2-D Minimum Variance Based Plane Wave Compounding with Generalized Coherence Factor in Ultrafast Ultrasound Imaging
Abstract
1. Introduction
2. Backgrounds
2.1. Mathematic Model of the Plane Wave Compounding
2.2. Minimum Variance Beamformers
2.3. Coherence Factor Beamformers
3. Methods
3.1. Joint Transmitting-Receiving Beamforming
3.2. 2-D generalized Coherence Factor Weighting
3.3. Implementation Summary
- (1)
- Calculate time delays for each channel of different plane wave firing events. The echo data matrix (1) can be obtained after the calculation.
- (2)
- Compute the JTR weighting vector, and , using Equations (5), (15), and (16). The JTR beamformed output matrix (19) can be acquired from Equation (18).
- (3)
- Use the 2-D FFT to get the spatial spectrum and then calculate the 2-D GCF using Equation (21). The final output of the proposed algorithm is obtained from (22).
- (4)
- Repeat the procedure I to III over each imaging pixel to generate the final B-mode image.
4. Experiments and Results
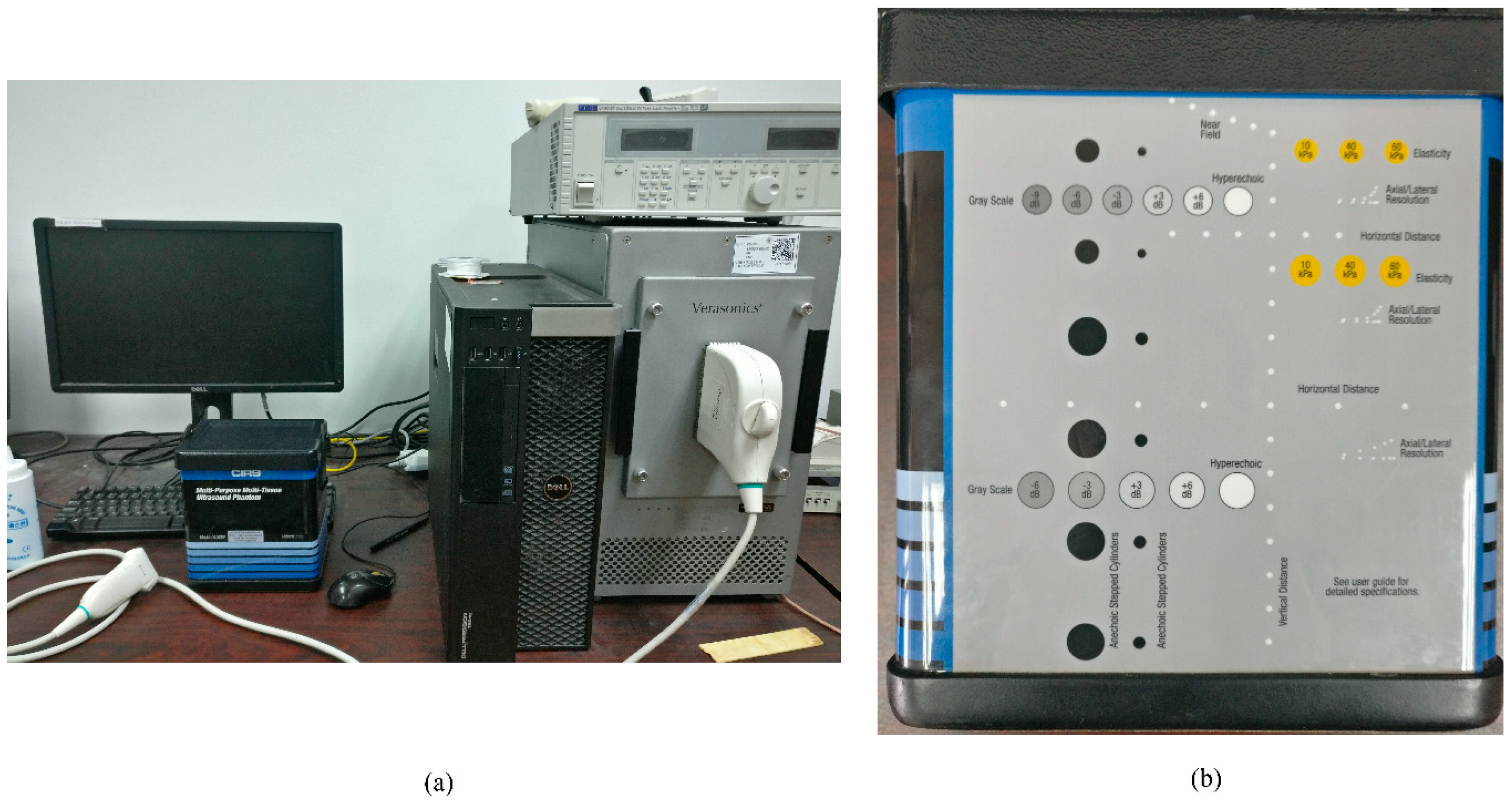
4.1. Experimental Setup
4.2. Parameters and Evaluation Metrics
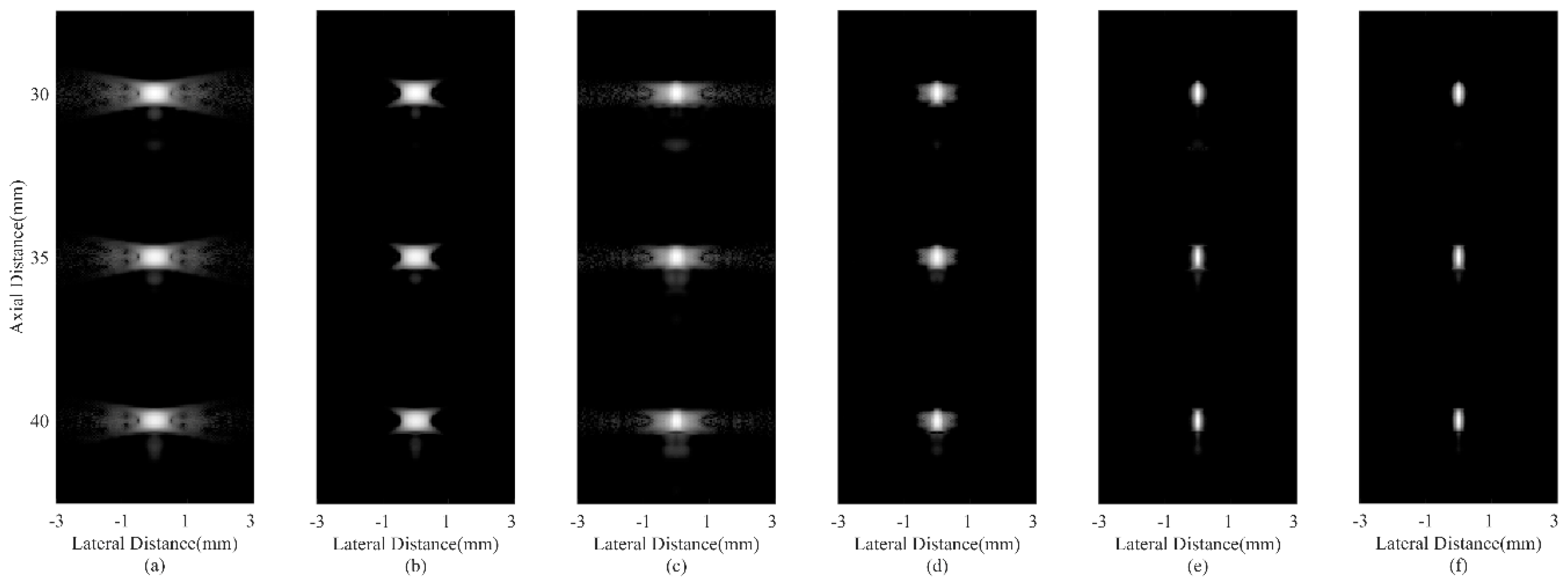
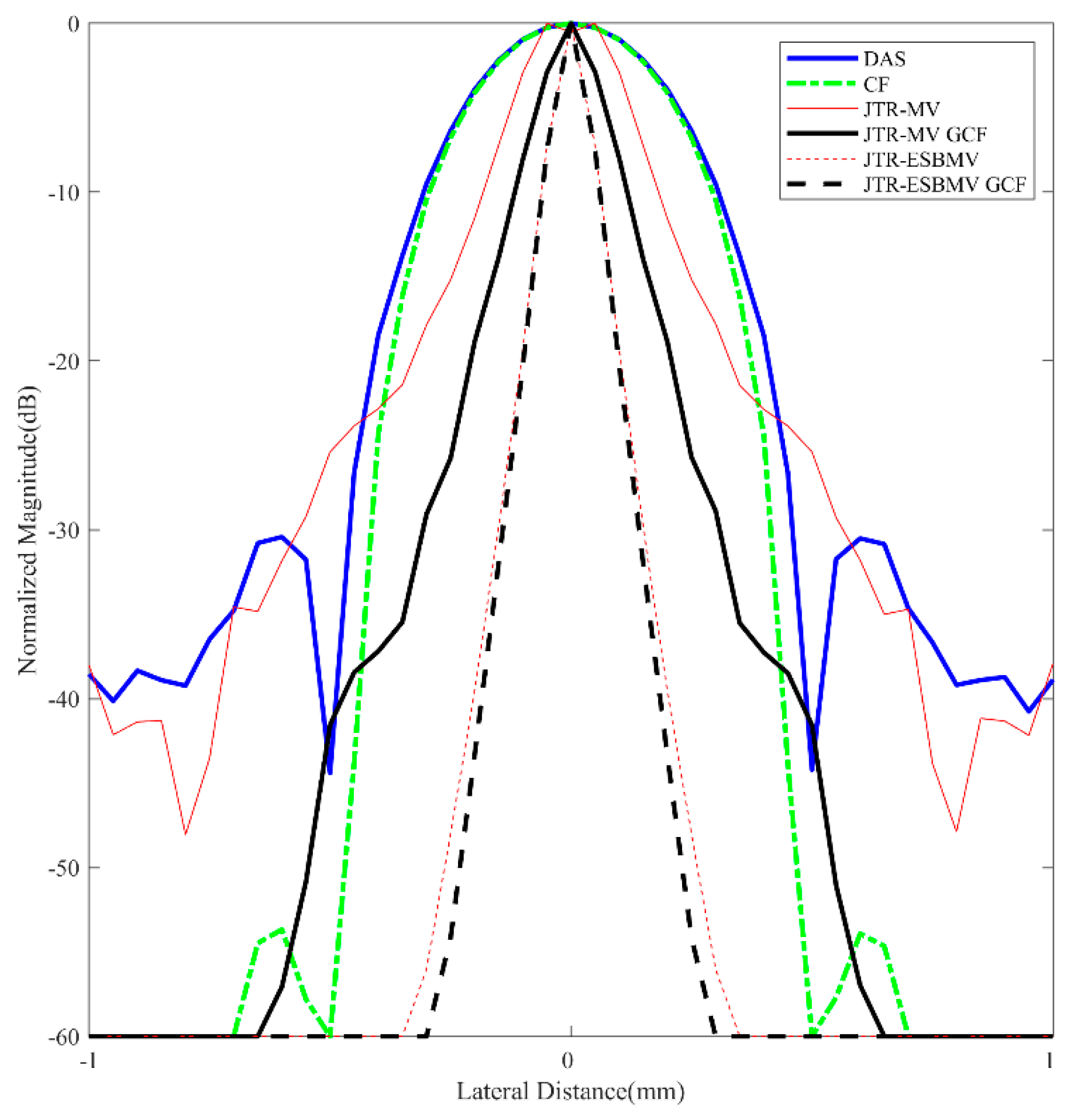
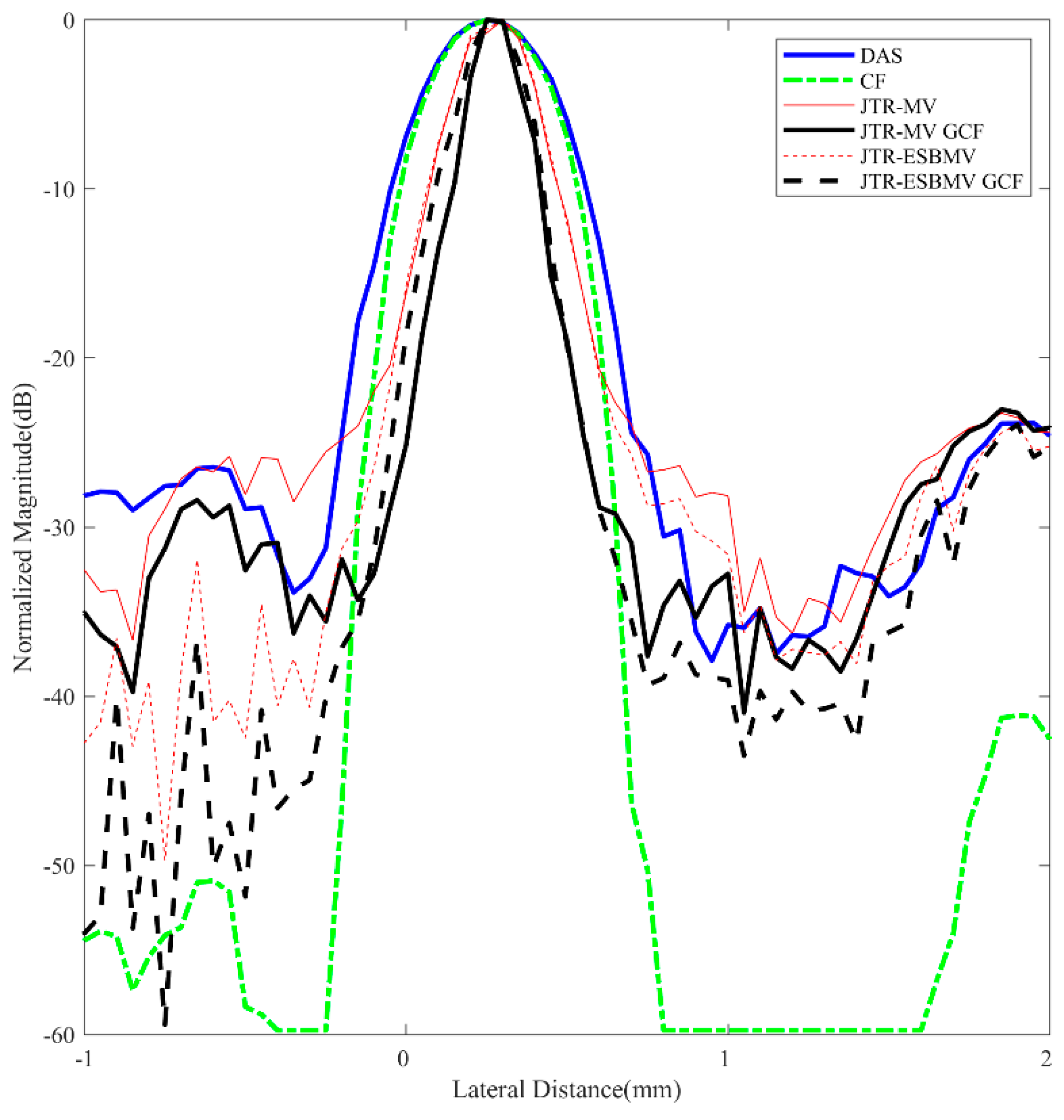
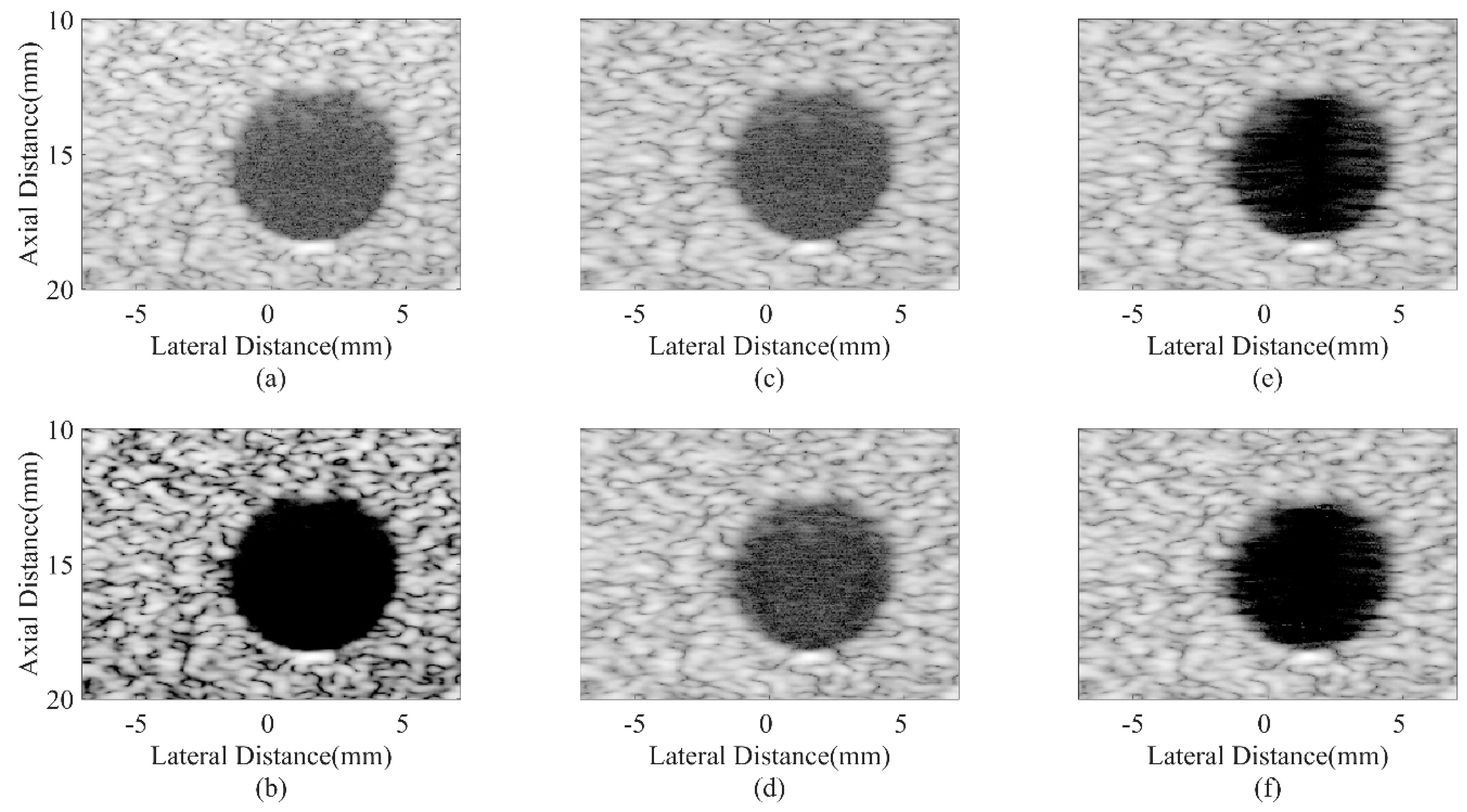
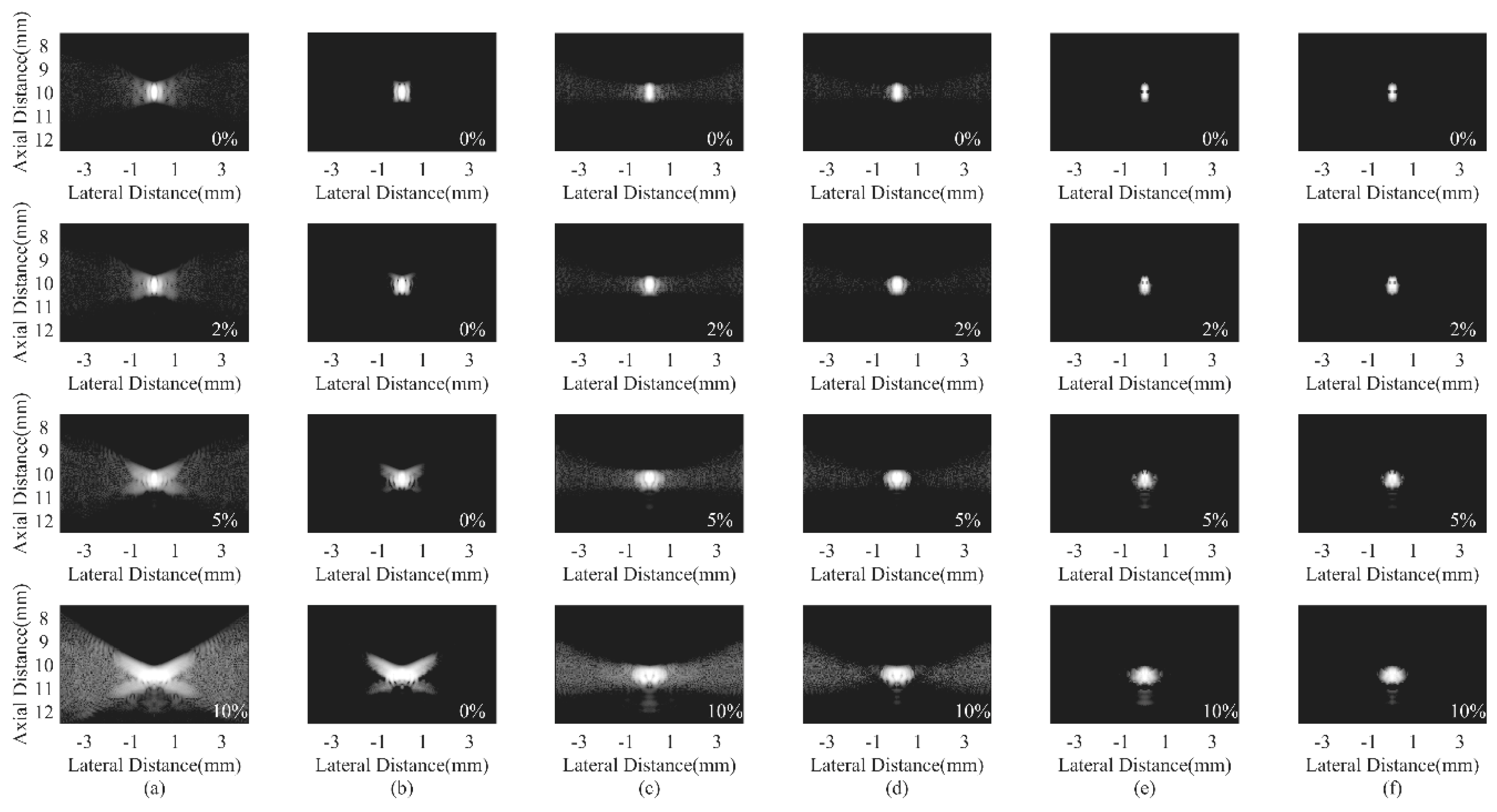
4.3. Simulated Study
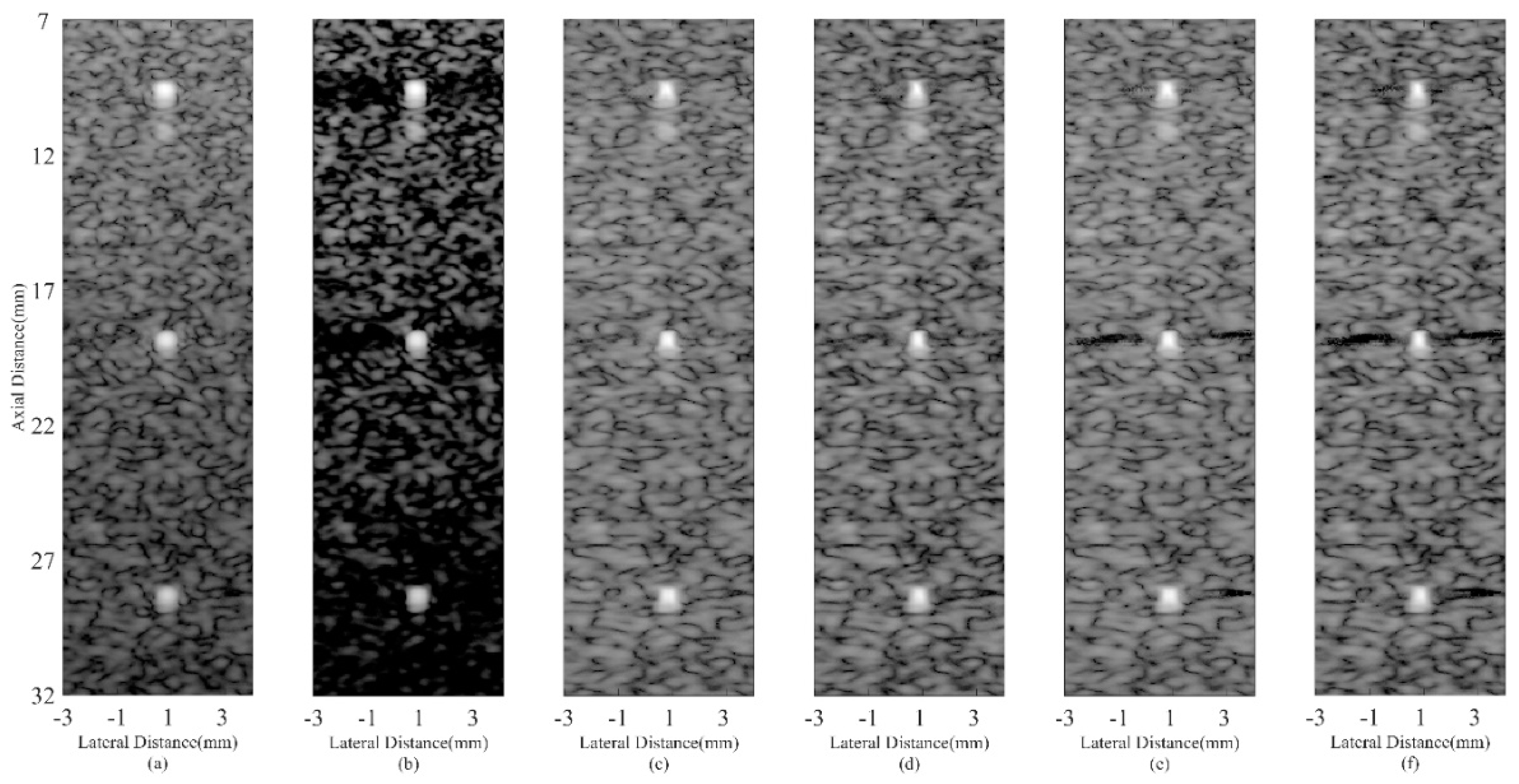
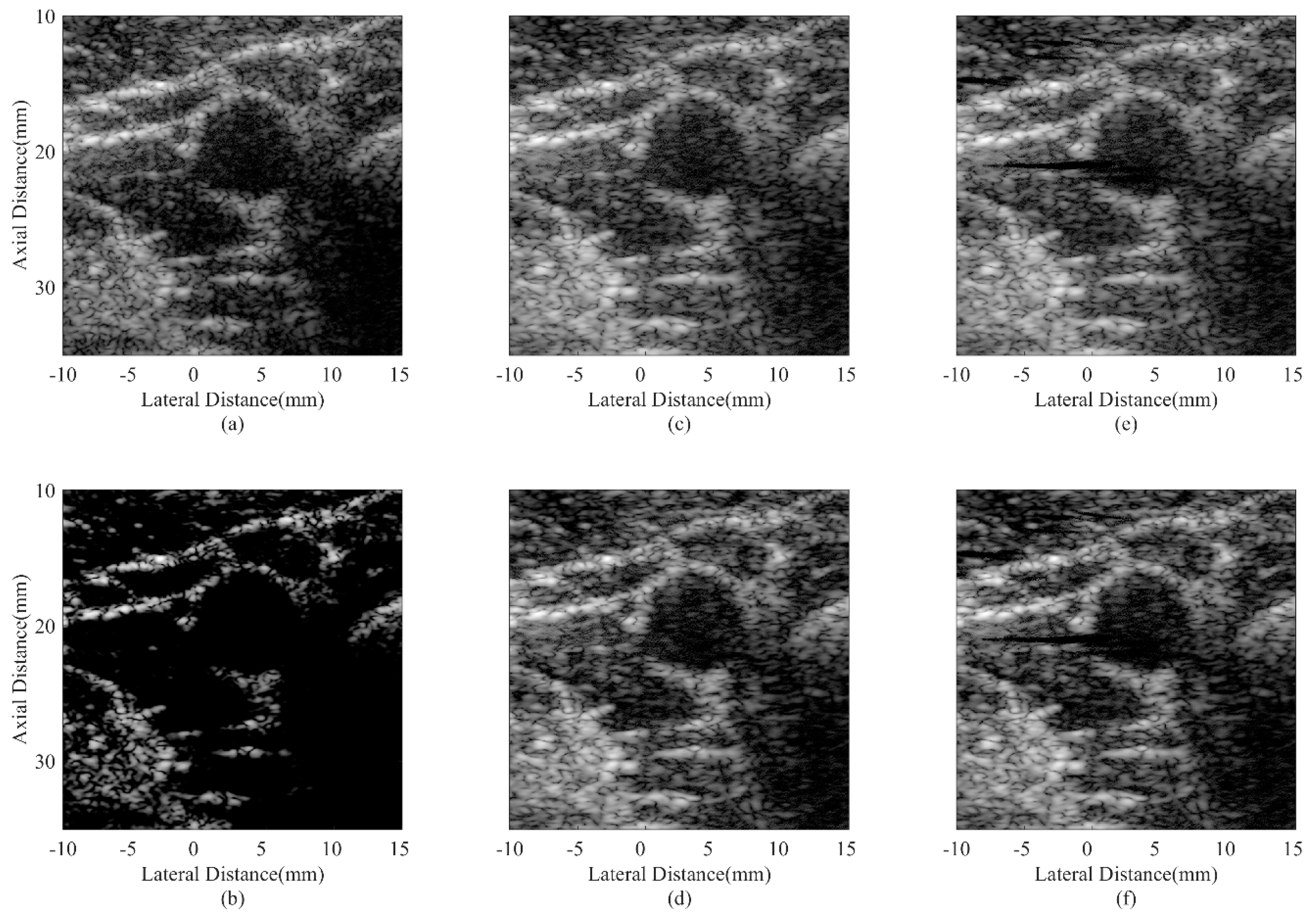
4.4. Experimental Phantom Study
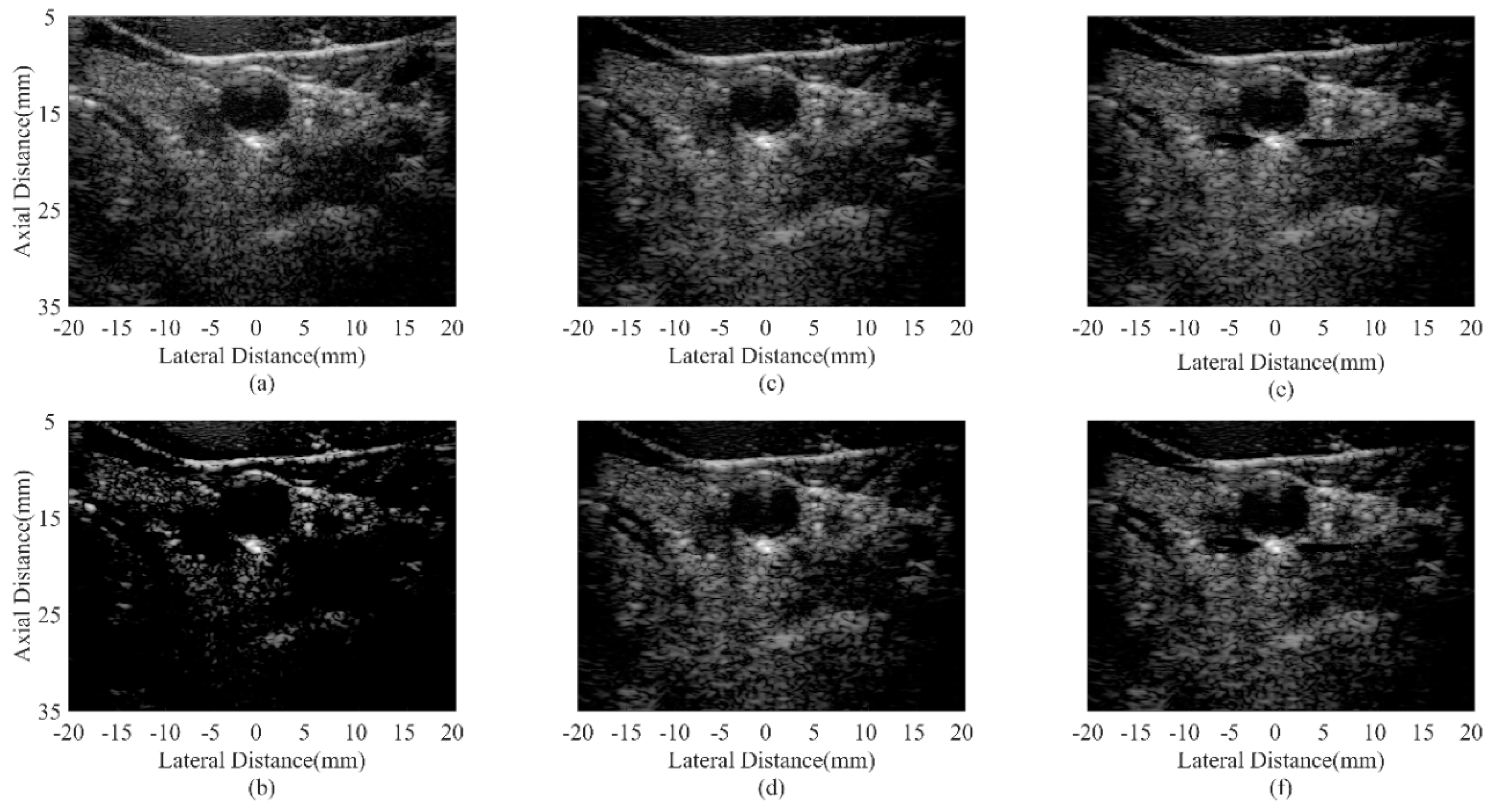
4.5. In Vivo Study
4.6. Robustness to Sound Velocity Inhomogenetities
