Pencil Lead as a Material for Microfluidic 3D-Electrode Assemblies
Abstract
1. Introduction
2. Materials and Methods
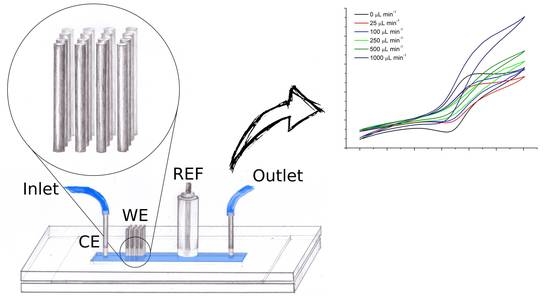
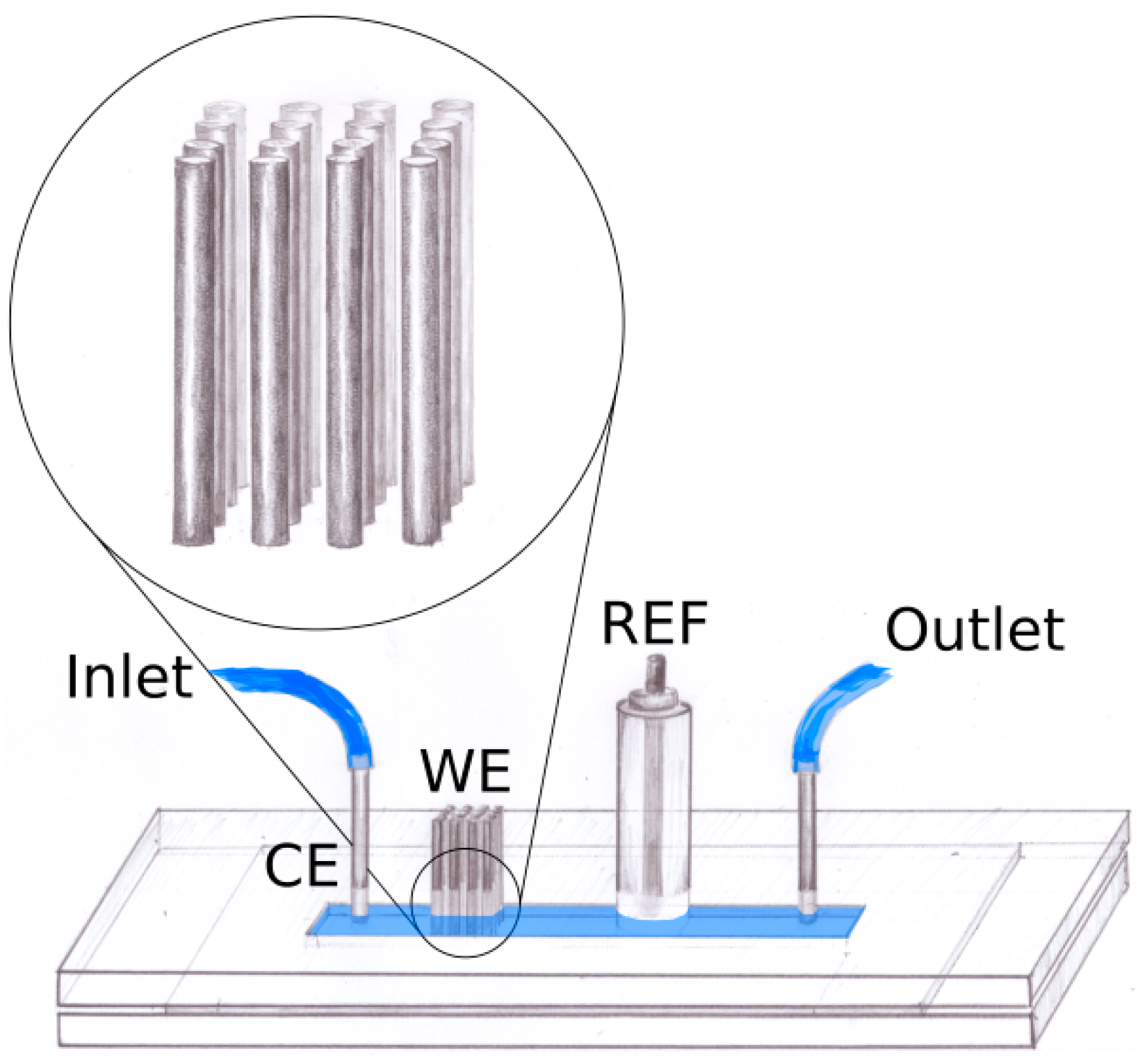
2.1. Device Fabrication
2.2. Electrochemical Characterization
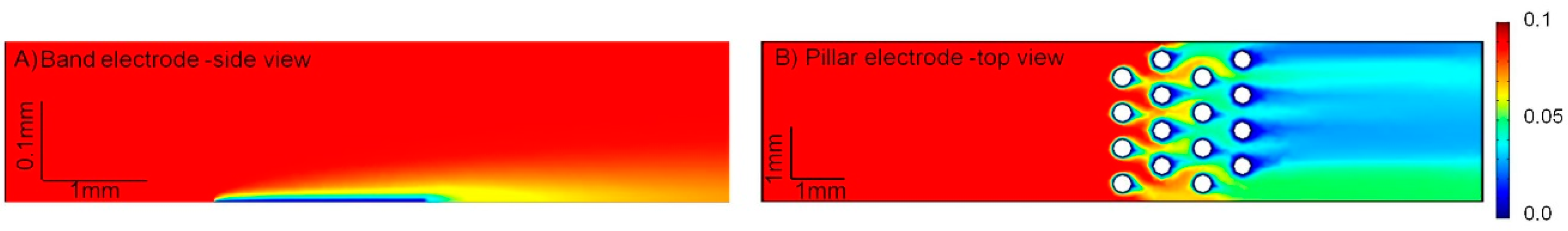
2.3. Computer Modeling Using COMSOL Multiphysics
2.4. Neurotransmitter Sensing
- (1)
- the standard pretreatment procedure –cycling between −0.1 to 0.5 V in 1 mmol L−1 FcDM, 0.1 mol L−1 KNO3,
- (2)
- oxidized by cycling between 1.2 to 1.8 V in 0.1 mol L−1 NaOH solution, 25 cycles, 100 mV s−1
- (3)
- oxidized by application of constant potential of 1.8 V in PBS solution (pH 7.4) for 600 s, the latter was shown to result in a graphene like surface when applied to glassy carbon electrodes [25]. In this case pre-treatment was applied to enhance the electrode performance (we evaluated the impact on the current and peak separation while performing measurement of the interferents).
