proteins on various solid surfaces using NTA-modified chitosan. Open J. Polym. Chem. 2013, 3, 6–10. [Google Scholar] [CrossRef][Green Version]
- Miyata, H.; Yumoto, K.; Itoh, K.; Sasahara, M.; Kawaura, H.; Oshima, N.; Suzuki, T.; Takahashi, S.; Oshige, M.; Katsura, S. Immobilization of His-Tagged Proteins through Interaction with L-Cysteine Electrodeposited on Modified Gold Surfaces. Key Eng. Mater. 2014, 596, 219–223. [Google Scholar] [CrossRef]
- Hochuli, E.; Bannwarth, W.; Döbeli, H.; Gentz, R.; Stüber, D. Genetic approach to facilitate purification of recombinant proteins with a novel metal chelate adsorbent. Nat. Biotechnol. 1988, 6, 1321–1325. [Google Scholar] [CrossRef]
- Kinpara, T.; Mizuno, R.; Murakami, Y.; Kobayashi, M.; Yamaura, S.; Hasan, Q.; Morita, Y.; Nakano, H.; Yamane, T.; Tamiya, E. A picoliter chamber array for cell-free protein synthesis. J. Biochem. 2004, 136, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Chaga, G.S. Twenty-five years of immobilized metal ion affinity chromatography: Past, present and future. J. Biochem. Biophys. Methods 2001, 49, 313–334. [Google Scholar] [CrossRef]
- Auer, S.; Azizi, L.; Faschinger, F.; Blazevic, V.; Vesikari, T.; Gruber, H.J.; Hytönen, V.P. Stable immobilisation of His-tagged proteins on BLI biosensor surface using cobalt. Sens. Actuators B Chem. 2017, 243, 104–113. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, S.; Kishi, K.; Hiraga, R.; Hayashi, K.; Mamada, Y.; Oshige, M.; Katsura, S. A New Method for Immobilization of His-Tagged Proteins with the Application of Low-Frequency AC Electric Field. Sensors 2018, 18, 784. https://doi.org/10.3390/s18030784
Takahashi S, Kishi K, Hiraga R, Hayashi K, Mamada Y, Oshige M, Katsura S. A New Method for Immobilization of His-Tagged Proteins with the Application of Low-Frequency AC Electric Field. Sensors. 2018; 18(3):784. https://doi.org/10.3390/s18030784
Chicago/Turabian StyleTakahashi, Shunsuke, Kazuki Kishi, Ryota Hiraga, Kazuki Hayashi, Youhei Mamada, Masahiko Oshige, and Shinji Katsura. 2018. "A New Method for Immobilization of His-Tagged Proteins with the Application of Low-Frequency AC Electric Field" Sensors 18, no. 3: 784. https://doi.org/10.3390/s18030784
APA StyleTakahashi, S., Kishi, K., Hiraga, R., Hayashi, K., Mamada, Y., Oshige, M., & Katsura, S. (2018). A New Method for Immobilization of His-Tagged Proteins with the Application of Low-Frequency AC Electric Field. Sensors, 18(3), 784. https://doi.org/10.3390/s18030784