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2-D | Two-dimensional |
| CC | Computational complexity |
| CF | Coherence factor |
| CNR | Contrast-to-noise ratio |
| CR | Contrast ratio |
| DAS | Delay-and-sum |
| DFT | Discrete Fourier transform |
| ESBMV | Eigenspace-based minimum variance |
| FFT | Fast Fourier transform |
| FWHM | Full width at half maximum |
| GCF | Generalized coherence factor |
| JTR | Joint-transmitting-receiving |
| MV | Minimum variance |
| PSF | Point spread function |
| PWC | Plane wave compounding |
| PWI | Plane wave imaging |
| ROI | Region of interest |
| SNR | Signal-to-noise ratio |
References
- Wells, P.N.T. Ultrasonics in medicine and biology. Phys. Med. Biol. 1977, 22, 629–669. [Google Scholar] [CrossRef] [PubMed]
- Wells, P.N.T. Ultrasonic imaging of the human body. Rep. Prog. Phys. 1999, 62, 671–722. [Google Scholar] [CrossRef]
- Wells, P.N.T. Ultrasound imaging. Phys. Med. Biol. 2006, 51, R83–R98. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Y.; Greenleaf, J.F. Pulse-echo imaging using a nondiffracting beam transducer. Ultrasound Med. Biol. 1991, 17, 265–281. [Google Scholar] [CrossRef]
- Garcia, D.; Tarnec, L.L.; Muth, S.; Montagnon, E.; Porée, J.; Cloutier, G. Stolt’s f-k migration for plane wave ultrasound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 1853–1867. [Google Scholar] [CrossRef] [PubMed]
- Couture, O.; Fink, M.; Tanter, M. Ultrasound contrast plane wave imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 2676–2683. [Google Scholar] [CrossRef] [PubMed]