2.5. p-Nitrophenol Assay
- (1)
- electrochemical oxidation in 0.2 mol L−1 NaCl, pH 3, different potentials (up to 4.5 V) and times of treatment (up to 20 min) tested;
- (2)
- electrochemical oxidation in 1.5% H2O2, 0.2 mol L−1 NaCl, pH 3 [26], different potentials (up to 4.5 V) and times of treatment (up to 20 min) tested;
- (3)
- adsorptive displacement using iodine and thiosulfate [27]. After each measurement solution of 40 mmol L−1 of iodine in ethanol was passed for 10 min (25 µL min−1), next electrochemical cell was cleaned with deionized water. Chemical reduction of iodine to iodide was achieved using 0.5 mol L−1 sodium thiosulfate in water (3 min, 25 µL min−1), after which the system was cleaned with distilled water and considered ready for the next measurement.
- (4)
- NaOH oxidative cycling which was earlier applied as a pretreatment step in the dopamine assay. After each measurement, the surface was regenerated with 25 scans between 1.2 to 1.8 V in 0.1 mol L−1 NaOH solution.
3. Results
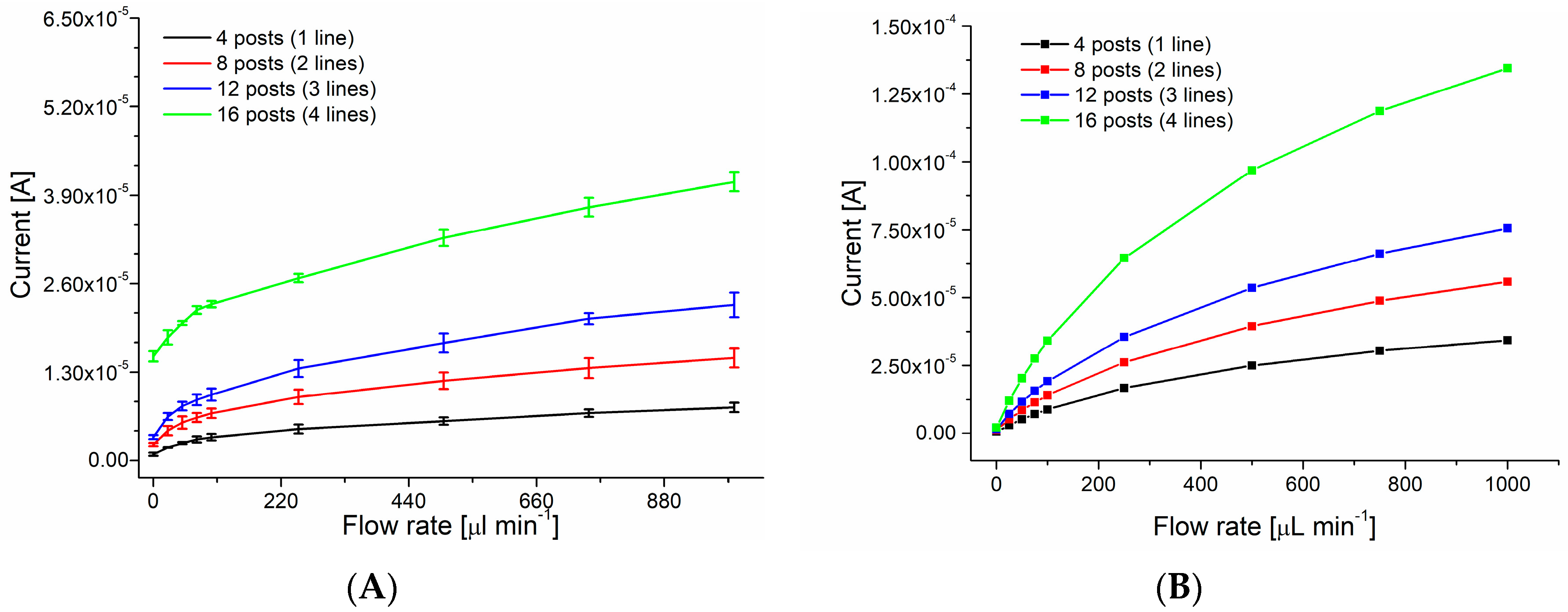
3.1. Characterization
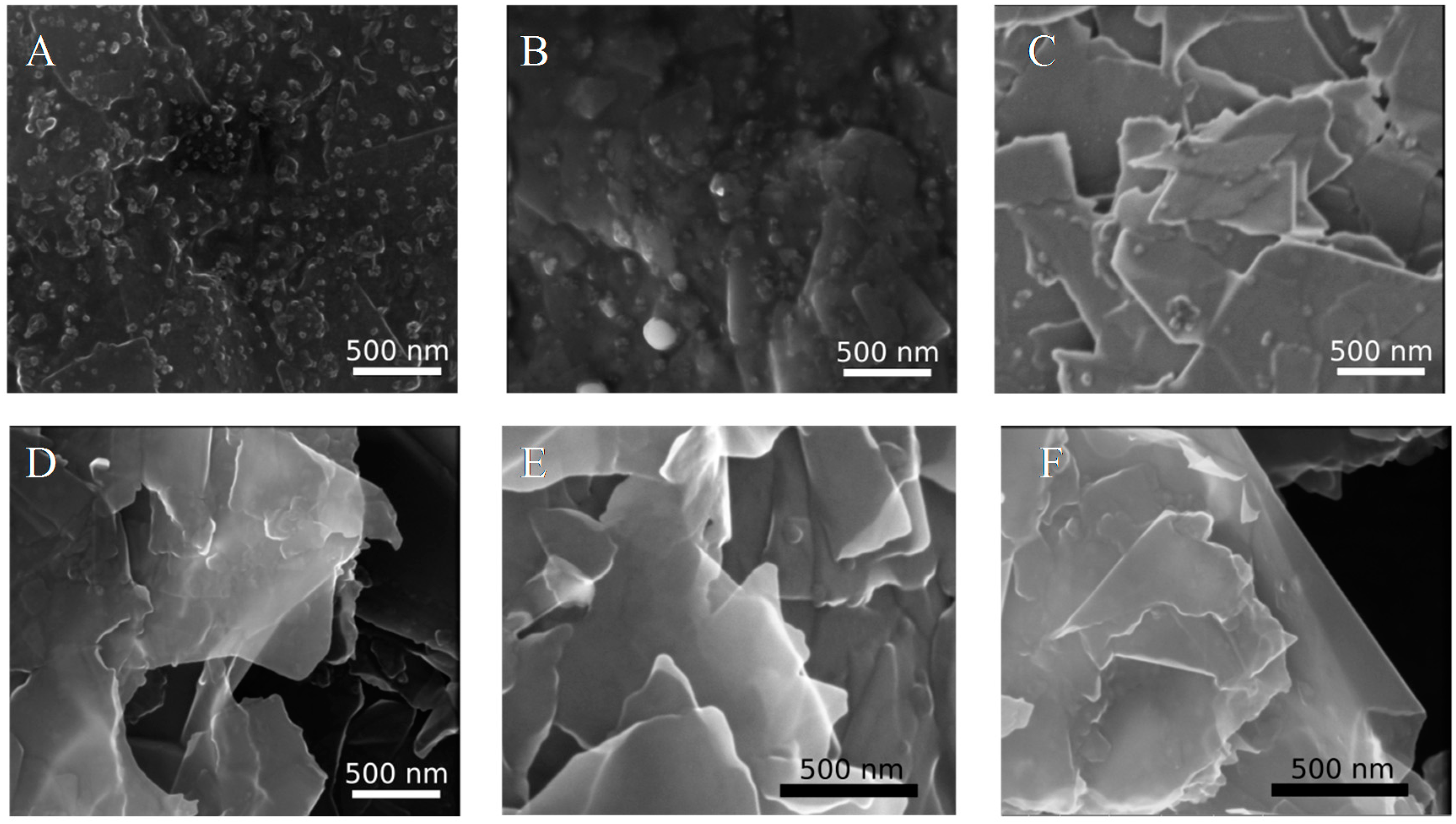
3.2. SEM Analysis
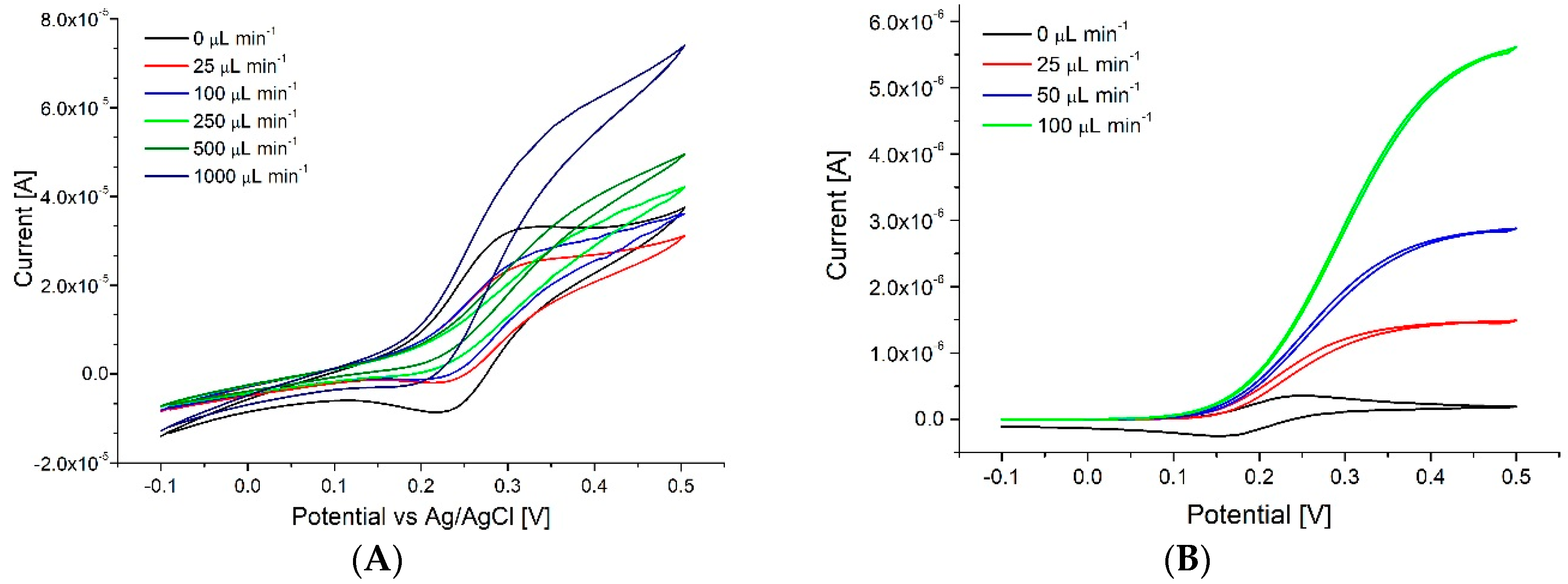
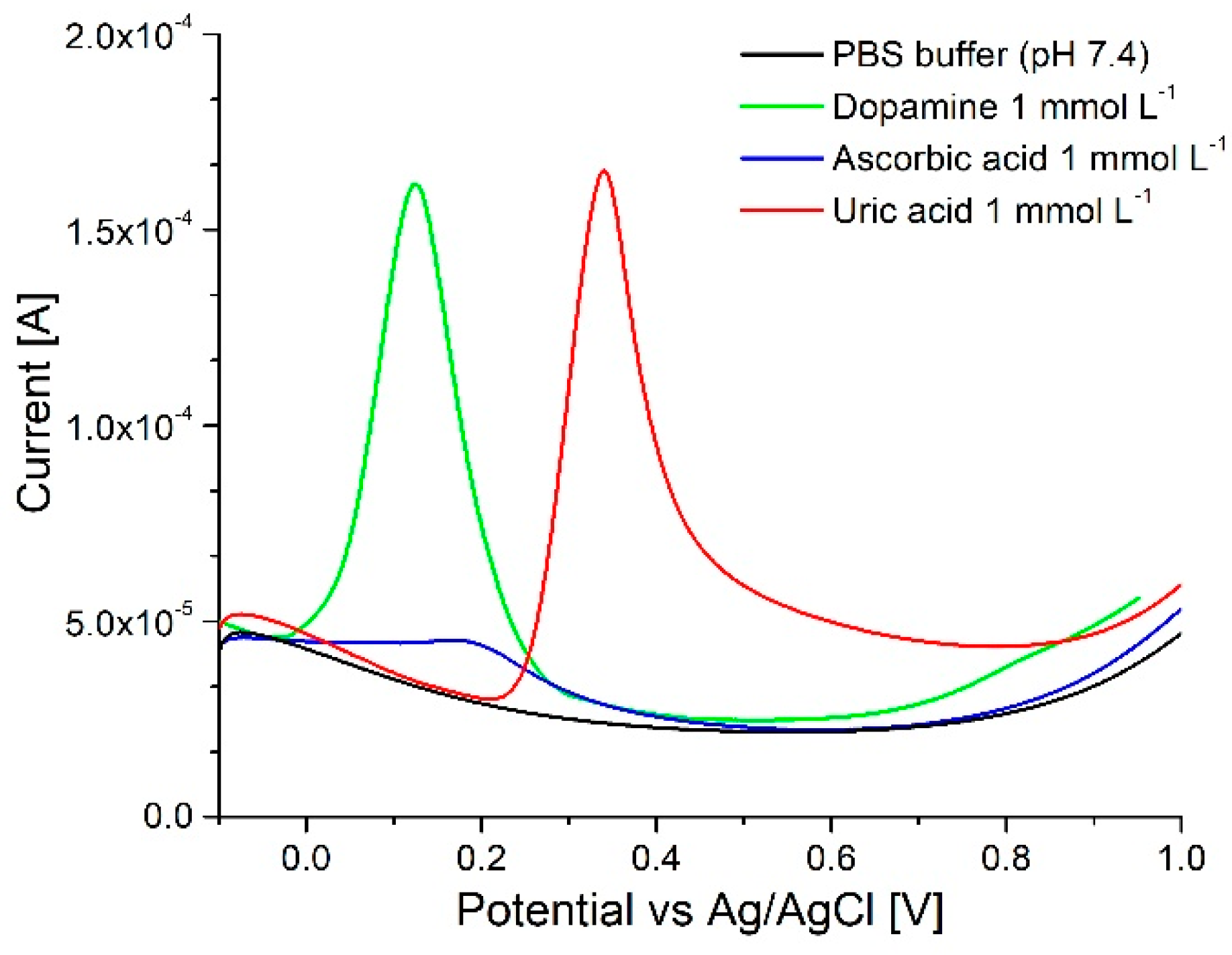
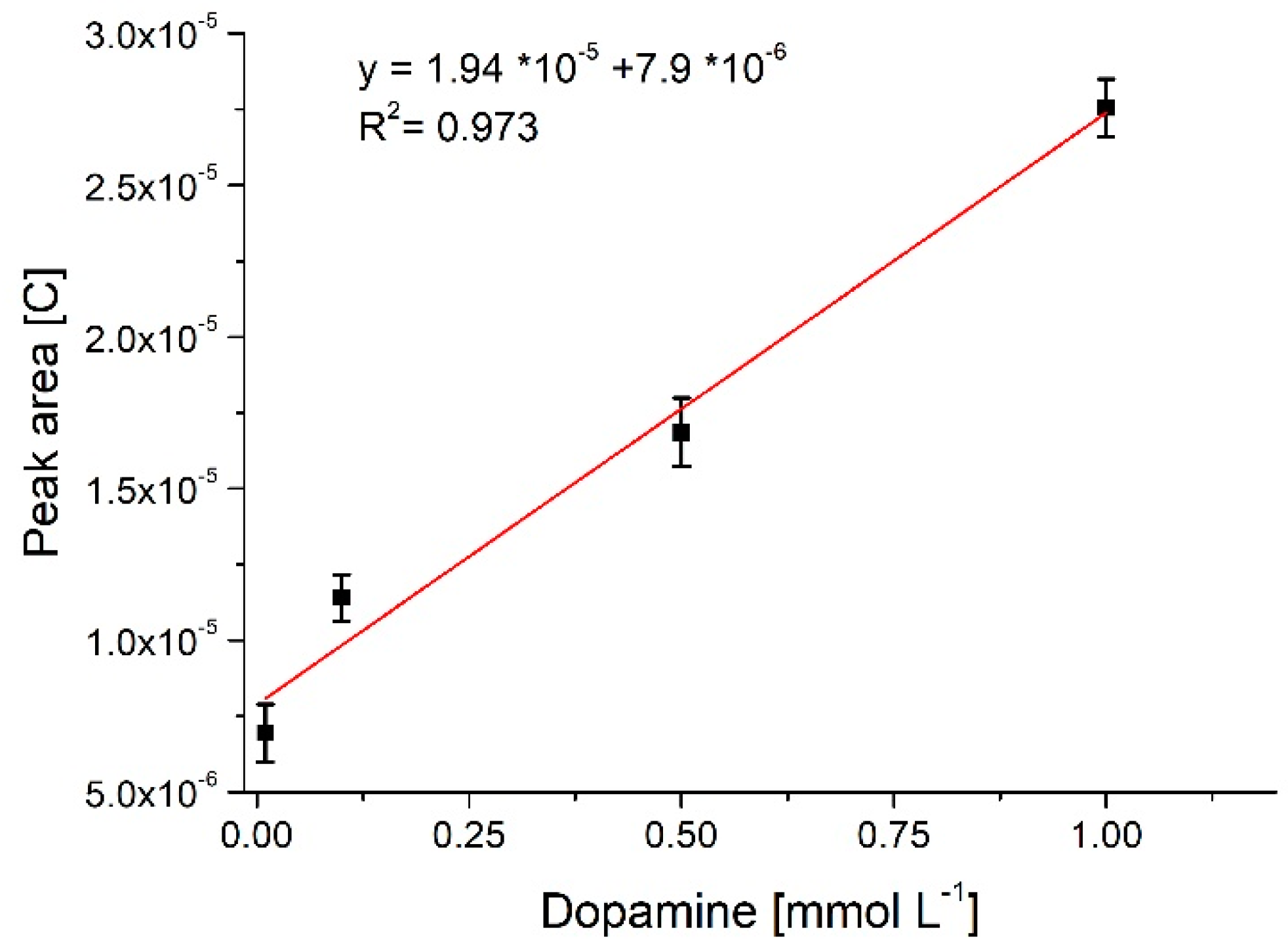
3.3. Neurotransmitter Detection
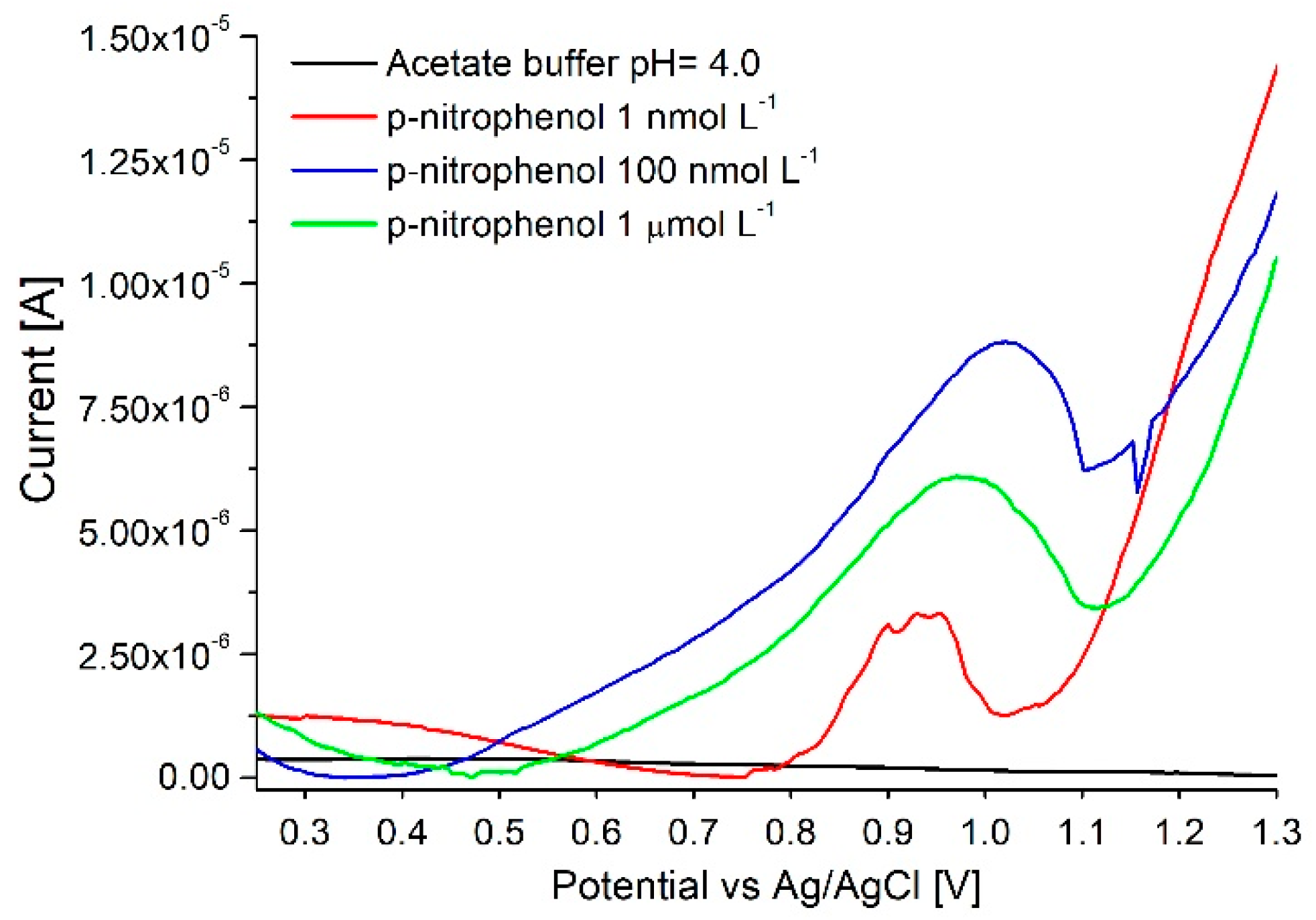
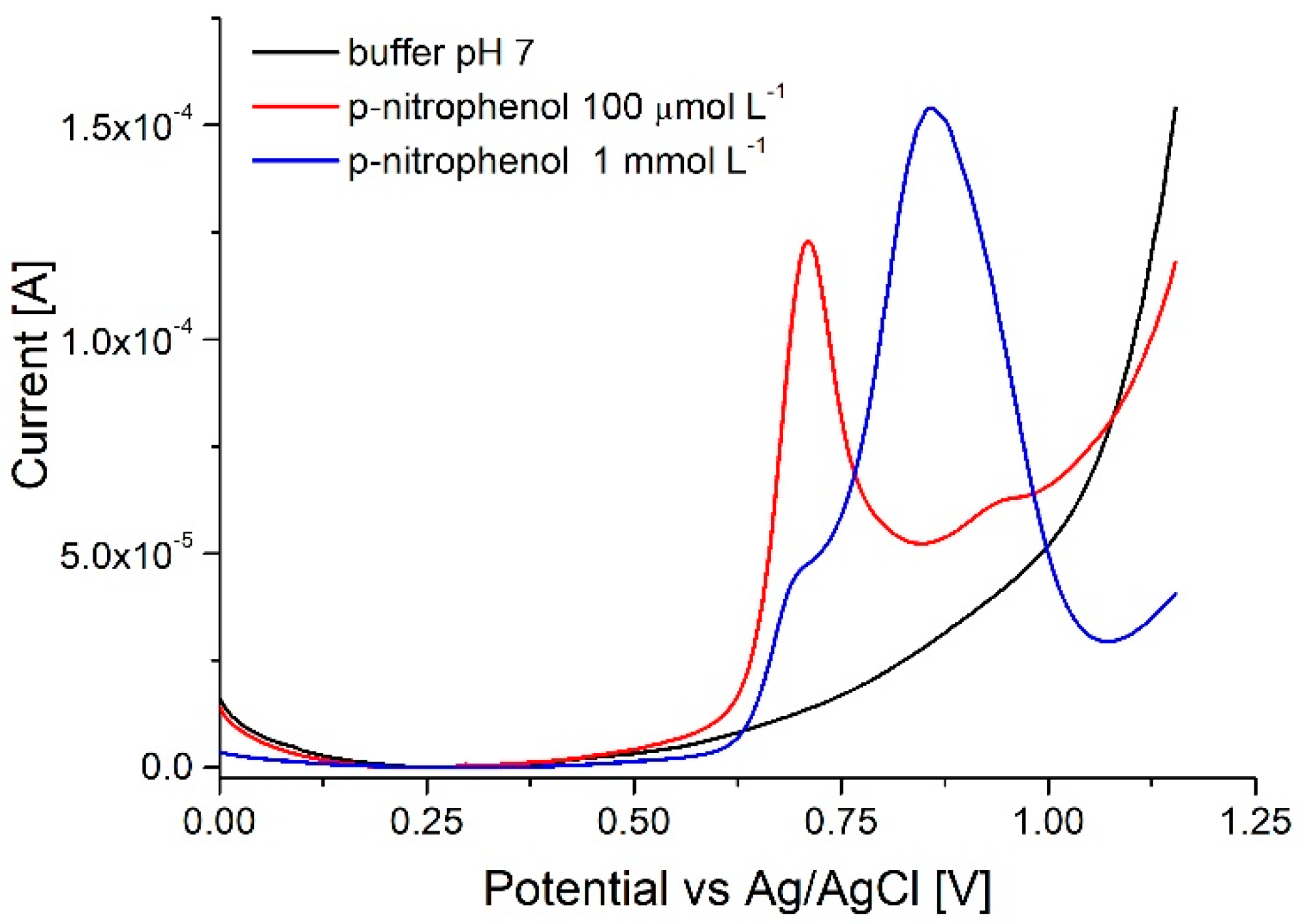
3.4. p-Nitrophenol Assay
3.4.1. Adsorptive Displacement (Method n° 3)
