An Ultra-Rapid Biosensory Point-of-Care (POC) Assay for Prostate-Specific Antigen (PSA) Detection in Human Serum
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
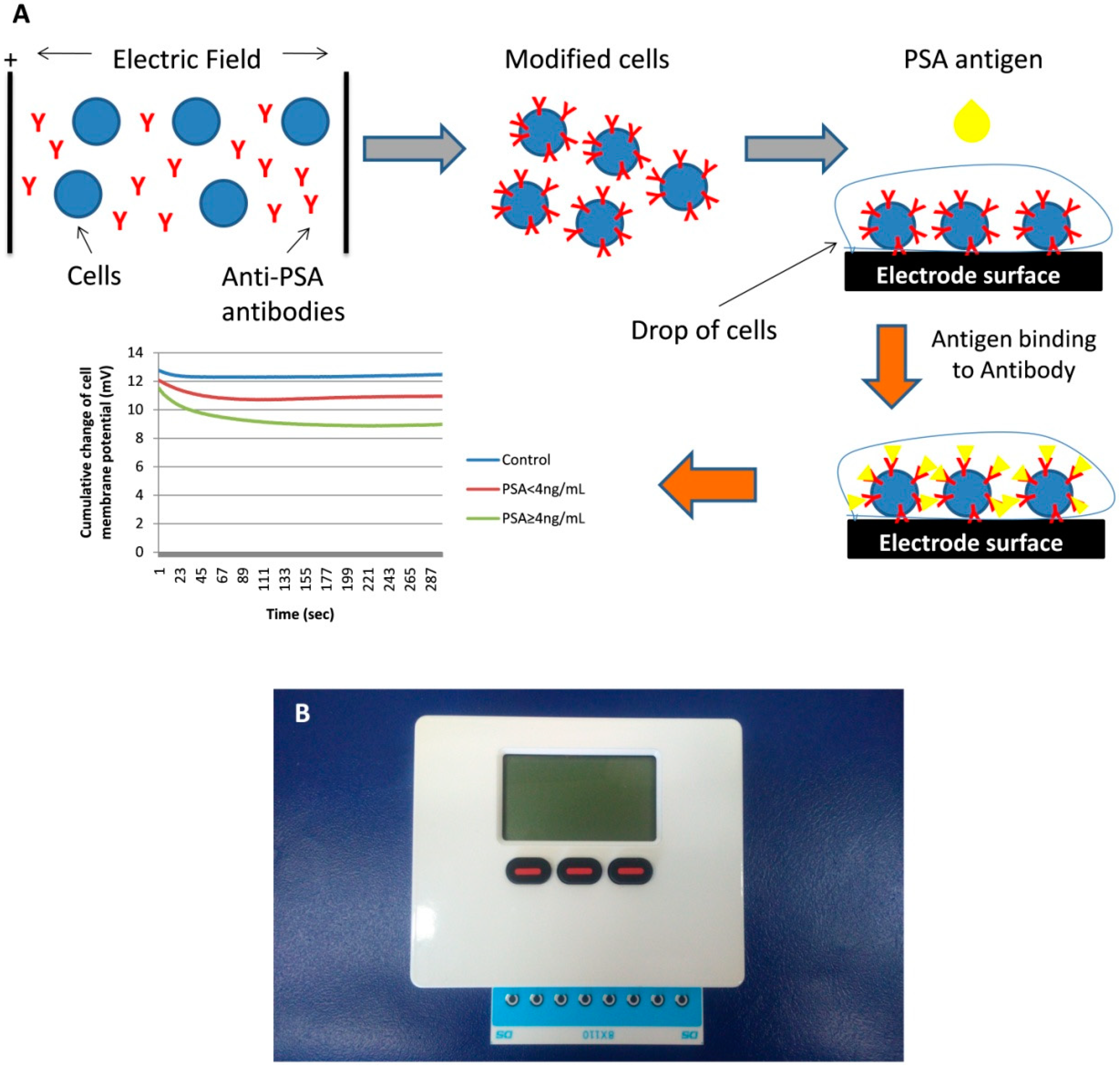
2.2. Manufacture of the Biosensing Element
2.3. Point-of-Care (POC) System Configuration
2.4. Assay Procedure
2.5. Immunoradiometric Assay (IRMA) for PSA Detection in Serum
2.6. Electrochemical Immunoassay
Antibody Immobilization on the Electrode’s Surface
2.7. Statistical Analysis
3. Results
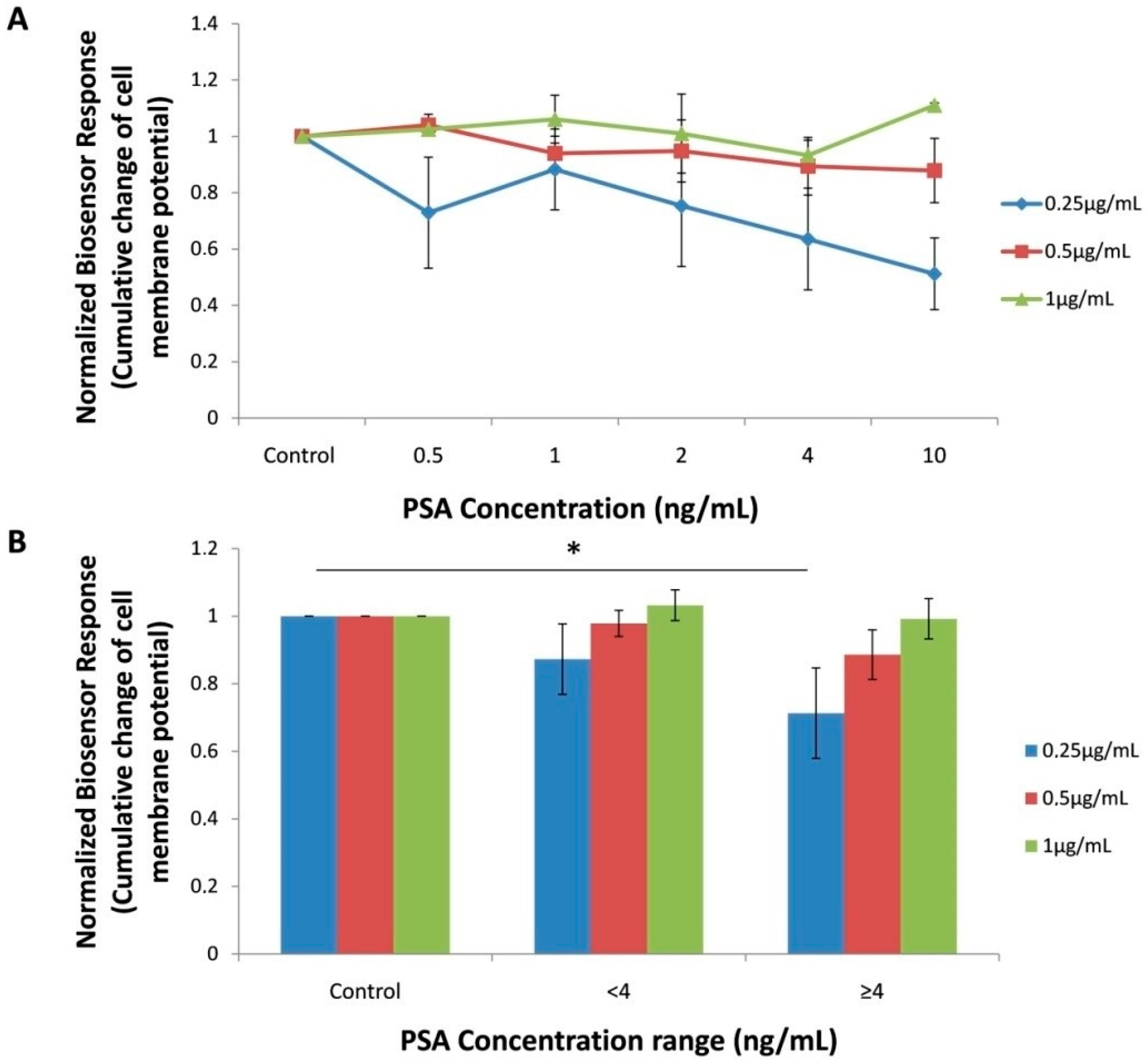
3.1. Biosensor Response against PSA Standard Solution
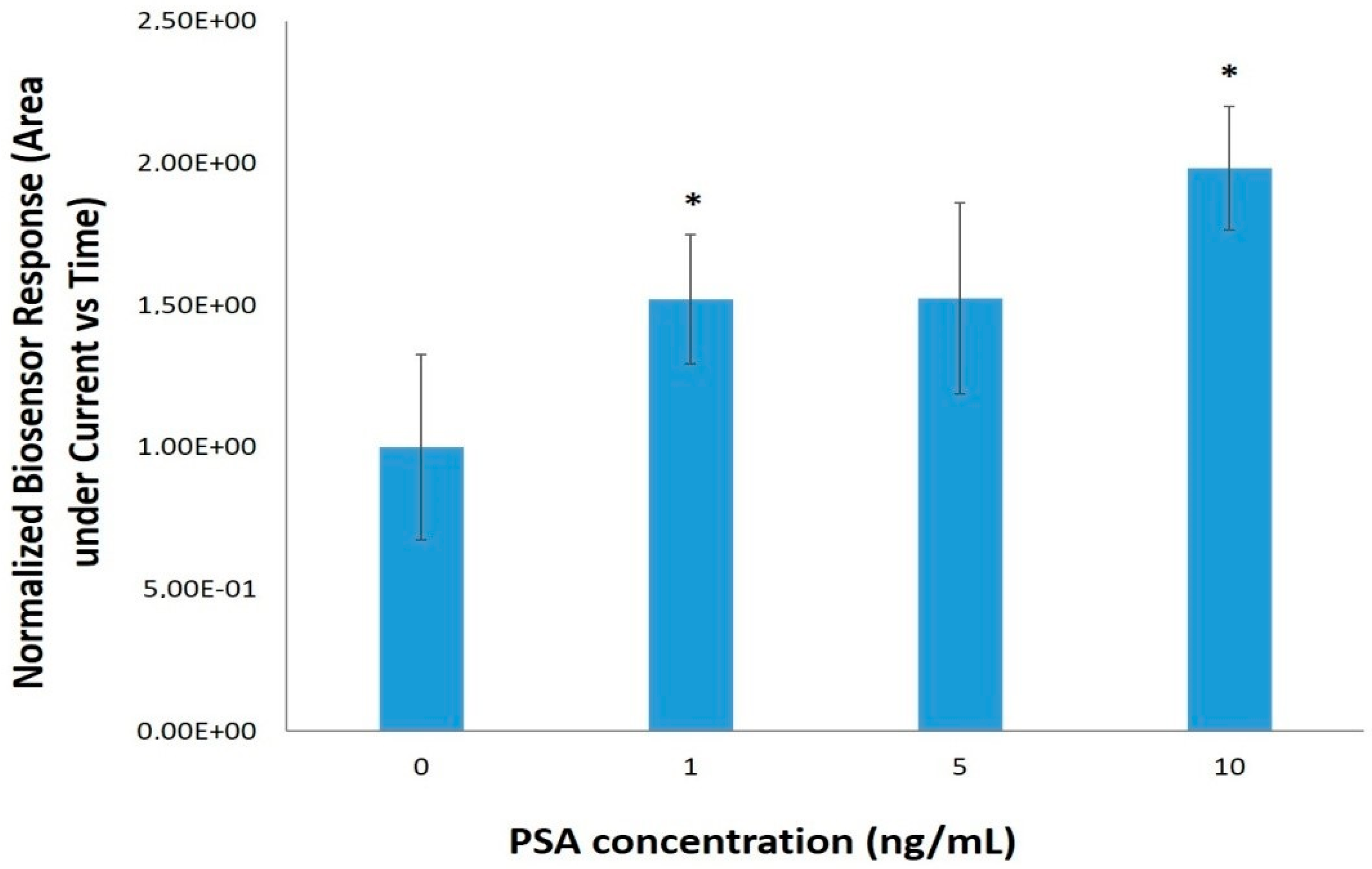
3.2. PSA Detection with the Electrochemical Immunoassay
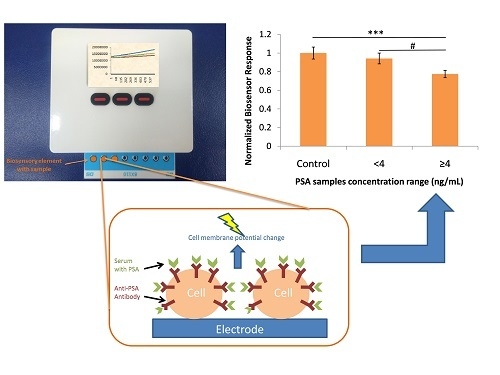
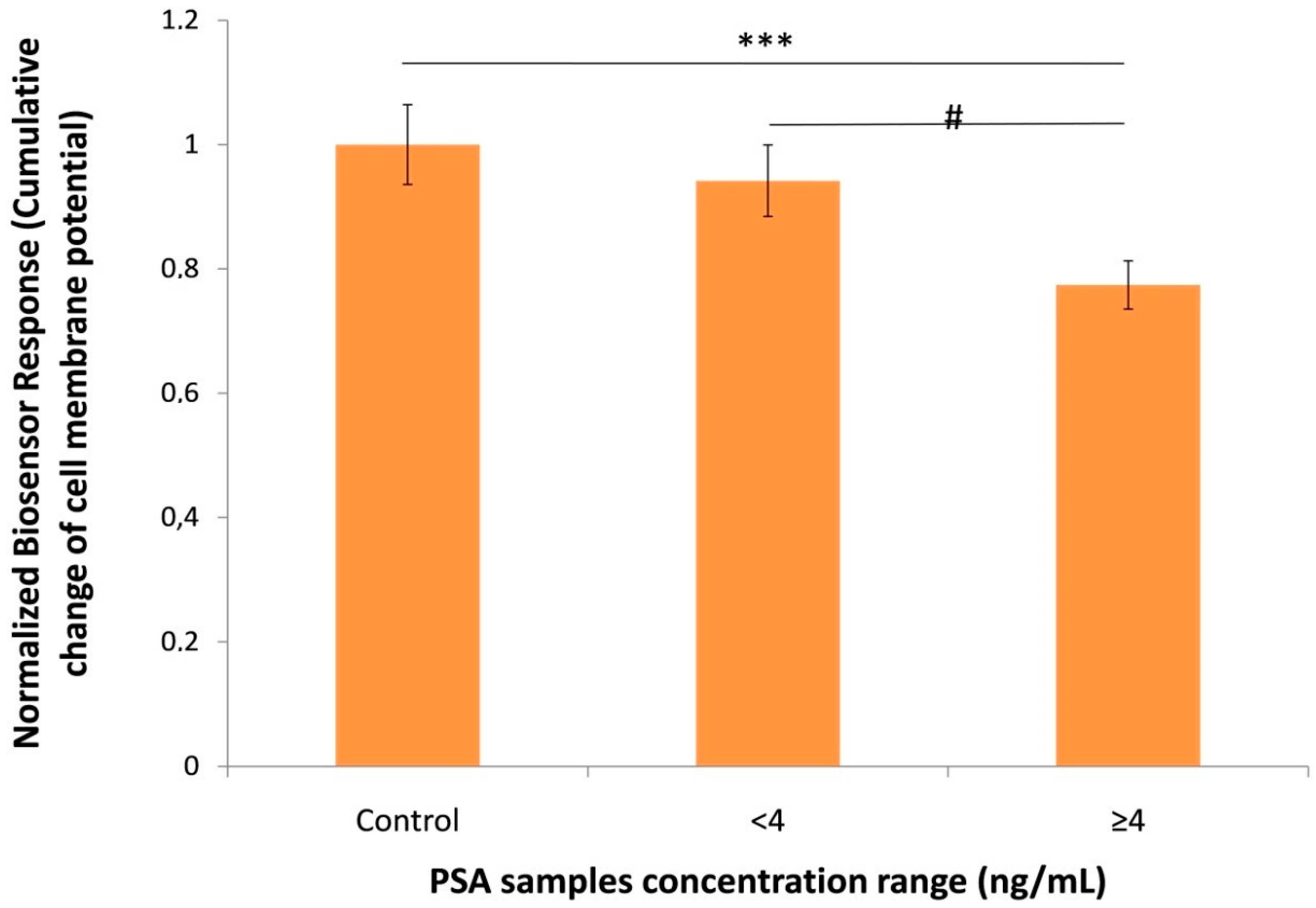
3.3. Biosensor Response against Human Serum Samples
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
Ethical Statements
References
- Fust, K. The Gale Encyclopedia of Cancer, 4th ed.; Gale: Farmington Hills, MI, USA, 2015; p. 2083. ISBN 978-1-4103-1740-7. [Google Scholar]
- Hanna, L.; Crosby, T.; Macbeth, F. Practical Clinical Oncology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2015; p. 625. ISBN 978-1-107-68362-4. [Google Scholar]
- Ankerst, D.P.; Tangen, C.M.; Thompson, I.M., Jr. Prostate Cancer Screening, 2nd ed.; Humana Press: New York, NY, USA, 2009; p. 391. ISBN 978-1-60327-280-3. [Google Scholar]
- Makarov, D.V.; Humphreys, E.B.; Mangold, L.A.; Walsh, P.C.; Partin, A.W.; Epstein, J.I.; Freedland, S.J. Pathological outcomes and biochemical progression in men with T1c prostate cancer undergoing radical prostatectomy with prostate specific antigen 2.6 to 4.0 vs. 4.1 to 6.0 ng/mL. J. Urol. 2006, 176, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Carter, H.B.; Pearson, J.D.; Metter, E.J.; Brant, L.J.; Chan, D.W.; Andres, R.; Fozard, J.L.; Walsh, P.C. Longitudinal evaluation of prostate-specific antigen levels in men with and without prostate disease. JAMA 1992, 267, 2215–2220. [Google Scholar] [CrossRef] [PubMed]