- Holfort, I.K.; Gran, F.; Jensen, J.A. Broadband minimum variance beamforming for ultrasound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Berson, M.; Roncin, A.; Pourcelot, L. Compound scanning with an electrically steered beam. Ultrason. Imaging 1981, 3, 303–308. [Google Scholar] [CrossRef]
- Lu, J.Y. 2D and 3D high frame rate imaging with limited diffraction beams. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1997, 44, 839–856. [Google Scholar] [CrossRef]
- Lu, J.Y. Experimental study of high frame rate imaging with limited diffraction beams. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1998, 45, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Lu, J.Y. Extended high frame rate imaging method with limited diffraction beams. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 53, 880–899. [Google Scholar] [CrossRef] [PubMed]
- Entrekin, R.R.; Porter, B.A.; Sillesen, H.H.; Wong, A.D.; Cooperberg, P.L.; Fix, C.H. Real-time spatial compound imaging: Application to breast, vascular, and musculoskeletal ultrasound. Semin. Ultrasound CT MRI 2001, 22, 50–64. [Google Scholar] [CrossRef]
- Forsberg, F. Ultrasonic biomedical technology; marketing versus clinical reality. Ultrasonics 2004, 42, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Montaldo, G.; Tanter, M.; Bercoff, J.; Benech, N.; Fink, M. Coherent plane-wave compounding for very high frame rate ultrasonography and transient elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Capon, J. High resolution frequency-wavenumber spectrum analysis. Proc. IEEE 1969, 57, 1408–1418. [Google Scholar] [CrossRef]