3.4.2. NaOH Oxidative Cycling (Method n° 4)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bhowmik, R.N. Ferromagnetism in lead graphite-pencils and magnetic composite with CoFe2O4 particles. Compos. Part B 2012, 43, 503–509. [Google Scholar] [CrossRef]
- Kawde, A.; Baig, N.; Sajid, M. Graphite pencil electrodes as electrochemical sensors for environmental analysis: A review of features, developments, and applications. RSC Adv. 2016, 6, 91325–91340. [Google Scholar] [CrossRef]
- David, I.G.; Popa, D.; Buleandra, M. Pencil Graphite Electrodes: A Versatile Tool in Electroanalysis. J. Anal. Methods Chem. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Mašek, J. A simple microcoulometric arrangement for polarographic purposes using the three-electrode system. J. Electroanal. Chem. 1960, 1, 416–421. [Google Scholar] [CrossRef]
- Demana, T.; Peterman, D.; Shaffer, J.; Melaragno, P.R. Flow-Through Spectroelectrochemical Detector Based on Diffraction at a Cylindrical Electrode. Anal. Chem. 1989, 61, 1216–1221. [Google Scholar] [CrossRef]
- Blum, D.; Leyffer, W.; Holze, R. Pencil-Leads as new electrodes for abrasive stripping voltammetry. Electroanalysis 1996, 8, 296–297. [Google Scholar] [CrossRef]
- WitkowskaNery, E.; Santhiago, M.; Kubota, L.T. Flow in a Paper-based Bioactive Channel—Study on Electrochemical Detection of Glucose and Uric Acid. Electroanalysis 2016, 28, 1–9. [Google Scholar] [CrossRef]
- Sharp Announcement “0.1” Which Is Not Broken Even with Ultra-fine 0.2 Is also Reference Exhibited. Available online: www.nichima.co.jp/news/entry/1747.html (accessed on 1 September 2018).