- Vural, T.; Yaman, Y.T.; Ozturk, S.; Abaci, S.; Denkbas, E.B. Electrochemical immunoassay for detection of prostate specific antigen based on peptide nanotube-gold nanoparticle-polyaniline immobilized pencil graphite electrode. J. Colloid Interface Sci. 2018, 510, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Pihikova, D.; Kasak, P.; Kubanikova, P.; Sokol, R.; Tkac, J. Aberrant sialylation of a prostate-specific antigen: Electrochemical label-free glycoprofiling in prostate cancer serum samples. Anal. Chim. Acta 2016, 934, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, J.; Fam, D.W.H.; Tok, A.I.Y. Horizontally aligned carbon nanotube based biosensors for protein detection. Bioengineering 2016, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Lee, M.; Kim, D. Detection of early stage prostate cancer by using a simple carbon nanotube@paper biosensor. Biosens. Bioelectron. 2018, 102, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Li, L.; Cui, K.; Zhang, Y.; Zhang, L.; Ge, S.; Yu, J. Ultrasensitive enzyme-free biosensor by coupling cyclodextrin functionalized au nanoparticles and high-performance au-paper electrode. ACS Appl. Mater. Interfaces 2018, 10, 3333–3340. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, N.; Wang, K.; Hai, X.; Liu, J.; Dang, F. A novel peptide/Fe3O4@SiO2-Au nanocomposite-based fluorescence biosensor for the highly selective and sensitive detection of prostate-specific antigen. Talanta 2018, 179, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Song, Y.; Gao, W.; Wu, T.; Xu, L.P.; Zhang, X.; Wang, S. Superwettable electrochemical biosensor toward detection of cancer biomarkers. ACS Sens. 2018, 3, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Jolly, P.; Formisano, N.; Tkac, J.; Kasak, P.; Frost, C.G.; Estrela, P. Label-free impedimetric aptasensor with antifouling surface chemistry: A prostate specific antigen case study. Sens. Actuators B Chem. 2015, 209, 306–312. [Google Scholar] [CrossRef]
- Pihíková, D.; Belicky, Š.; Kasák, P.; Bertok, T.; Tkac, J. Sensitive detection and glycoprofiling of a prostate specific antigen using impedimetric assays. Analyst 2016, 141, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Spain, E.; Gilgunn, S.; Sharma, S.; Adamson, K.; Carthy, E.; O’Kennedy, R.; Forster, R.J. Detection of prostate specific antigen based on electrocatalytic platinum nanoparticles conjugated to a recombinant scFv antibody. Biosens. Bioelectron. 2016, 77, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Kasprzyk-Hordern, B.; Goggins, S.; Frost, C.G.; Estrela, P. A novel immobilization strategy for electrochemical detection of cancer biomarkers: DNA-directed immobilization of aptamer sensors for sensitive detection of prostate specific antigen. Analyst 2015, 140, 2628–2633. [Google Scholar] [CrossRef] [PubMed]
- Tamboli, V.K.; Bhalla, N.; Jolly, P.; Bowen, C.R.; Taylor, J.T.; Bowen, J.L.; Allender, C.J.; Estrela, P. Hybrid synthetic receptors on MOSFET devices for detection of prostate specific antigen in human plasma. Anal. Chem. 2016, 88, 11486–11490. [Google Scholar] [CrossRef] [PubMed]