- Synnevag, J.F.; Austeng, A.; Holm, S. Adaptive beamforming applied to medical ultrasound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2007, 54, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Synnevag, J.F.; Austeng, A.; Holm, S. Benefits of minimum-variance beamforming in medical ultrasound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 1868–1879. [Google Scholar] [CrossRef] [PubMed]
- Asl, B.M.; Mahloojifar, A. Eigenspace-based minimum variance beamforming applied to medical ultrasound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2010, 57, 2381–2390. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, S.; Austeng, A.; Johansen, T.F.; Holm, S. Eigenspace based minimum variance beamforming applied to ultrasound imaging of acoustically hard tissues. IEEE Trans. Med. Imaging 2012, 31, 1912–1921. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.C.; Austeng, A. An approach to multibeam covariance matrices for adaptive beamforming in ultrasonography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Y.; Zeng, X.; Yu, J.; Billy, Y.S.Y.; Yu, A.C.H. Plane wave compounding based on a joint transmitting-receiving adaptive beamformer. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 1440–1452. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, C.C.; Holm, S. Wiener beamforming and the coherence factor in ultrasound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2010, 57, 1329–1346. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, M. Adaptive imaging using the generalized coherence factor. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2003, 50, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Deylami, A.M.; Jensen, J.A.; Asl, B.M. An improved minimum variance beamforming applied to plane-wave imaging in medical ultrasound. In Proceedings of the 2016 IEEE Ultrasonics Symposium, Tours, France, 18–21 September 2016. [Google Scholar] [CrossRef]
- Asl, B.M.; Mahloojifar, A. Minimum variance beamforming combined with adaptive coherence weighting applied to medical ultrasound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Chen, C.; Wang, Y. Eigenspace-based minimum variance beamformer combined with Wiener postfilter for medical ultrasound imaging. Ultrasonics 2012, 52, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Y.; Yu, J.; Guo, W.; Li, T.; Zheng, Y. Subarray coherence based postfilter for eigenspace based minimum variance beamformer in ultrasound plane-wave imaging. Ultrasonics 2016, 65, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Holfort, I.K.; Austeng, A.; Synnevåg, J.F.; Holm, S.; Gran, F.; Jensen, J.A. Adaptive Receive and Transmit Apodization for Synthetic Aperture Ultrasound Imaging. In Proceedings of the 2009 IEEE Ultrasonics Symposium, Rome, Italy, 20–23 September 2009. [Google Scholar] [CrossRef]
- Matrone, G.; Savoia, A.; Caliano, G.; Magenes, G. The delay multiply and sum beamforming algorithm in ultrasound B-mode medical imaging. IEEE Trans. Med. Imaging 2015, 34, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Mucci, R. A comparison of efficient beamforming algorithms. IEEE Trans. Acoust. Speech Signal Process. 1984, 32, 548–558. [Google Scholar] [CrossRef]