- Amatore, C.; Da Mota, N.; Sella, C.; Thouin, L. Theory and experiments of transport at channel microband electrodes under laminar flows. 1. Steady-state regimes at a single electrode. Anal. Chem. 2007, 79, 8502–8510. [Google Scholar] [CrossRef] [PubMed]
- Amatore, C.; Da Mota, N.; Sella, C.; Thouin, L. Theory and experiments of transport at channel microband electrodes under laminar flow. 3. Electrochemical detection at electrode arrays under steady state. Anal. Chem. 2010, 82, 2434–2440. [Google Scholar] [CrossRef] [PubMed]
- Rozniecka, E.; Jonsson-Niedziolka, M.; Celebanska, A.; Niedziolka-Jonsson, J.; Opallo, M. Selective electrochemical detection of dopamine in a microfluidic channel on carbon nanoparticulate electrodes. Analyst 2014, 139, 2896–2903. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Duarte, R. Microfabrication technologies in dielectrophoresis applications—A review. Electrophoresis 2012, 33, 3110–3132. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lee, S.; Li, C. 3D carbon based nanostructures for advanced supercapacitors. Energy Environ. Sci. 2013, 6, 41–53. [Google Scholar] [CrossRef]
- La O’, G.J.; In, H.J.; Crumlin, E.; Barbastathis, G.; Shao-Horn, Y. Recent advances in microdevices for electrochemical energy conversion and storage. Int. J. Energy Res. 2007, 31, 548–575. [Google Scholar] [CrossRef]
