A Comparative Study of Interdigitated Electrode and Quartz Crystal Microbalance Transduction Techniques for Metal–Organic Framework-Based Acetone Sensors
Abstract
1. Introduction
2. Materials and Methods
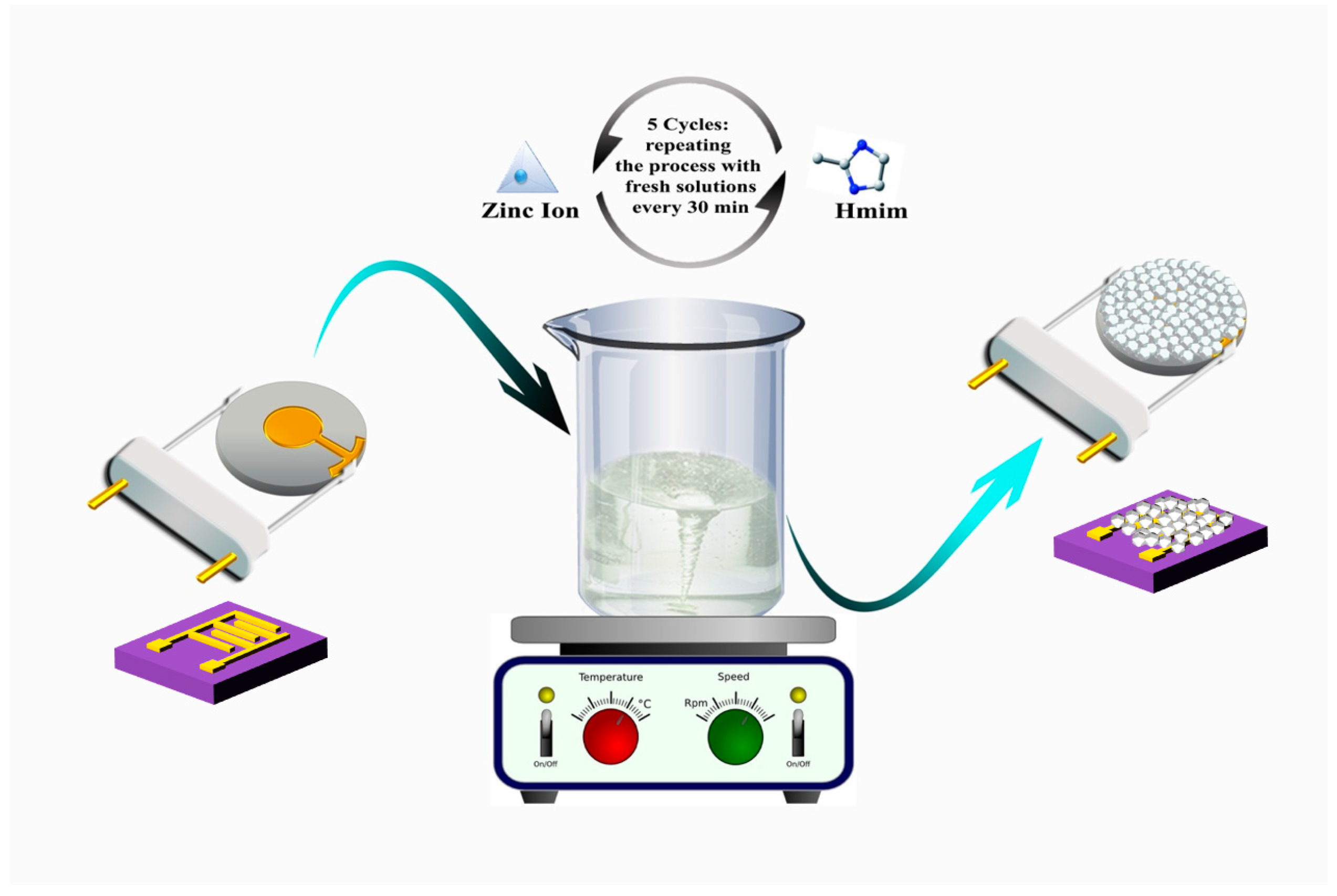
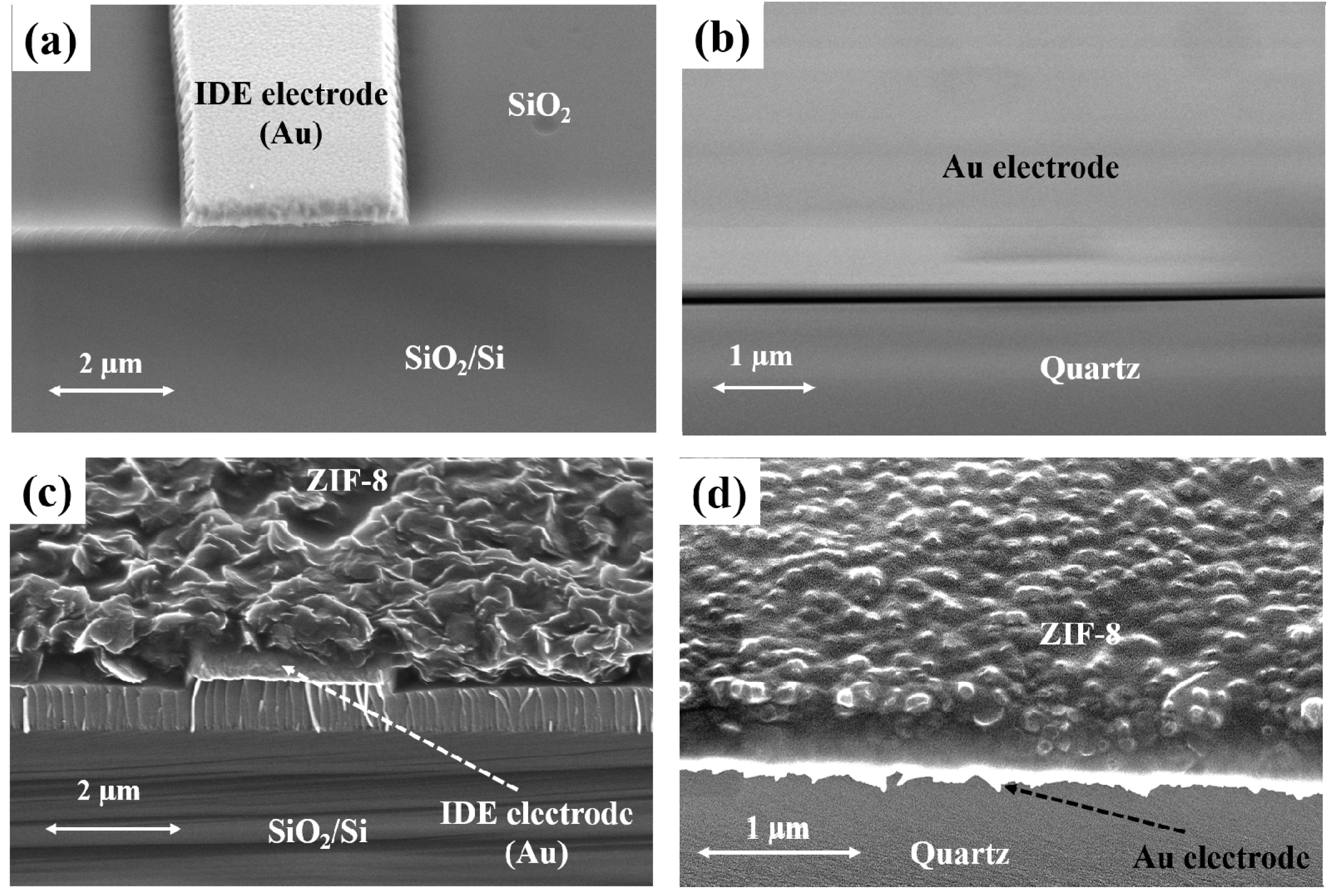
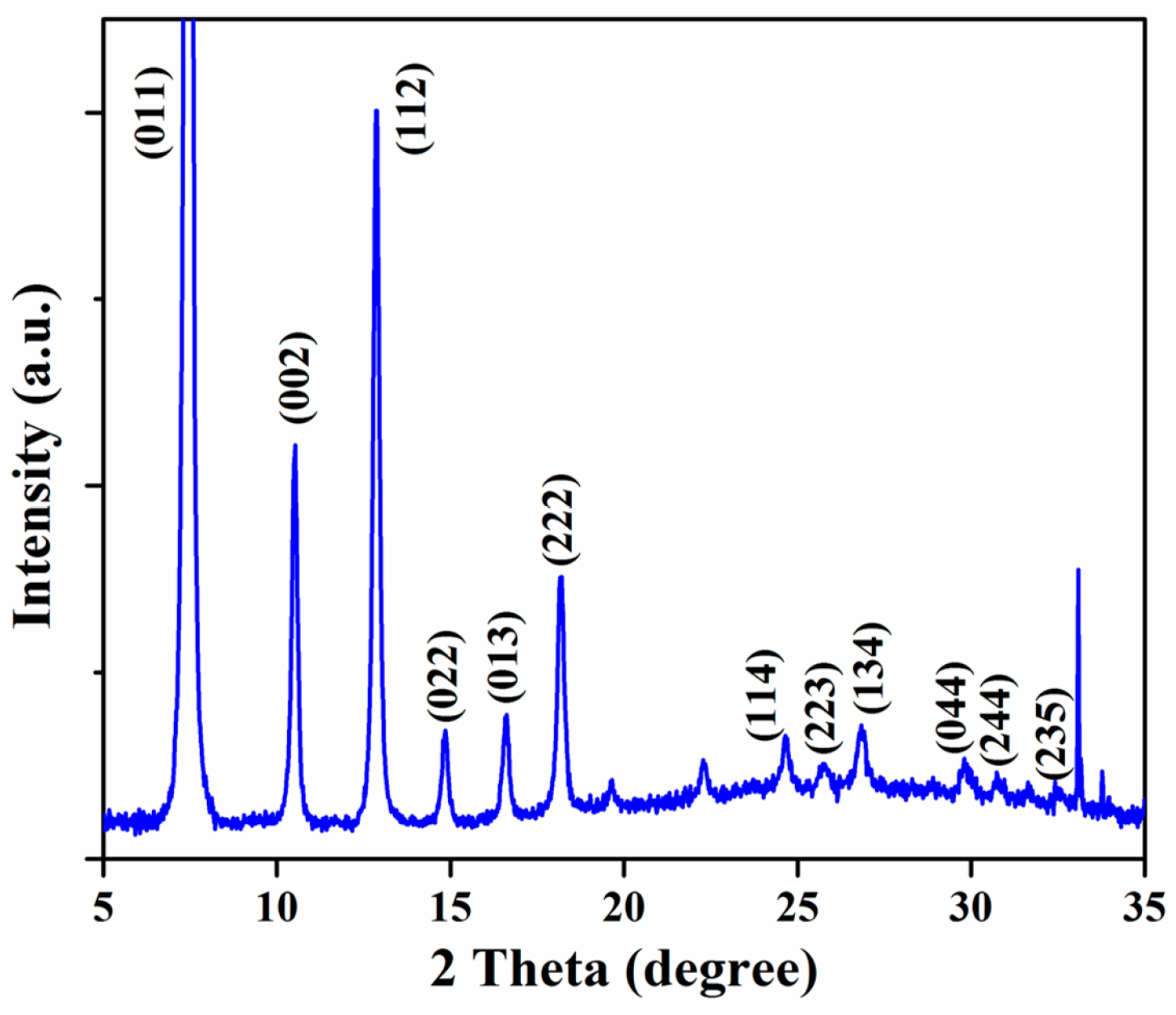
2.1. Material Preparation and Characterization
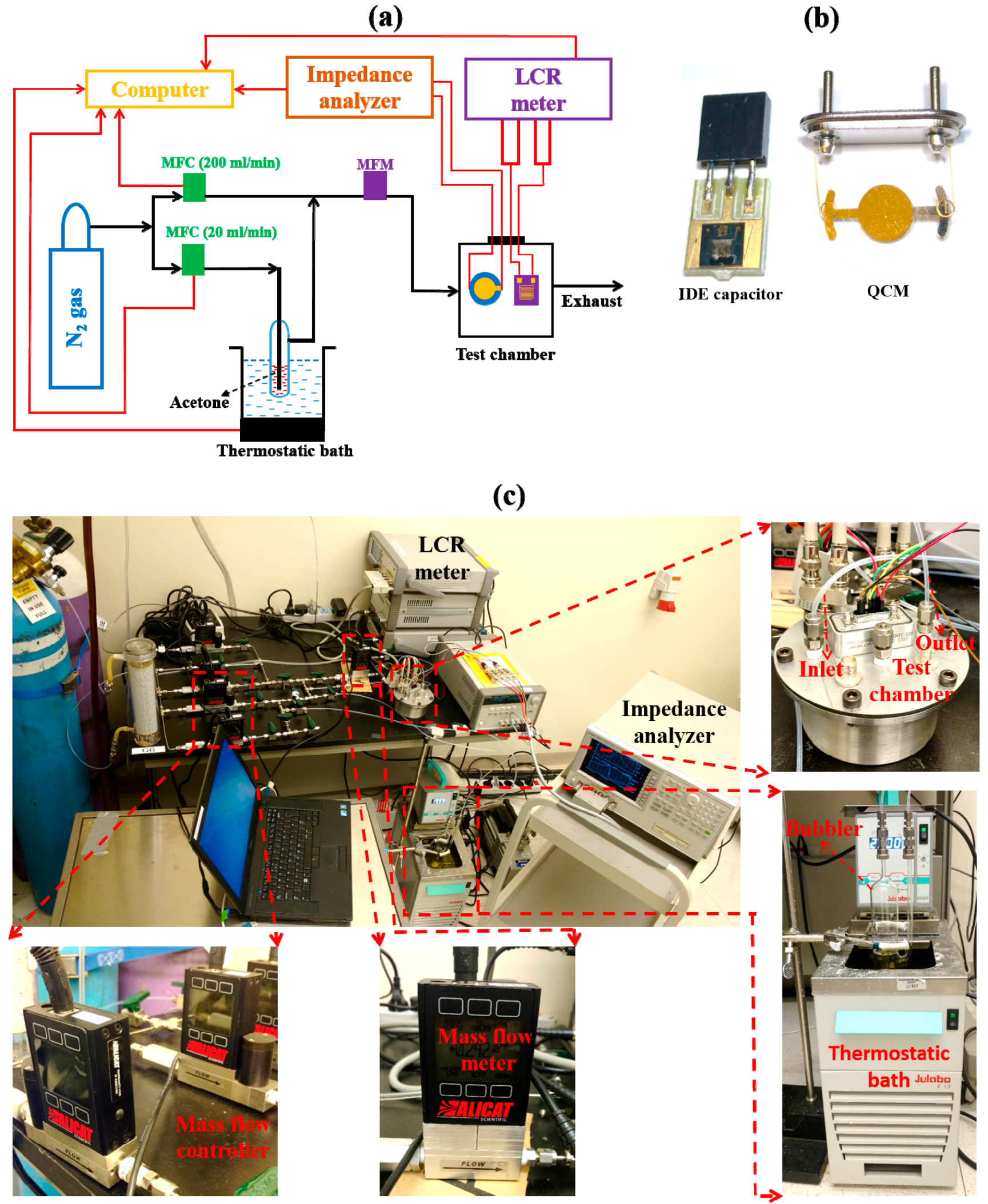
2.2. Experimental Setup
3. Results and Discussions
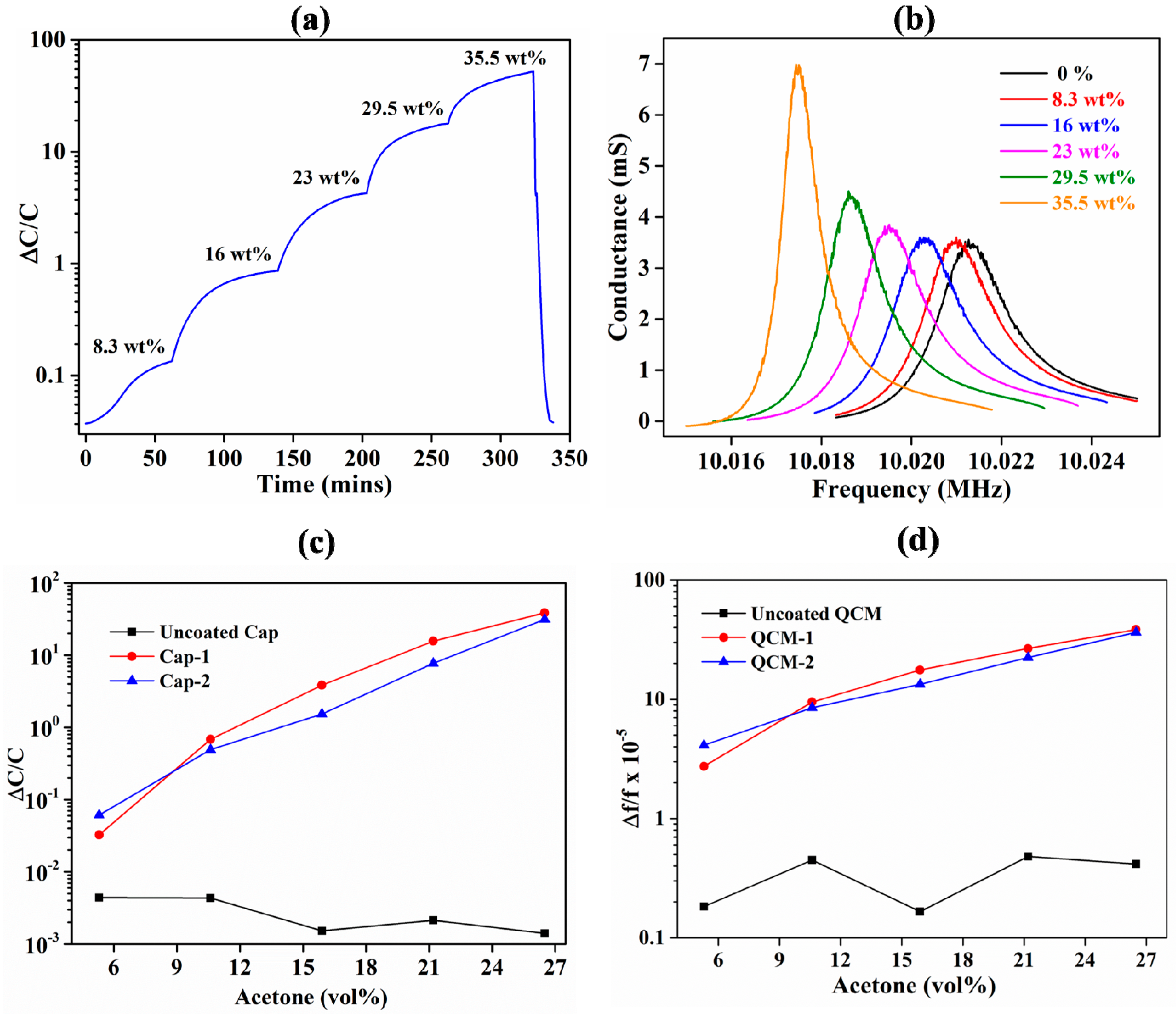
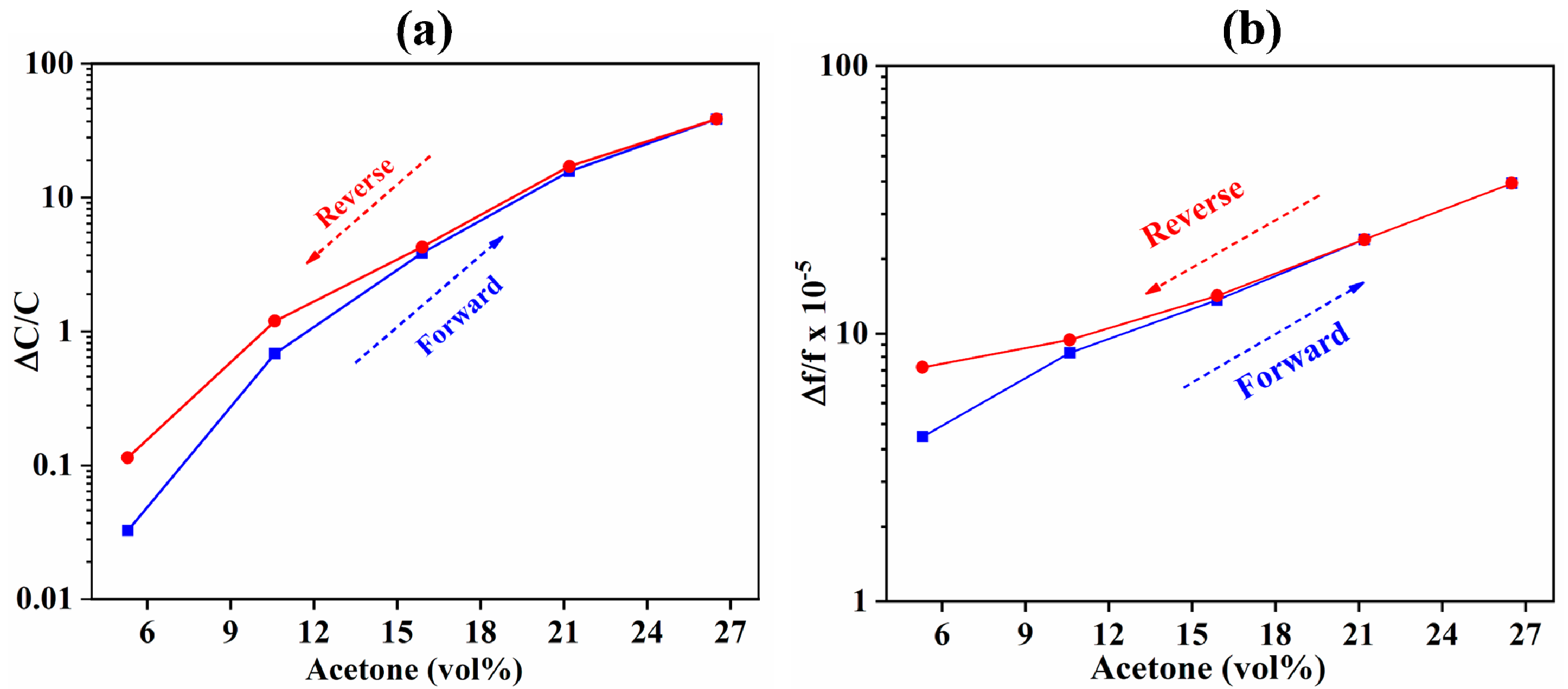
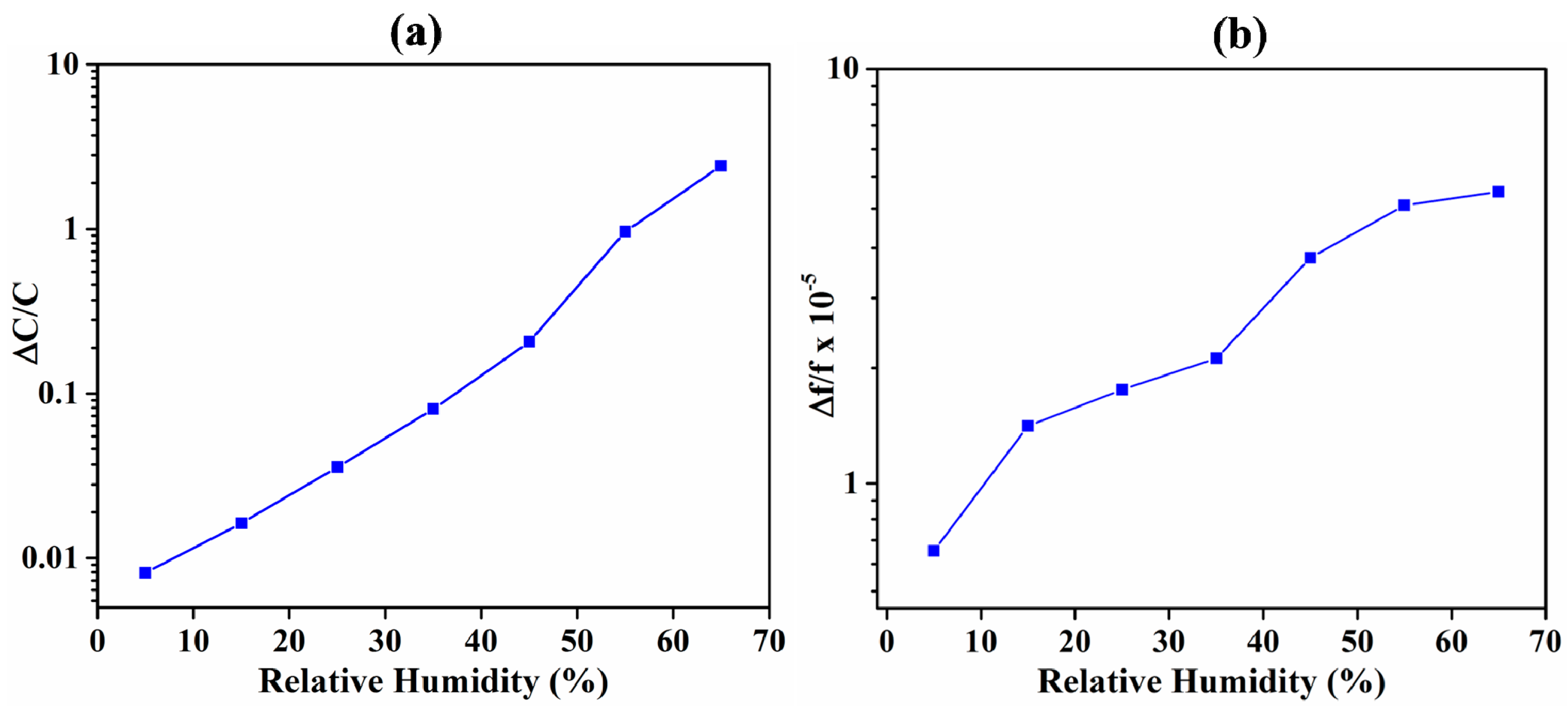
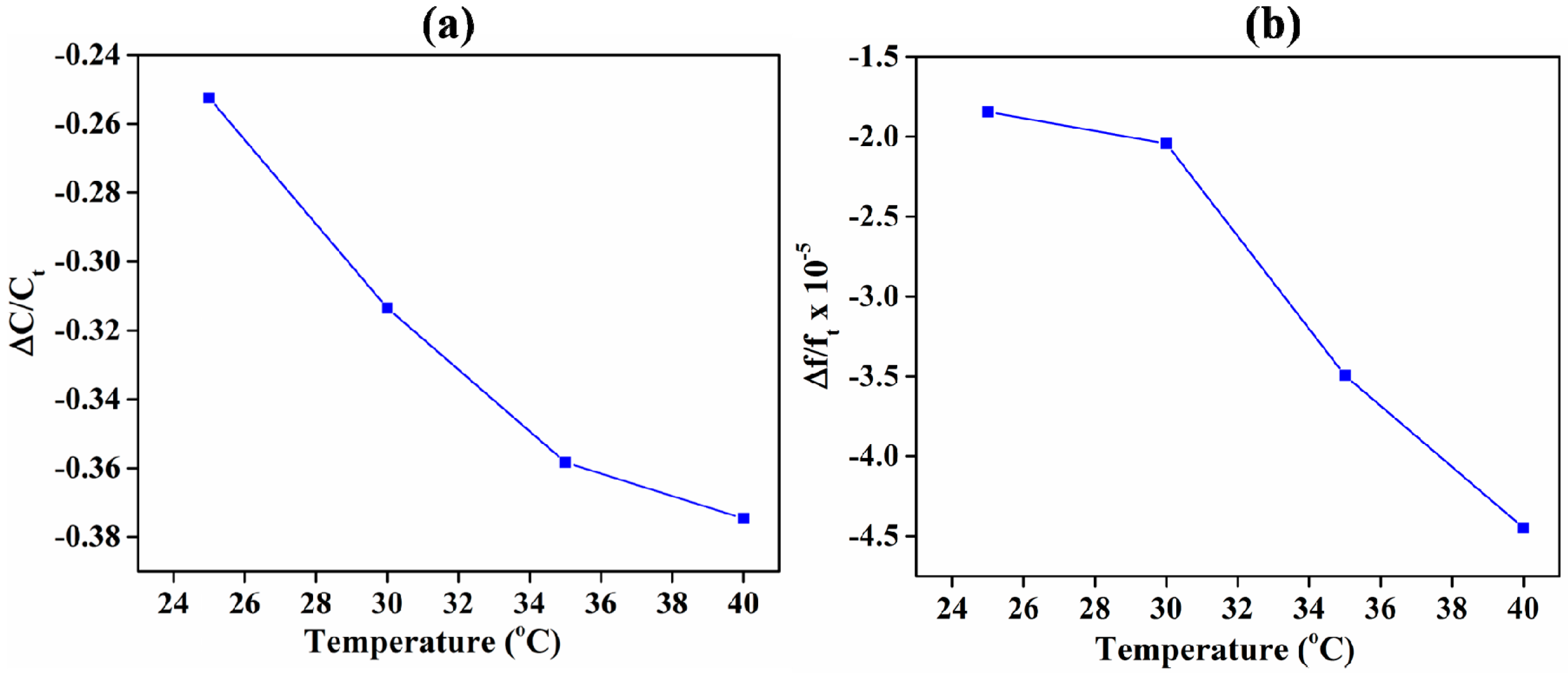
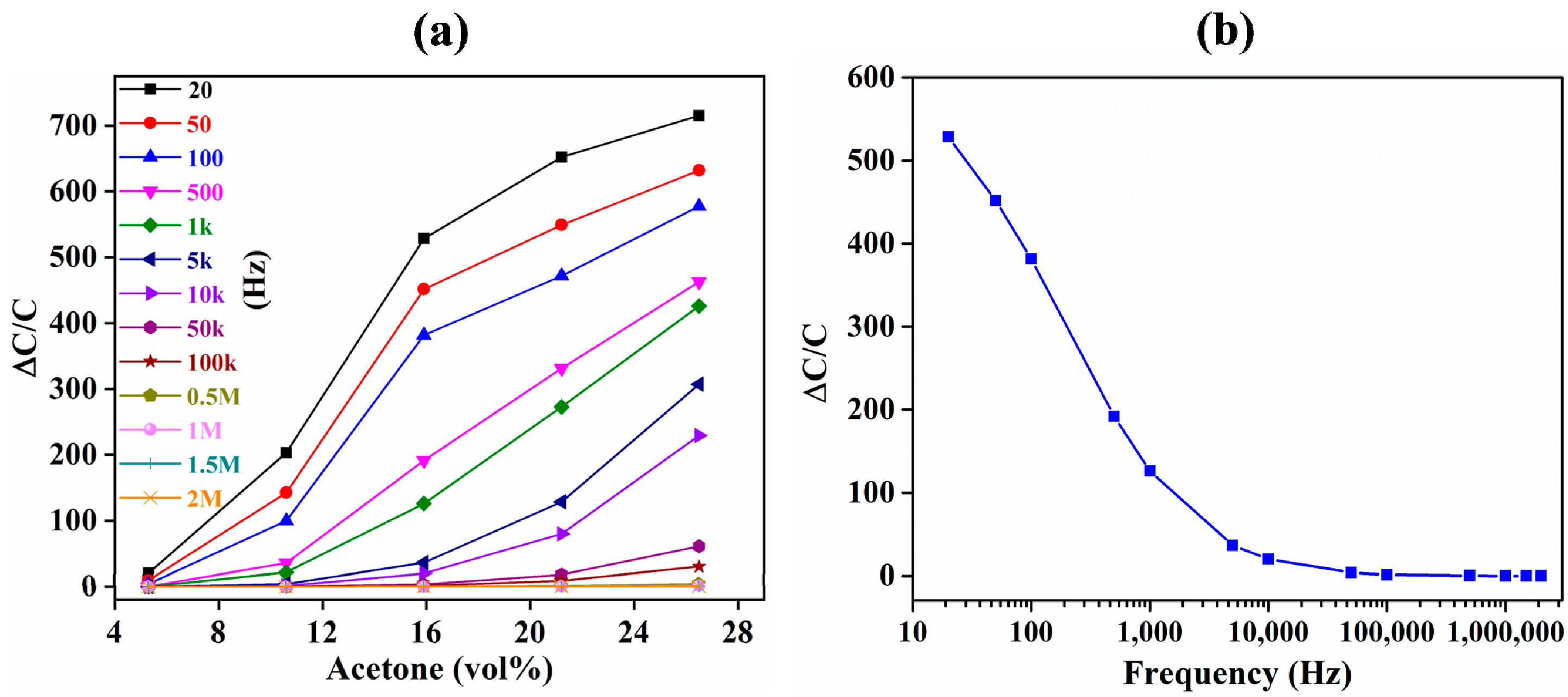
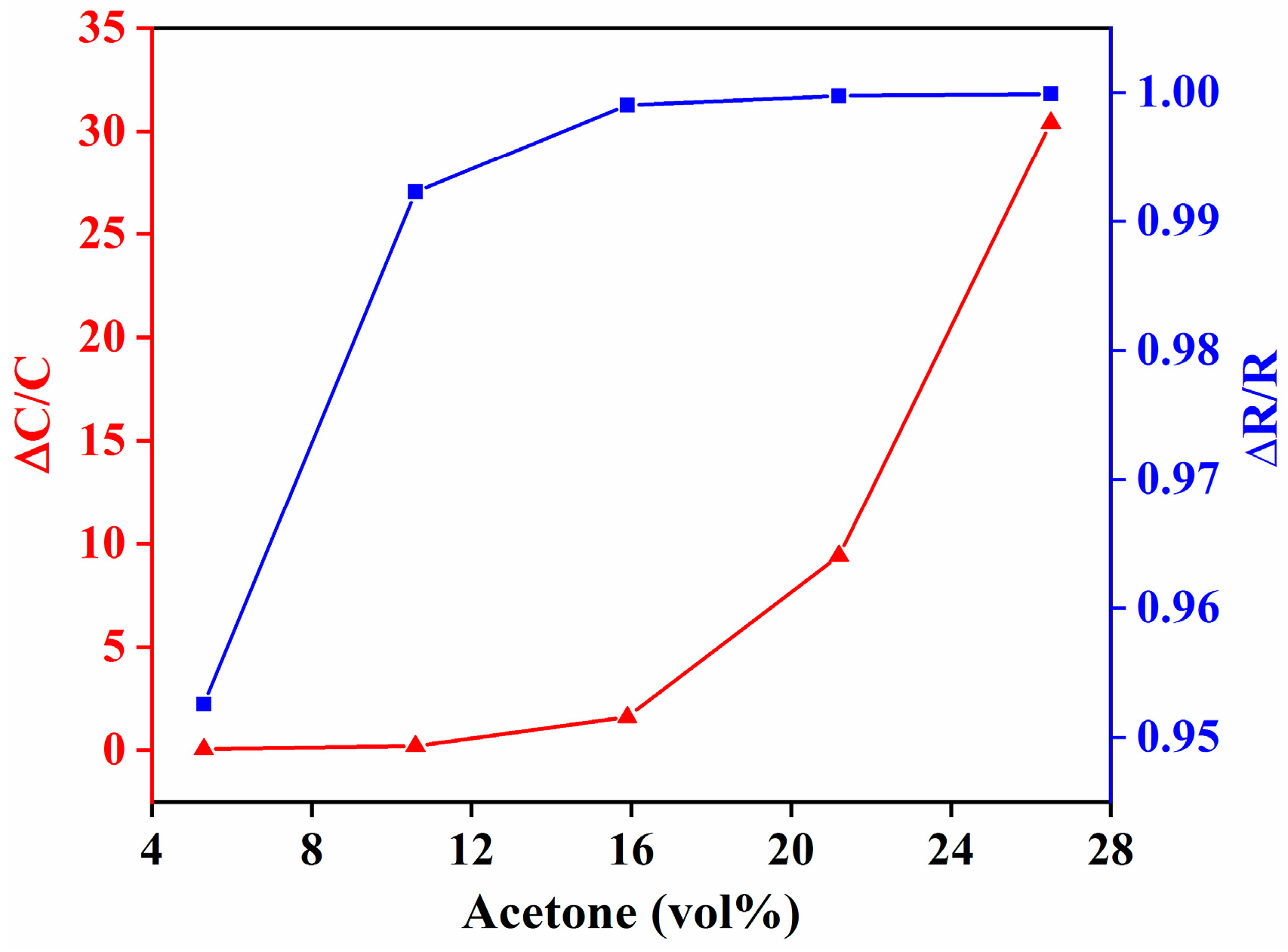
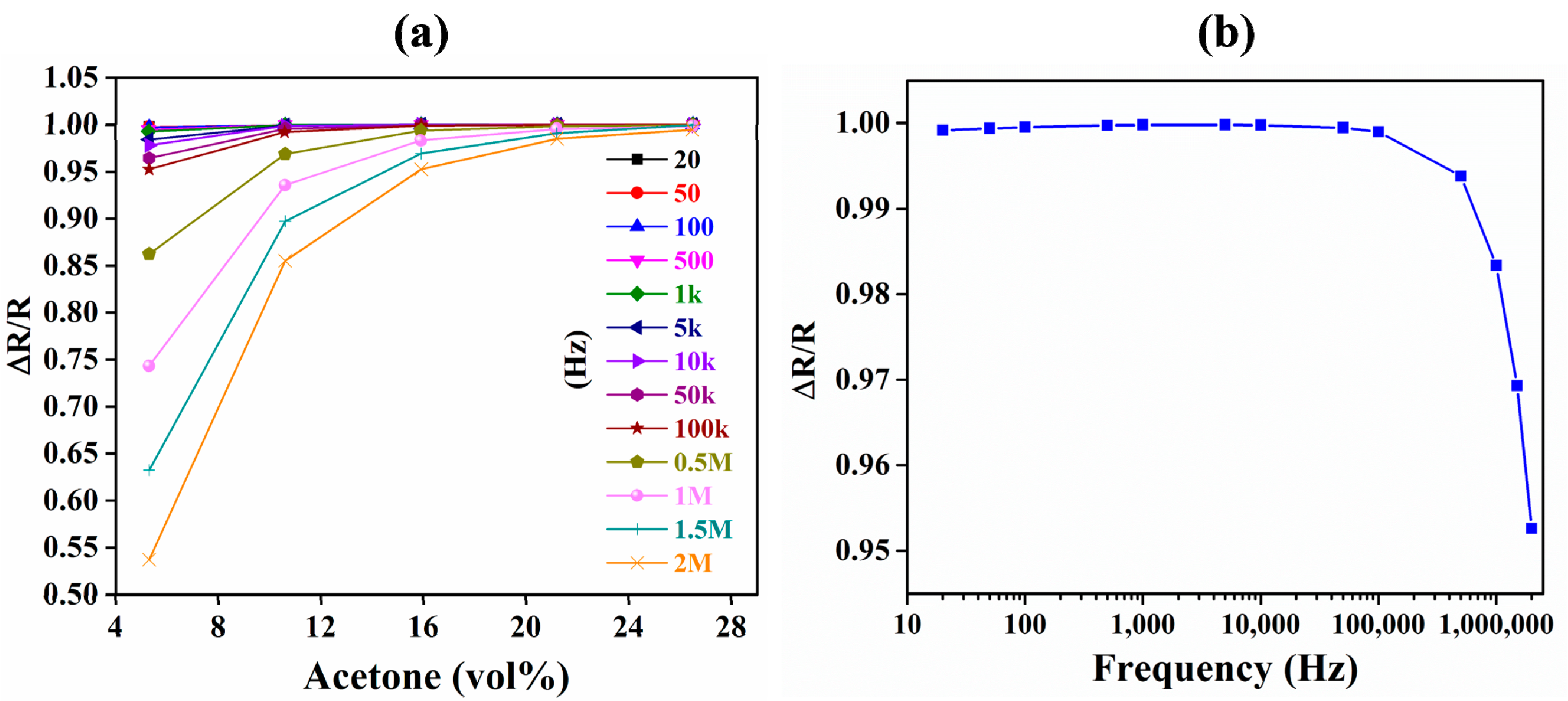
3.1. Comparison of IDE-Capacitive- and QCM-Based Sensing
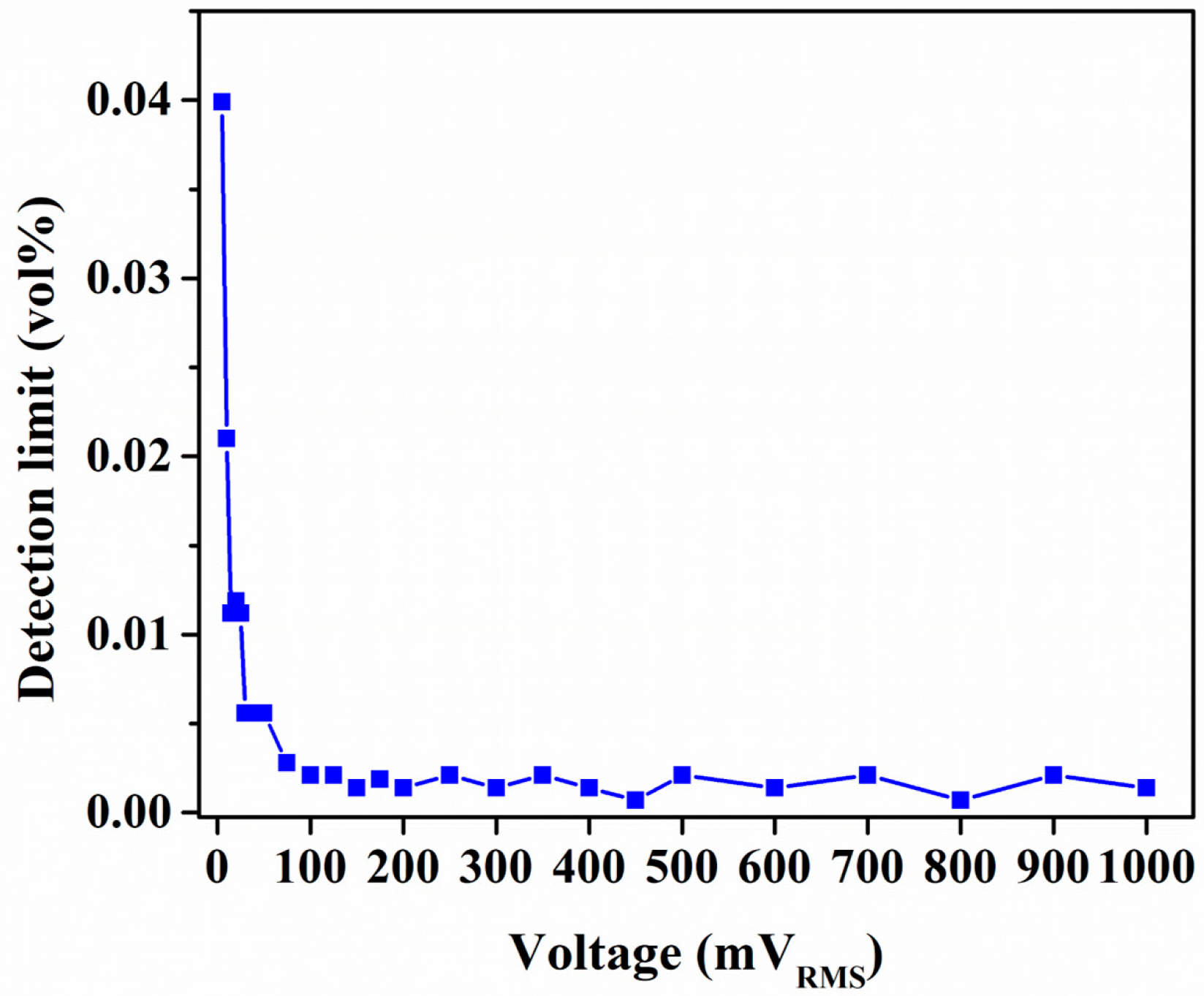
3.2. Comparison of Capacitive and Resistive Transduction-Based Sensing Using IDE Substrates
3.3. ZIF-8 as an Acetone-Sensing Material
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, E.; Mohammadi, A.V.; Prorok, B.C.; Yoon, Y.S.; Beidaghi, M.; Kim, D.-J. Room Temperature Gas Sensing of Two-Dimensional Titanium Carbide (MXene). ACS Appl. Mater. Interfaces 2017, 9, 37184–37190. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.-S.; Tang, W.-X.; Wang, G.-E.; Nath, B.; Xu, G. MOF Thin Film-Coated Metal Oxide Nanowire Array: Significantly Improved Chemiresistor Sensor Performance. Adv. Mater. 2016, 28, 5229–5234. [Google Scholar] [CrossRef] [PubMed]
- Rydosz, A.; Szkudlarek, A.; Ziabka, M.; Domanski, K.; Maziarz, W.; Pisarkiewicz, T. Performance of Si-Doped WO3 Thin Films for Acetone Sensing Prepared by Glancing Angle DC Magnetron Sputtering. IEEE Sens. J. 2016, 16, 1004–1012. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, A.; Chang, H.; Xi, B. Room-temperature high-performance acetone gas sensor based on hydrothermal synthesized SnO2-reduced graphene oxide hybrid composite. RSC Adv. 2015, 5, 3016–3022. [Google Scholar] [CrossRef]
- Li, X.B.; Ma, S.Y.; Li, F.M.; Chen, Y.; Zhang, Q.Q.; Yang, X.H.; Wang, C.Y.; Zhu, J. Porous spheres-like ZnO nanostructure as sensitive gas sensors for acetone detection. Mater. Lett. 2013, 100, 119–123. [Google Scholar] [CrossRef]
- Righettoni, M.; Tricoli, A.; Gass, S.; Schmid, A.; Amann, A.; Pratsinis, S.E. Breath acetone monitoring by portable Si: WO3 gas sensors. Anal. Chim. Acta 2012, 738, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Bamsaoud, S.F.; Rane, S.B.; Karekar, R.N.; Aiye, R.C. Nano particulate SnO2 based resistive films as a hydrogen and acetone vapour sensor. Sens. Actuators B 2011, 153, 382–391. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Zhuang, J.; Wang, L.; Zhang, J.; Li, H.; Liu, Z.; Han, Y.; Jiang, X.; Zhang, P. Improved selective acetone sensing properties of Co-doped ZnO nanofibers by electrospinning. Sens. Actuators B 2011, 155, 782–788. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, T.; Yuan, M.; Kang, M.; Lu, G.; Wang, R.; Fan, H.; He, Y.; Yang, H. Growth and selective acetone detection based on ZnO nanorod arrays. Sens. Actuators B 2009, 143, 93–98. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, T.; Liu, L.; Zheng, X.; Yu, Q.; Zeng, Y.; Yang, H. Selective acetone sensor based on dumbbell-like ZnO with rapid response and recovery. Sens. Actuators B 2008, 134, 166–170. [Google Scholar] [CrossRef]
- Rezlescu, N.; Iftimie, N.; Rezlescu, E.; Doroftei, C.; Popa, P.D. Semiconducting gas sensor for acetone based on the fine grained nickel ferrite. Sens. Actuators B 2006, 114, 427–432. [Google Scholar] [CrossRef]
- Zhao, J.; Huo, L.-H.; Gao, S.; Zhao, H.; Zhao, J.-G. Alcohols and acetone sensing properties of SnO2 thin films deposited by dip-coating. Sens. Actuators B 2006, 115, 460–464. [Google Scholar] [CrossRef]
- Bene, R.; Perczel, I.V.; Réti, F.; Meyer, F.A.; Fleisher, M.; Meixner, H. Chemical reactions in the detection of acetone and NO by a CeO2 thin film. Sens. Actuators B 2000, 71, 36–41. [Google Scholar] [CrossRef]
- Savagatrup, S.; Schroeder, V.; He, X.; Lin, S.; He, M.; Yassine, O.; Salama, K.N.; Zhang, X.-X.; Swager, T.M. Bio-Inspired Carbon Monoxide Sensors with Voltage-Activated Sensitivity. Angew. Chem. 2017, 56, 14066–14070. [Google Scholar] [CrossRef] [PubMed]
- Minh, Q.N.; Tong, H.D.; Kuijk, A.; Bent, F.V.D.; Beekman, P.; van Rijn, C.J.M. Gas sensing performance at room temperature of nanogap interdigitated electrodes for detection of acetone at low concentration. RSC Adv. 2017, 7, 50279–50286. [Google Scholar] [CrossRef]
- Sapsanis, C.; Omran, H.; Chernikova, V.; Shekhah, O.; Belmabkhout, Y.; Buttner, U.; Eddaoudi, M.; Salama, K.N. Insights on Capacitive Interdigitated Electrodes Coated with MOF Thin Films: Humidity and VOCs Sensing as a Case Study. Sensors 2015, 15, 18153–18166. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-Z.; Dai, C.-L.; Shih, P.-J. An Acetone Microsensor with a Ring Oscillator Circuit Fabricated Using the Commercial 0.18 μm CMOS Process. Sensors 2014, 14, 12735–12747. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.A.; Snow, E.S.; Perkins, F.K. Improved chemical detection using single-walled carbon nanotube network capacitors. Sens. Actuators A 2007, 135, 309–314. [Google Scholar] [CrossRef]
- Snow, E.S.; Perkins, F.K.; Houser, E.J.; Badescu, S.C.; Reinecke, T.L. Chemical detection with a single-walled carbon nanotube capacitor. Science 2005, 307, 1942–1945. [Google Scholar] [CrossRef] [PubMed]
- Amrani, M.E.H.; Persaud, K.C.; Payne, P.A. High-frequency measurements of conducting polymers: Development of a new technique for sensing volatile chemicals. Meas. Sci. Technol. 1995, 6, 1500. [Google Scholar] [CrossRef]
- Chernikova, V.; Yassine, O.; Shekhah, O.; Eddaoudi, M.; Salama, K.N. Highly sensitive and selective SO2 MOF sensor: The integration of MFM-300 MOF as a sensitive layer on a capacitive interdigitated electrode. J. Mater. Chem. A 2018, 6, 5550–5554. [Google Scholar] [CrossRef]