- Moschopoulou, G.; Vitsa, K.; Bem, F.; Vassilakos, N.; Perdikaris, A.; Blouhos, P.; Yialouris, C.; Frossiniotis, D.; Anthopoulos, I.; Maggana, O.; et al. Engineering of the membrane of fibroblast cells with virus-specific antibodies: A novel biosensor tool for virus detection. Biosens. Bioelectron. 2008, 24, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Perdikaris, A.; Alexandropoulos, N.; Kintzios, S. Development of a Novel, Ultra-rapid Biosensor for the Qualitative Detection of Hepatitis B Virus-associated Antigens and Anti-HBV, Based on “Membrane-engineered” Fibroblast Cells with Virus-Specific Antibodies and Antigens. Sensors 2009, 9, 2176–2186. [Google Scholar] [CrossRef] [PubMed]
- Moschopoulou, G.; Valero, T.; Kintzios, S. Superoxide determination using membrane-engineered cells: An example of a novel concept for the construction of cell sensors with customized target recognition properties. Sens. Actuators B Chem. 2012, 175, 78–84. [Google Scholar] [CrossRef]
- Apostolou, T.; Pascual, N.; Marco, M.P.; Moschos, A.; Petropoulos, A.; Kaltsas, G.; Kintzios, S. Extraction-less, rapid assay for the direct detection of 2,4,6-trichloroanisole (TCA) in cork samples. Talanta 2014, 125, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Mavrikou, S.; Flampouri, E.; Iconomou, D.; Kintzios, S. Development of a cellular biosensor for the detection of aflatoxin B1, based on the interaction of membrane engineered Vero cells with anti-AFB1 antibodies on the surface of gold nanoparticle screen printed electrodes. Food Control 2017, 73, 64–70. [Google Scholar] [CrossRef]
- Foroutan, H.; Najafi, R.; Babaei, M.H.; Shafii, M. Preparation of prostate specific antigen standards for immunoradiometric assay. Iran. J. Radiat. Res. 2008, 6, 51–58. [Google Scholar]