- Bashirzadeh, Y.; Maruthamuthu, V.; Qian, S. Electrokinetic Phenomena in Pencil Lead-based Microfluidics. Micromachines 2016, 7, 235. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Li, Z.-Q.; Zhang, J.; Wang, C.; Wang, J.; Xia, X.-H.; Zhou, G.-J. A rapid and sensitive method for hydroxyl radical detection on a micro fl uidic chip using an N-doped porous carbon nano fiber modified pencil graphite electrode. Analyst 2014, 139, 3416–3422. [Google Scholar] [CrossRef] [PubMed]
- Kjeang, E.; McKechnie, J.; Sinton, D.; Djilali, N. Planar and three-dimensional microfluidic fuel cell architectures based on graphite rod electrodes. J. Power Sources 2007, 168, 379–390. [Google Scholar] [CrossRef]
- Navratil, R.; Kotzianova, A.; Halouzka, V.; Opletal, T.; Triskova, I.; Trnkova, L.; Hrbac, J. Polymer lead pencil graphite as electrode material: Voltammetric, XPS and Raman study. J. Electroanal. Chem. 2016, 783, 152–160. [Google Scholar] [CrossRef]
- Kariuki, J.K. An Electrochemical and Spectroscopic Characterization of Pencil Graphite Electrodes. J. Electrochem. Soc. 2012, 159, 747–751. [Google Scholar] [CrossRef]
- Wu, Z.; Yuan, X.; Zhong, H.; Wang, H.; Zeng, G.; Chen, X.; Wang, H.; Zhang, L.; Shao, J. Enhanced adsorptive removal of p-nitrophenol from water by aluminum metal–organic framework/reduced graphene oxide composite. Sci. Rep. 2016, 6, 25638. [Google Scholar] [CrossRef] [PubMed]
- Santhiago, M.; Henry, C.S.; Kubota, L.T. Low cost, simple three dimensional electrochemical paper-based analytical device for determination of p-nitrophenol. Electrochim. Acta 2014, 130, 771–777. [Google Scholar] [CrossRef]