- Assen, A.H.; Yassine, O.; Shekhah, O.; Eddaoudi, M.; Salama, K.N. MOFs for the Sensitive Detection of Ammonia: Deployment of fcu-MOF Thin Films as Effective Chemical Capacitive Sensors. ACS Sens. 2017, 2, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Chappanda, K.N.; Tabib-Azar, M. Electrical and AFM Structural Studies of a Humidity Sensors Based on Keratin (Human Hair). In Proceedings of the IEEE Sensors, Limerick, Ireland, 28–31 October 2011; pp. 1062–1065. [Google Scholar]
- Alhoshany, A.; Sivashankar, S.; Mashraei, Y.; Omran, H.; Salama, K.N. A Biosensor-CMOS Platform and Integrated Readout Circuit in 0.18-μm CMOS Technology for Cancer Biomarker Detection. Sensors 2017, 17, 1942. [Google Scholar] [CrossRef] [PubMed]
- Yassine, O.; Shekhah, O.; Assen, A.H.; Belmabkhout, Y.; Salama, K.N.; Eddaoudi, M. H2S Sensors: Fumarate-Based fcu-MOF Thin Film Grown on a Capacitive Interdigitated Electrode. Angew. Chem. Int. Ed. 2016, 55, 15879–15883. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Fanguy, J.C.; Soni, K.; Tao, S. Optical fiber humidity sensor based on evanescent-wave scattering. Opt. Lett. 2004, 29, 1191–1193. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, F.K.; Chappanda, K.N.; Tabib-Azar, M. High enhancement SERS substrates created using DEP-DLA & annealing Au-W. In Proceedings of the IEEE Sensors, Limerick, Ireland, 28–31 October 2011; pp. 1329–1332. [Google Scholar]
- Yuan, W.; Chappanda, K.N.; Tabib-Azar, M. Microfabricated atmospheric RF microplasma devices for gas spectroscopy. In Proceedings of the 15th International Conference on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS, Seattle, WA, USA, 2–6 October 2011; pp. 568–570. [Google Scholar]
- Hromadka, J.; Tokay, B.; Correia, R.; Morgan, S.P.; Korposh, S. Highly sensitive volatile organic compounds vapour measurements using a long period grating optical fibre sensor coated with metal organic framework ZIF-8. Sens. Actuators B 2018, 260, 685–692. [Google Scholar] [CrossRef]
- Matatagui, D.; Kolokoltsev, O.V.; Qureshi, N.; Mejía-Uriarte, E.V.; Saniger, J.M. A novel ultra-high frequency humidity sensor based on a magnetostatic spin wave oscillator. Sens. Actuators B 2015, 210, 297–301. [Google Scholar] [CrossRef]
- Kimura, M. A new method to measure absolute humidity independently of the ambient temperature. Sens. Actuators B 1996, 33, 156–160. [Google Scholar] [CrossRef]
- Staszek, K.; Rydosz, A.; Maciak, E.; Wincza, K.; Gruszczynski, S. Six-port microwave system for volatile organic compounds detection. Sens. Actuators B 2017, 245, 882–894. [Google Scholar] [CrossRef]
- Rydosz, A.; Maciak, E.; Wincza, K.; Gruszczynski, S. Microwave-based sensors with phthalocyanine films for acetone, ethanol and methanol detection. Sens. Actuators B 2016, 237, 876–886. [Google Scholar] [CrossRef]