- Lin, Y.; Chen, S.; Chuang, Y.; Lu, Y.C.; Shen, T.Y.; Chang, C.A.; Lin, C.S. Disposable amperometricimmunosensing strips fabricated by Au nanoparticles-modified screen-printed carbon electrodes for the detection of foodborne pathogen Escherichia coli O157: H7. Biosens. Bioelectron. 2008, 23, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Chuang, Y.C.; Lu, Y.C.; Lin, H.C.; Yang, Y.L.; Lin, C.S. A method of layer-by-layer gold nanoparticles hybridization in a quartz crystal microbalance DNA sensing system used to detect dengue virus. Nanotechnology 2009, 20, 215501. [Google Scholar] [CrossRef] [PubMed]
- Pingarro’n, J.M.; Ya’nez-Sedeno, P.; Araceli, G. Gold-nanopartcle-based electrochemical biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- Goyal, R.N.; Gupta, V.K.; Oyama, M.; Bachheti, N. Differential pulse voltammetric determination of atenolol in pharmaceutical formulations and urine using nanogold modified indium tin oxide electrode. Electrochem. Commun. 2006, 8, 65–70. [Google Scholar] [CrossRef]
- Jacobs, M.; Jacobs, M.J.; Selvam, A.P.; Craven, J.E.; Prasad, S. Antibody-conjugated gold nanoparticle-based immunosensor for ultra-sensitive detection of troponin-T. J. Lab. Autom. 2014, 19, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Polonschii, C.; David, S.; Tombelli, S.; Mascini, M.; Gheorghiu, M. A novel low-cost and easy to develop functionalization platform. Case study: Aptamer-based detection of thrombin by surface plasmon resonance. Talanta 2010, 80, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Badea, M.; Floroian, L.; Restani, P.; Cobzac, S.C.A.; Moga, M. Ochratoxin A Detection on Antibody-Immobilized on BSA-Functionalized Gold Electrodes. PLoS ONE 2016, 11, e0160021. [Google Scholar] [CrossRef] [PubMed]
- Kadir, M.K.; Tothill, I.E. Development of an electrochemical immunosensor for fumonisins detection in foods. Toxins 2010, 2, 382–398. [Google Scholar] [CrossRef] [PubMed]