- Hryhorczuk, D.O.; Moomey, M.; Burton, A.; Runkle, K.; Chen, E.; Saxer, T.; Slightom, J.; Dimos, J.; Mccann, K.; Barr, D. Urinary p -Nitrophenol As a Biomarker of Household Exposure to Methyl Parathion. Environ. Health Perspect. 2002, 110, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.; Li, Q.; Kennedy, R.T. Review of recent advances in analytical techniques for the determination of neurotransmitters. Anal. Chim. Acta 2009, 653, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.L.; Hermans, A.; Seipel, A.T.; Wightman, R.M. Monitoring rapid chemical communication in the brain. Chem. Rev. 2008, 108, 2554–2584. [Google Scholar] [CrossRef] [PubMed]
- Santhiago, M.; Maroneze, C.M.; Silva, C.C.C.; Camargo, M.N.L.; Kubota, L.T. Electrochemical Oxidation of Glassy Carbon Provides Similar Electrochemical Response as Graphene Oxide Prepared by Tour or Hummers Routes. ChemElectroChem 2015, 2, 761–767. [Google Scholar] [CrossRef]
- Zhou, M.H.; Lei, L.C. Electrochemical regeneration of activated carbon loaded with p -nitrophenol in a fluidized electrochemical reactor. Electrochim. Acta 2006, 51, 4489–4496. [Google Scholar] [CrossRef]
- Manes, M. Recovery of Activated Carbon. U.S. Patent 4,058,457, 15 November 1977. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Potential sweep methods. In Electrochemical Methods Fundamentals and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; pp. 226–260. ISBN 9780123813749. [Google Scholar]
- Friedlander, S.K. Mass and heat transfer to single spheres and cylinders at low Reynolds numbers. AIChE J. 1957, 3, 43–48. [Google Scholar] [CrossRef]