- Chappanda, K.N.; Shekhah, O.; Yassine, O.; Patole, S.P.; Eddaoudi, M.; Salama, K.N. The quest for highly sensitive QCM humidity sensors: The coating of CNT/MOF composite sensing films as case study. Sens. Actuators B 2018, 257, 609–619. [Google Scholar] [CrossRef]
- Öztürk, S.; Kösemen, A.; Kösemen, Z.A.; Kılınç, N.; Öztürk, Z.Z.; Penzag, M. Electrochemically growth of Pd doped ZnO nanorods on QCM for room temperature VOC sensors. Sens. Actuators B 2016, 222, 280–289. [Google Scholar] [CrossRef]
- Xu, X.; Li, C.; Pei, K.; Zhao, K.; Zhao, Z.K.; Li, H. Ionic liquids used as QCM coating materials for the detection of alcohols. Sens. Actuators B 2008, 134, 258–265. [Google Scholar] [CrossRef]
- Si, P.; Mortensen, J.; Komolov, A.; Denborg, J.; Møller, P.J. Polymer coated quartz crystal microbalance sensors for detection of volatile organic compounds in gas mixtures. Anal. Chim. Acta 2007, 597, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Koshets, I.A.; Kazantseva, Z.I.; Shirshov, Y.M.; Cherenok, S.A.; Kalchenko, V.I. Calixarene films as sensitive coatings for QCM-based gas sensors. Sens. Actuators B 2005, 106, 177–181. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, J.; Chen, S.; Zeng, L.; Huang, Y. A highly sensitive QCM sensor coated with Ag+-ZSM-5 film for medical diagnosis. Sens. Actuators B 2004, 101, 316–321. [Google Scholar] [CrossRef]
- Zeinali, S.; Homayoonnia, S.; Homayoonnia, G. Comparative investigation of interdigitated and Parallel-plate capacitive gas sensors based on Cu-BTC nanoparticles for selective detection of polar and apolar VOCs indoors. Sens. Actuators B 2019, 278, 153–164. [Google Scholar] [CrossRef]
- Silva, P.; Vilela, S.M.F.; Tomé, J.P.C.; Paz, F.A.A. Multifunctional metal–organic frameworks: From academia to industrial applications. Chem. Soc. Rev. 2015, 44, 6774–6803. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Y.; Li, D.-S.; Bu, X.; Feng, P. Metal–Organic Frameworks for Separation. Adv. Mater. 2018, 30, 1705189. [Google Scholar] [CrossRef] [PubMed]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Féreya, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular chemistry: Secondary building units as a basis for the design of highly porous and robust metal–organic carboxylate frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to metal–organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Shekhah, O.; Liu, J.; Fischer, R.A.; Wöll, C. MOF thin films: Existing and future applications. Chem. Soc. Rev. 2011, 40, 1081–1106. [Google Scholar] [CrossRef] [PubMed]
- Shekhah, O.; Eddaoudi, M. The liquid phase epitaxy method for the construction of oriented ZIF-8 thin films with controlled growth on functionalized surfaces. Chem. Commun. 2013, 49, 10079–10081. [Google Scholar] [CrossRef] [PubMed]