- Kokla, A.; Blouchos, P.; Livaniou, E.; Zikos, C.; Kakabakos, S.E.; Petrou, P.S.; Kintzios, S. Visualization of the membrane engineering concept: Evidence for the specific orientation of electroinserted antibodies and selective binding of target analytes. J. Mol. Recognit. 2013, 26, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Malati, T.; Kumari, G.R. Racial and ethnic variation of PSA in global population: Age specific reference intervals for serum prostate specific antigen in healthy South Indian males. Indian J. Clin. Biochem. 2004, 19, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Luboldt, H.J.; Schindler, J.M.; Rubben, H. Age-Specific Reference Ranges for Prostate-Specific Antigen as a Marker for Prostate Cancer. EAU-EBU Updat. Ser. 2007, 5, 38–48. [Google Scholar] [CrossRef]
- Erol, B.; Gulpinar, M.T.; Bozdogan, G.; Ozkanli, S.; Onem, K.; Mungan, G.; Bektasf, S.; Tokgoz, H.; Akduman, B.; Mungan, A. The cutoff level of free/total prostate specific antigen (f/t PSA) ratios in the diagnosis of prostate cancer: A validation study on a Turkish patient population in different age categories. Kaohsiung J. Med. Sci. 2014, 30, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Shahyad, S.; Saadat, S.H.; Hosseini-Zijoud, S.M. The Clinical Efficacy of Prostate Cancer Screening in Worldwide and Iran: Narrative Review. World J. Oncol. 2018, 9, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Benoit, R.M.; Gronberg, H.; Naslund, M.J. A quantitative analysis of the costs and benefits of prostate cancer screening. Prostate Cancer Prostatic Dis. 2001, 4, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Globocan 2018 Graph Production: IARC. Available online: http://gco.iarc.fr/today (accessed on 1 September 2018).