- Wung, T.-S.; Chen, C.J. Finite Analytic Solution of Convective Heat Transfer for Tube Arrays in Crossflow: Part II—Heat Transfer Analysis. J. Heat Transf. 1989, 111, 641. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, X.; Li, H.; Ni, J. Effect of nitro substituent on electrochemical oxidation of phenols at boron-doped diamond anodes. Chemosphere 2010, 78, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Asadpour-Zeynali, K.; Najafi-Marandi, P. Bismuth modified disposable pencil-lead electrode for simultaneous determination of 2-nitrophenol and 4-nitrophenol by net analyte signal standard addition method. Electroanalysis 2011, 23, 2241–2247. [Google Scholar] [CrossRef]
- Kawde, A.N.; Aziz, M.A. Porous Copper-Modified Graphite Pencil Electrode for the Amperometric Detection of 4-Nitrophenol. Electroanalysis 2014, 26, 2484–2490. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Khodadadian, M.; Bahrami, G. Molecularly Imprinted Polymer Preconcentration and Flow Injection Amperometric Determination of 4-Nitrophenol in Water. Anal. Lett. 2015, 48, 2856–2869. [Google Scholar] [CrossRef]
- Wo, A.; Hogan, C.; Elton, D.; Loke, S. Mobile voltammetric analysis. International Patent Application No. PCT/AU20 17/050232, 1 September 2017. [Google Scholar]
- East, G.A.; del Valle, M.A. Easy-to-Make Ag/AgCl Reference Electrode. J. Chem. Educ. 2000, 77, 97. [Google Scholar] [CrossRef]
- Galván-Valencia, M.; Albino-Navarro, G.; Flores-Morales, V.; Durón-Torres, S.M. Pencil Electrodes for Determination of Dopamine, Serotonine and Ascorbic Acid. ECS Trans. 2008, 15, 479–487. [Google Scholar]