- Azzama, E.M.S.; Bashir, A.; Shekhah, O.; Alawady, A.R.E.; Birkner, A.; Grunwald, C.; Wöll, C. Fabrication of a surface plasmon resonance biosensor based on gold nanoparticles chemisorbed onto a 1, 10-decanedithiol self-assembled monolayer. Thin Solid Films 2009, 518, 387–391. [Google Scholar] [CrossRef]
- Chernikova, V.; Shekhah, O.; Eddaoudi, M. Advanced fabrication method for the preparation of MOF thin films: Liquid-phase epitaxy approach meets spin coating method. ACS Appl. Mater. Interfaces 2016, 8, 20459–20464. [Google Scholar] [CrossRef] [PubMed]
- Shekhah, O.; Arslan, H.K.; Chen, K.; Schmittel, M.; Maul, R.; Wenzele, W.; Wölla, C. Post-synthetic modification of epitaxially grown, highly oriented functionalized MOF thin films. Chem. Commun. 2011, 47, 11210–11212. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Hupp, J.T. Metal–organic frameworks as sensors: A ZIF-8 based Fabry–Pérot device as a selective sensor for chemical vapors and gases. J. Am. Chem. Soc. 2010, 132, 7832–7833. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhu, X.; Liu, T.; Zhao, H.; Lan, M. Encapsulating Cu nanoparticles into metal-organic frameworks for nonenzymatic glucose sensing. Sens. Actuators B 2016, 227, 583–590. [Google Scholar] [CrossRef]
- Zhan, W.-W.; Kuang, Q.; Zhou, J.-Z.; Kong, X.-J.; Xie, Z.-X.; Zheng, L.-S. Semiconductor@ metal–organic framework core–shell heterostructures: A case of ZnO@ ZIF-8 nanorods with selective photoelectrochemical response. J. Am. Chem. Soc. 2013, 135, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Drobek, M.; Kim, J.-H.; Bechelany, M.; Vallicari, C.; Julbe, A.; Kim, S.S. MOF-based membrane encapsulated ZnO nanowires for enhanced gas sensor selectivity. ACS Appl. Mater. Interfaces 2016, 8, 8323–8328. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.-X.; Yang, H.; Zhang, J. Zeolitic imidazolate framework as formaldehyde gas sensor. Inorg. Chem. 2014, 53, 5411–5413. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.H.; Fan, H.; Li, M.; Ma, L. Zeolitic imidazolate framework coated ZnO nanorods as molecular sieving to improve selectivity of formaldehyde gas sensor. ACS Sens. 2016, 1, 243–250. [Google Scholar] [CrossRef]
- Xia, J.; Diao, K.; Zhenga, Z.; Cui, X. Porous Au/ZnO nanoparticles synthesised through a metal organic framework (MOF) route for enhanced acetone gas-sensing. RSC Adv. 2017, 7, 38444–38451. [Google Scholar] [CrossRef]
- Shia, G.M.; Yang, T.; Chung, T.S. Polybenzimidazole (PBI)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of alcohols. J. Membr. Sci. 2012, 415–416, 577–586. [Google Scholar] [CrossRef]
- Kwon, H.T.; Jeong, H.-K. In situ synthesis of thin zeolitic–imidazolate framework ZIF-8 membranes exhibiting exceptionally high propylene/propane separation. J. Am. Chem. Soc. 2013, 135, 10763–10768. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Liu, Q.; Wang, N.; Zhu, Y.; Caro, J. Bicontinuous zeolitic imidazolate framework ZIF-8@ GO membrane with enhanced hydrogen selectivity. J. Am. Chem. Soc. 2014, 136, 14686–14689. [Google Scholar] [CrossRef] [PubMed]