- Anonymous. Grand View Research 2018. Prostate Cancer Diagnostics Market Analysis Report by Type (Preliminary Tests, Confirmatory Tests), by Region (North America, APAC, Europe, MEA, Latin America), And Segment Forecasts, 2018–2025. Available online: https://www.grandviewresearch.com/industry-analysis/prostate-cancer-diagnostics-market (accessed on 1 September 2018).
- Malati, T.; Kumari, G.R.; Murthy, P.V.; Reddy, C.R.; Prakash, B.S. Prostate specific antigen in patients of benign prostate hypertrophy and carcinoma prostate. Indian J. Clin. Biochem. 2006, 21, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Hibi, H.; Miyake, K. Prostate-specific antigen levels in acute and chronic bacterial prostatitis. Hinyokika Kiyo 1993, 39, 445–449. [Google Scholar] [PubMed]
- Stamey, T.A.; Yang, N.; Hay, A.R.; McNeal, J.E.; Freiha, F.S.; Redwine, E. Prostate-specific antigen as a serum marker for adenocarcinomaof the prostate. N. Engl. J. Med. 1987, 317, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Oesterling, J.E.; Jacobsen, S.J.; Chute, C.G.; Guess, H.A.; Girman, C.J.; Panser, L.A.; Lieber, M.M. Serum prostate-specific antigenin a community-based population of healthy men. Establishmentof age-specific reference ranges. JAMA 1993, 270, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Aref, A.T.; Vincent, A.D.; O’Callaghan, M.E.; Martin, S.A.; Sutherland, P.D.; Hoy, A.J.; Butler, L.M.; Wittert, G.A. The inverse relationship between prostate specific antigen (PSA) and obesity. Endocr. Relat. Cancer 2018, 25, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, K.; Hashimoto, M.; Kubota, M.; Yamashita, S.; Nakamura, M.; Usui, S.; Sugiyama, T.; Hirano, K. Effects of 14 frequently used drugs on prostate-specific antigen expression in prostate cancer LNCaP cells. Oncol. Lett. 2014, 7, 1665–1668. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.L.; Harshman, L.C.; Presti, J.C. Impact of common medications on serum total prostate-specific antigen levels: Analysis of the National Health and Nutrition Examination Survey. J. Clin. Oncol. 2010, 28, 3951–3957. [Google Scholar] [CrossRef] [PubMed]
- Schröder, F.H.; Hugosson, J.; Roobol, M.J.; Tammela, T.L.; Ciatto, S.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Lilja, H.; Zappa, M.; et al. ERSPC Investigators. Prostate-cancer mortality at 11 years of follow-up. N. Engl. J. Med. 2012, 366, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Roobol, M.J.; Kranse, R.; Bangma, C.H.; van Leenders, A.G.; Blijenberg, B.G.; van Schaik, R.H.; Kirkels, W.J.; Otto, S.J.; van der Kwast, T.H.; de Koning, H.J.; et al. ERSPC Rotterdam Study Group. Screening for prostate cancer: Results of the Rotterdam section of the European randomized study of screening for prostate cancer. Eur. Urol. 2013, 64, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Wilt, T.J.; Jones, K.M.; Barry, M.J.; Andriole, G.L.; Culkin, D.; Wheeler, T.; Aronson, W.J.; Brawer, M.K. Follow-up of prostatectomy versus observation for early prostate cancer. N. Engl. J. Med. 2017, 377, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Yamamoto, T.; Ohi, M.; Kurokawa, K.; Suzuki, K.; Yamanaka, H. Free/total PSA ratio is a powerful predictor of future prostatecancer morbidity in men with initial PSA levels of 4.1 to 10.0ng/mL. Urology 2003, 61, 760–764. [Google Scholar] [CrossRef]
- Catalona, W.J.; Partin, A.W.; Slawin, K.M.; Brawer, M.K.; Flanigan, R.C.; Patel, A.; Richie, J.P.; deKernion, J.B.; Walsh, P.C.; Scardino, P.T.; et al. Use of the percentage of free prostatespecificantigen to enhance differentiation of prostate cancer frombenign prostatic disease: A prospective multicenter clinical trial. JAMA 1998, 279, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Borque-Fernando, A.; Rubio-Briones, J.; Esteban, L.M.; Dong, Y.; Calatrava, A.; Gómez-Ferrer, Á.; Gómez-Gómez, E.; Gil Fabra, J.M.; Rodríguez-García, N.; López González, P.Á.; et al. Role of the 4Kscore test as a predictor of reclassification in prostate cancer active surveillance. Prostate Cancer Prostatic Dis. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Vertosick, E.A.; Sjoberg, D.D. Value of a Statistical Model Based on Four Kallikrein Markers in Blood, Commercially Available as 4Kscore, in All Reasonable Prostate Biopsy Subgroups. Eur. Urol. 2018, 74, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.; Sanda, M.G.; Broyles, D.L.; Shin, S.S.; Bangma, C.H.; Wei, J.T.; Partin, A.W.; Klee, G.G.; Slawin, K.M.; Marks, L.S.; et al. The Prostate Health Index Selectively Identifies Clinically Significant Prostate Cancer. J. Urol. 2015, 193, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Huo, Q.; Litherland, S.A.; Sullivan, S.; Hallquist, H.; Decker, D.A.; Rivera-Ramirez, I. Developing a nanoparticle test for prostate cancer scoring. J. Transl. Med. 2012, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Anceschi, U.; Tuderti, G.; Lugnani, F.; Biava, P.; Malossini, G.; Luciani, L.; Cai, T.; Marsiliani, D.; Filianoti, A.; Mattevi, D.; et al. Novel diagnostic biomarkers of prostate cancer: An update. Curr. Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, R.J.; van Oort, I.M.; Schalken, J.A. Blood-based and urinary prostate cancer biomarkers: A review and comparison of novel biomarkers for detection and treatment decisions. Prostate Cancer Prostatic Dis. 2017, 20, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Gadzinski, A.J.; Cooperberg, M.R. Prostate Cancer Markers. Cancer Treat. Res. 2018, 175, 55–86. [Google Scholar] [CrossRef] [PubMed]
- Pugongchai, A.; Bychkov, A.; Sampatanukul, P. Promoter hypermethylation of SOX11 correlates with adverse clinicopathological features of human prostate cancer. Int. J. Exp. Pathol. 2017, 98, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Ashour, N.; Angulo, J.C.; Andrés, G.; Alelú, R.; González-Corpas, A.; Toledo, M.V.; Rodríguez-Barbero, J.M.; López, J.I.; Sánchez-Chapado, M.; Ropero, S. A DNA hypermethylation profile reveals new potential biomarkers for prostate cancer diagnosis and prognosis. Prostate 2014, 74, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Aref-Eshghi, E.; Schenkel, L.C.; Ainsworth, P.; Lin, H.; Rodenhiser, D.I.; Cutz, J.C.; Sadikovic, B. Genomic DNA Methylation-Derived Algorithm Enables Accurate Detection of Malignant Prostate Tissues. Front. Oncol. 2018, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Baden, J.; Adams, S.; Astacio, T.; Jones, J.; Markiewicz, J.; Painter, J.; Trust, C.; Wang, Y.; Green, G. Predicting prostate biopsy result in men with prostate specific antigen 2.0 to 10.0 ng/mL using an investigational prostate cancer methylation assay. J. Urol. 2011, 186, 2101–2106. [Google Scholar] [CrossRef] [PubMed]
- Maunder, R.J.; Baron, M.G.; Owen, S.F.; Jha, A.N. Investigations to extend viability of a rainbow trout primary gill cell culture. Ecotoxicology 2017, 26, 1314–1326. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavrikou, S.; Moschopoulou, G.; Zafeirakis, A.; Kalogeropoulou, K.; Giannakos, G.; Skevis, A.; Kintzios, S. An Ultra-Rapid Biosensory Point-of-Care (POC) Assay for Prostate-Specific Antigen (PSA) Detection in Human Serum. Sensors 2018, 18, 3834. https://doi.org/10.3390/s18113834
Mavrikou S, Moschopoulou G, Zafeirakis A, Kalogeropoulou K, Giannakos G, Skevis A, Kintzios S. An Ultra-Rapid Biosensory Point-of-Care (POC) Assay for Prostate-Specific Antigen (PSA) Detection in Human Serum. Sensors. 2018; 18(11):3834. https://doi.org/10.3390/s18113834
Chicago/Turabian StyleMavrikou, Sophie, Georgia Moschopoulou, Athanasios Zafeirakis, Konstantina Kalogeropoulou, Georgios Giannakos, Athanasios Skevis, and Spyridon Kintzios. 2018. "An Ultra-Rapid Biosensory Point-of-Care (POC) Assay for Prostate-Specific Antigen (PSA) Detection in Human Serum" Sensors 18, no. 11: 3834. https://doi.org/10.3390/s18113834
APA StyleMavrikou, S., Moschopoulou, G., Zafeirakis, A., Kalogeropoulou, K., Giannakos, G., Skevis, A., & Kintzios, S. (2018). An Ultra-Rapid Biosensory Point-of-Care (POC) Assay for Prostate-Specific Antigen (PSA) Detection in Human Serum. Sensors, 18(11), 3834. https://doi.org/10.3390/s18113834