- Shan, T.J.; Wax, M.; Kailath, T. On spatial smoothing for direction-of-arrival estimation of coherent signals. IEEE Trans. Acoust. Speech Signal Process. 1985, 33, 806–811. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Wu, R. Time-delay- and time-reversal-based robust Capon beamformers for ultrasound imaging. IEEE Trans. Med. Imaging 2005, 24, 1308–1322. [Google Scholar] [CrossRef] [PubMed]
- Vignon, F.; Burcher, M.R. Capon beamforming in medical ultrasound imaging with focused beams. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2008, 55, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.A. Field: A program for simulating ultrasound systems. Med. Biol. Eng. Comput. 1996, 34 (Suppl. 1), 351–353. [Google Scholar]
- Jensen, J.A.; Svendsen, N.B. Calculation of pressure fields from arbitrarily shaped, apodized, and excited ultrasound transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1992, 39, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wang, Y.; Guo, W. Joint subarray coherence and minimum variance beamformer for multitransmission ultrasound imaging modalities. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 1600–1617. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.; Lee, Y.; Chang, J.H.; Song, T.; Yoo, Y. In vitro estimation of mean sound speed based on minimum average phase variance in medical ultrasound imaging. Ultrasonics 2011, 51, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, D.; Chou, C.H.; McLaughlin, G.; Ji, T.L.; Mo, L.; DeBusschere, D.; Steins, R. Sound speed correction in ultrasound imaging. Ultrasonics 2006, 44, e43–e46. [Google Scholar] [CrossRef] [PubMed]
- Asen, J.P.; Buskenes, J.I.; Colombo, N.I.C.; Austeng, A.; Holm, S. Implementing Capon beamforming on a GPU for real-time cardiac ultrasound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Yiu, B.Y.S.; Yu, A.C.H. GPU-based minimum variance beamformer for synthetic aperture imaging of the eye. Ultrasound Med. Biol. 2015, 41, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.M.; Tomé, A.M. On the use of backward adaptation in a perceptual audio coder. IEEE Trans. Speech Audio Process. 2000, 8, 488–490. [Google Scholar] [CrossRef]



| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Sound speed c Number of elements M Transducer center frequency Element pitch Cycles in emitted pulse Range of steering angles Number of plane waves N | 1540 m/s 128 5 MHz 0.3 mm 2 −12°–12° 49 | Sampling frequency Receive F-number Diagonal loading factor Receiving subarray length Transmitting subarray length ESBMV threshold Cut-off frequency of GCF | 40 MHz 1.4 0.01 38 (0.3M) 15 (0.3N) 0.02 0 (point)/1 (others) |
| Beamformer | FWHM (mm) |
|---|---|
| DAS | 0.40 |
| CF JTR-MV JTR-MV GCF JTR-ESBMV JTR-ESBMV GCF | 0.37 0.13 0.12 0.05 0.04 |
| Beamformer | CR (dB) | CNR |
|---|---|---|
| DAS | 30.61/33.98 | 1.58/1.67 |