- Tran, U.P.N.; Le, K.K.A.; Phan, N.T.S. Expanding applications of metal−organic frameworks: Zeolite imidazolate framework ZIF-8 as an efficient heterogeneous catalyst for the knoevenagel reaction. ACS Catal. 2011, 1, 120–127. [Google Scholar] [CrossRef]
- Sorribas, S.; Zornoza, B.; Téllez, C.; Coronas, J. Mixed matrix membranes comprising silica-(ZIF-8) core–shell spheres with ordered meso–microporosity for natural-and bio-gas upgrading. J. Membr. Sci. 2014, 452, 184–192. [Google Scholar] [CrossRef]
- Buchan, I.; Ryder, M.R.; Tan, J.-C. Micromechanical behavior of polycrystalline metal–organic framework thin films synthesized by electrochemical reaction. Cryst. Growth Des. 2015, 15, 1991–1999. [Google Scholar] [CrossRef]
- Omran, H.; Arsalan, M.; Salama, K.N. An integrated energy-efficient capacitive sensor digital interface circuit. Sens. Actuators A 2014, 216, 43–51. [Google Scholar] [CrossRef]
- Alhoshany, A.; Omran, H.; Salama, K.N. A 45.8fJ/Step, Energy-Efficient, Differential SAR Capacitance-to-Digital Converter for Capacitive Pressure Sensing. Sens. Actuators A 2016, 245, 10–18. [Google Scholar] [CrossRef]
- Omran, H.; Alhoshany, A.; Alahmadi, H.; Salama, K.N. A 33fJ/Step SAR Capacitance-to-Digital Converter Using a Chain of Inverter-Based Amplifiers. IEEE Trans. Circuits Syst. I 2017, 64, 310–321. [Google Scholar] [CrossRef]
- Sauerbrey, G. The use of quartz oscillators for weighing thin layers and for microweighing applications. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- González, G.; Kolosovas-Machuca, E.S.; López-Luna, E.; Hernández-Arriaga, H.; González, F.J. Design and fabrication of interdigital nanocapacitors coated with HfO2. Sensors 2015, 15, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Igreja, R.; Dias, C.J. Analytical evaluation of the interdigital electrodes capacitance for a multi-layered structure. Sens. Actuators A 2004, 112, 291–301. [Google Scholar] [CrossRef]
- Mohsen-Nia, M.; Amiri, H.; Jazi, B. Dielectric constants of water, methanol, ethanol, butanol and acetone: Measurement and computational study. J. Solut. Chem. 2010, 39, 701–708. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, X.; Guo, H.; Wu, Z. Graphene oxide thin film coated quartz crystal microbalance for humidity detection. Appl. Surf. Sci. 2011, 257, 7778–7782. [Google Scholar] [CrossRef]
- Zhu, Y.; Yuan, H.; Xua, J.; Xuc, P.; Pan, Q. Highly stable and sensitive humidity sensors based on quartz crystal microbalance coated with hexagonal lamelliform monodisperse mesoporous silica SBA-15 thin film. Sens. Actuators B 2010, 144, 164–169. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Jiang, T.; Wang, X.; Zhu, Z. Humidity detection by nanostructured ZnO: A wireless quartz crystal microbalance investigation. Sens. Actuators A 2007, 135, 209–214. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Li, N.; Ding, X.; Zhao, X. A QCM Humidity Sensors Based on GO/Nafion Composite Films with Enhanced Sensitivity. IEEE Sens. 2016, 16, 8874–8883. [Google Scholar] [CrossRef]