- Özcan, A.; Yücel, S. Selective and sensitive voltammetric determination of dopamine in blood by electrochemically treated pencil graphite electrodes. Electroanalysis 2009, 21, 2363–2370. [Google Scholar] [CrossRef]
- Shou, M.; Ferrario, C.R.; Schultz, K.N.; Robinson, T.E.; Kennedy, R.T. Monitoring dopamine in vivo by microdialysis sampling and on-line ce-laser-induced fluorescence. Anal. Chem. 2006, 78, 6717–6725. [Google Scholar] [CrossRef] [PubMed]
- Tukimin, N.; Abdullah, J.; Sulaiman, Y. Review—Electrochemical Detection of Uric Acid, Dopamine and Ascorbic Acid. J. Electrochem. Soc. 2018, 165, B258–B267. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witkowska Nery, E.; Kundys-Siedlecka, M.; Furuya, Y.; Jönsson-Niedziółka, M. Pencil Lead as a Material for Microfluidic 3D-Electrode Assemblies. Sensors 2018, 18, 4037. https://doi.org/10.3390/s18114037
Witkowska Nery E, Kundys-Siedlecka M, Furuya Y, Jönsson-Niedziółka M. Pencil Lead as a Material for Microfluidic 3D-Electrode Assemblies. Sensors. 2018; 18(11):4037. https://doi.org/10.3390/s18114037
Chicago/Turabian StyleWitkowska Nery, Emilia, Magdalena Kundys-Siedlecka, Yoshitaka Furuya, and Martin Jönsson-Niedziółka. 2018. "Pencil Lead as a Material for Microfluidic 3D-Electrode Assemblies" Sensors 18, no. 11: 4037. https://doi.org/10.3390/s18114037
APA StyleWitkowska Nery, E., Kundys-Siedlecka, M., Furuya, Y., & Jönsson-Niedziółka, M. (2018). Pencil Lead as a Material for Microfluidic 3D-Electrode Assemblies. Sensors, 18(11), 4037. https://doi.org/10.3390/s18114037