| CF JTR-MV JTR-MV GCF JTR-ESBMV JTR-ESBMV GCF | 81.23/85.34 31.04/33.50 38.06/40.65 54.08/61.44 76.50/86.36 | 1.01/1.26 1.65/1.74 1.59/1.67 1.63/1.72 1.58/1.66 |
| Beamformer | FWHM (mm) |
|---|---|
| DAS | 0.51 |
| CF JTR-MV JTR-MV GCF JTR-ESBMV JTR-ESBMV GCF | 0.48 0.32 0.20 0.30 0.25 |
| Beamformer | CR (dB) | CNR |
|---|---|---|
| DAS | 30.32 | 1.76 |
| CF JTR-MV JTR-MV GCF JTR-ESBMV JTR-ESBMV GCF | 76.77 29.87 33.27 50.97 64.01 | 1.13 1.82 1.74 1.86 1.78 |
| Beamformer | CR (dB) | CNR |
|---|---|---|
| DAS | 20.19 | 1.57 |
| CF JTR-MV JTR-MV GCF JTR-ESBMV JTR-ESBMV GCF | 59.69 24.50 27.11 25.23 28.03 | 0.89 1.60 1.55 1.61 1.56 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Wang, Y.; Yu, J.; Guo, Y. 2-D Minimum Variance Based Plane Wave Compounding with Generalized Coherence Factor in Ultrafast Ultrasound Imaging. Sensors 2018, 18, 4099. https://doi.org/10.3390/s18124099
Qi Y, Wang Y, Yu J, Guo Y. 2-D Minimum Variance Based Plane Wave Compounding with Generalized Coherence Factor in Ultrafast Ultrasound Imaging. Sensors. 2018; 18(12):4099. https://doi.org/10.3390/s18124099
Chicago/Turabian StyleQi, Yanxing, Yuanyuan Wang, Jinhua Yu, and Yi Guo. 2018. "2-D Minimum Variance Based Plane Wave Compounding with Generalized Coherence Factor in Ultrafast Ultrasound Imaging" Sensors 18, no. 12: 4099. https://doi.org/10.3390/s18124099
APA StyleQi, Y., Wang, Y., Yu, J., & Guo, Y. (2018). 2-D Minimum Variance Based Plane Wave Compounding with Generalized Coherence Factor in Ultrafast Ultrasound Imaging. Sensors, 18(12), 4099. https://doi.org/10.3390/s18124099