- Omran, H.; Salama, K.N. Design and fabrication of capacitive interdigitated electrodes for smart gas sensors. In Proceedings of the IEEE International Conference on Smart Instrumentation, Measurement and Application, Kuala Lumpur, Malaysia, 24–25 November 2015; pp. 1–4. [Google Scholar]
- Mondloch, J.E.; Karagiaridi, O.; Farha, O.K.; Hupp, J.T. Activation of metal–organic framework materials. CrystEngComm 2013, 15, 9258–9264. [Google Scholar] [CrossRef]
- Zouaoui, M.J.; Nait-Ali, B.; Glandut, N.; Smith, D.S. Effect of humidity on the dielectric constant and electrical impedance of mesoporous zirconia ceramics. J. Eur. Ceram. Soc. 2016, 36, 163–169. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Ye, Q.; Cinke, M.; Han, J.; Meyyappan, M. Carbon nanotube sensors for gas and organic vapor detection. Nano Lett. 2003, 3, 929–933. [Google Scholar] [CrossRef]
- Sapsanis, C.; Buttner, U.; Omran, H.; Belmabkhout, Y.; Shekhah, O.; Eddaoudi, M.; Salama, K.N. A Nafion Coated Capacitive Humidity Sensor on a Flexible PET Substrate. In Proceedings of the 2016 IEEE 59th International Midwest Symposium on Circuits and Systems (MWSCAS), Abu Dhabi, UAE, 16–19 October 2016; pp. 1–4. [Google Scholar]

| Device | ZIF-8 Film Thickness (µm) | ZIF-8 Mass (µg) |
|---|---|---|
| Cap-1 | 1.1 | 5.7 |
| Cap-2 | 1 | 5.2 |
| QCM-1 | 0.95 | 32 |
| QCM-2 | 0.9 | 30 |
| Comparison Parameter | IDE Capacitor | QCM |
|---|---|---|
| Average sensitivity (per vol %) | 1.3 (ΔC/C) | 1.4 × 10−5 (Δf/f) |
| Device size (mm2) | 4.3 | 28.3 |
| Detection limit (vol %) | 0.0014 | 0.13 |
| Avg. deviation (at 26.5 vol %) | 4.4 | 2 × 10−5 |
| Linearity | Nonlinear | Nonlinear |
| Energy (at 0.2 VRMS) | 0.5 pJ | 0.1 pJ |
| Hysteresis (vol %) | 3 | 3 |
| Selectivity (humidity) | Less | More |
| Temperature effect | More | Less |
| Response time at 20 sccm (min) | 60 | 60 |
| Cost ($) | 1 | 10 |
| Ref. | Sensing Material | Electrode Design | Sensitivity (ΔC/C)/vol % | Response Time (min) | Response Type | Hysteresis | Temperature (°C) |
|---|---|---|---|---|---|---|---|
| This work | ZIF-8 | IDE | 30 | 60 * | Nonlinear | 3 vol % in N2 | RT |
| [15] RSC Adv., 2017 | poly(4-vinyl phenol) | IDE | 9.7 | 2 | Nonlinear | NR | RT |
| [16] Sensors 2015 | HKUST-1 | IDE | 6.8 × 10−4 | NR | Linear | NR | RT |
| [17] Sensors 2014 | α-Fe2O3 | IDE | 340 | 0.32 | Linear | NR | 250 |
| [18] Sens. Actuators A 2007 | SWCNT | IDE | 0.65 | 0.1 | Nonlinear | NR | RT |
| [19] Science 2005 | SWCNT + polycarbosilane | IDE | 0.6 | 0.17 | Nonlinear | NR | RT |
| [20] Meas. Sci. Technol. 1995 | poly-N-(2-pyridy1)pyrro | Spiral | 1.85 | 0.25 | NR | NR | RT |
| Ref. | Sensing Material | Sensitivity (Δf/f)/vol % | Response Time (min) | Response Type | Hysteresis | Temperature (°C) |
|---|---|---|---|---|---|---|
| This work | ZIF-8 | 2 × 10−5 | 60 * | Nonlinear | 3 vol % in N2 | RT |
| [35] Sens. Actuators B 2016 | Pd doped ZnO | 3.8 × 10−4 | 0.2 | Nonlinear | NR | RT |
| [36] Sens. Actuators B 2008 | Imidazolium-based ionic liquids | 1.6 × 10−7 | 1 | Linear | NR | 30 |
| [37] Anal. Chim. Acta 2007 | Thiophene | 1.36 × 1−5 | 0.5 | Linear | NR | RT |
| [38] Sens. Actuators B 2005 | Calixarene | 1 × 10−5 | NR | NR | NR | RT |
| [39] Sens. Actuators B 2004 | Ag+-ZSM-5 zeolite | 9.3 × 10−3 | 4 | Linear | NR | RT |
| Ref. | Sensing Material | Sensitivity (ΔR/R)/vol % | Response Time (min) | Response Type | Temperature (°C) |
|---|---|---|---|---|---|
| This work | ZIF-8 | 5.5 × 10−2 | 60 * | Nonlinear | RT |
| [1] ACS Appl. Mater. Interfaces 2017 | MXene | 9.5 | 5 | NR | RT |
| [2] Adv. Mater. 2016 | ZIF-8 + ZnO | 4 × 104 | 6 | Nonlinear | 260 |
| [3] IEEE Sens. J. 2016 | Si-doped tungsten oxide | 3.5 × 105 | 4 | Nonlinear | 425 |
| [4] RSC Adv. 2015 | SnO2 reduced graphene oxide | 43 | 2 | Nonlinear | RT |
| [5] Mater. Lett. 2013 | ZnO | 2700 | 0.5 | Nonlinear | 310 |
| [6] Anal. Chim. Acta 2012 | Si doped WO3 | 15,000 | 1 | Linear | 350 |
| [7] Sens. Actuators B 2011 | SnO2 | 170 | 0.1 | Nonlinear | 200 |
| [8] Sens. Actuators B 2011 | Co doped ZnO | 1500 | 0.14 | Nonlinear | 360 |
| [9] Sens. Actuators B 2009 | ZnO | 3000 | 0.12 | Nonlinear | 320 |
| [10] Sens. Actuators B 2008 | ZnO | 560 | 0.05 | Nonlinear | 300 |
| [11] Sens. Actuators B 2006 | nickel ferrite | 0.3 | 3 | Nonlinear | 214 |
| [12] Sens. Actuators B 2006 | SnO2 | 25,000 | 0.34 | Nonlinear | RT |
| [13] Sens. Actuators B 2000 | CeO2 | 600 | NR | NR | 350 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chappanda, K.N.; Tchalala, M.R.; Shekhah, O.; Surya, S.G.; Eddaoudi, M.; Salama, K.N. A Comparative Study of Interdigitated Electrode and Quartz Crystal Microbalance Transduction Techniques for Metal–Organic Framework-Based Acetone Sensors. Sensors 2018, 18, 3898. https://doi.org/10.3390/s18113898
Chappanda KN, Tchalala MR, Shekhah O, Surya SG, Eddaoudi M, Salama KN. A Comparative Study of Interdigitated Electrode and Quartz Crystal Microbalance Transduction Techniques for Metal–Organic Framework-Based Acetone Sensors. Sensors. 2018; 18(11):3898. https://doi.org/10.3390/s18113898
Chicago/Turabian StyleChappanda, Karumbaiah N., Mohamed R. Tchalala, Osama Shekhah, Sandeep G. Surya, Mohamed Eddaoudi, and Khaled N. Salama. 2018. "A Comparative Study of Interdigitated Electrode and Quartz Crystal Microbalance Transduction Techniques for Metal–Organic Framework-Based Acetone Sensors" Sensors 18, no. 11: 3898. https://doi.org/10.3390/s18113898
APA StyleChappanda, K. N., Tchalala, M. R., Shekhah, O., Surya, S. G., Eddaoudi, M., & Salama, K. N. (2018). A Comparative Study of Interdigitated Electrode and Quartz Crystal Microbalance Transduction Techniques for Metal–Organic Framework-Based Acetone Sensors. Sensors, 18(11), 3898. https://doi.org/10.3390/s18113898







