Determination of the Optimal Sensing Temperature in Pt/Ta2O5/MoO3 Schottky Contacted Nanobelt Straddling Heterojunction
Abstract
1. Introduction
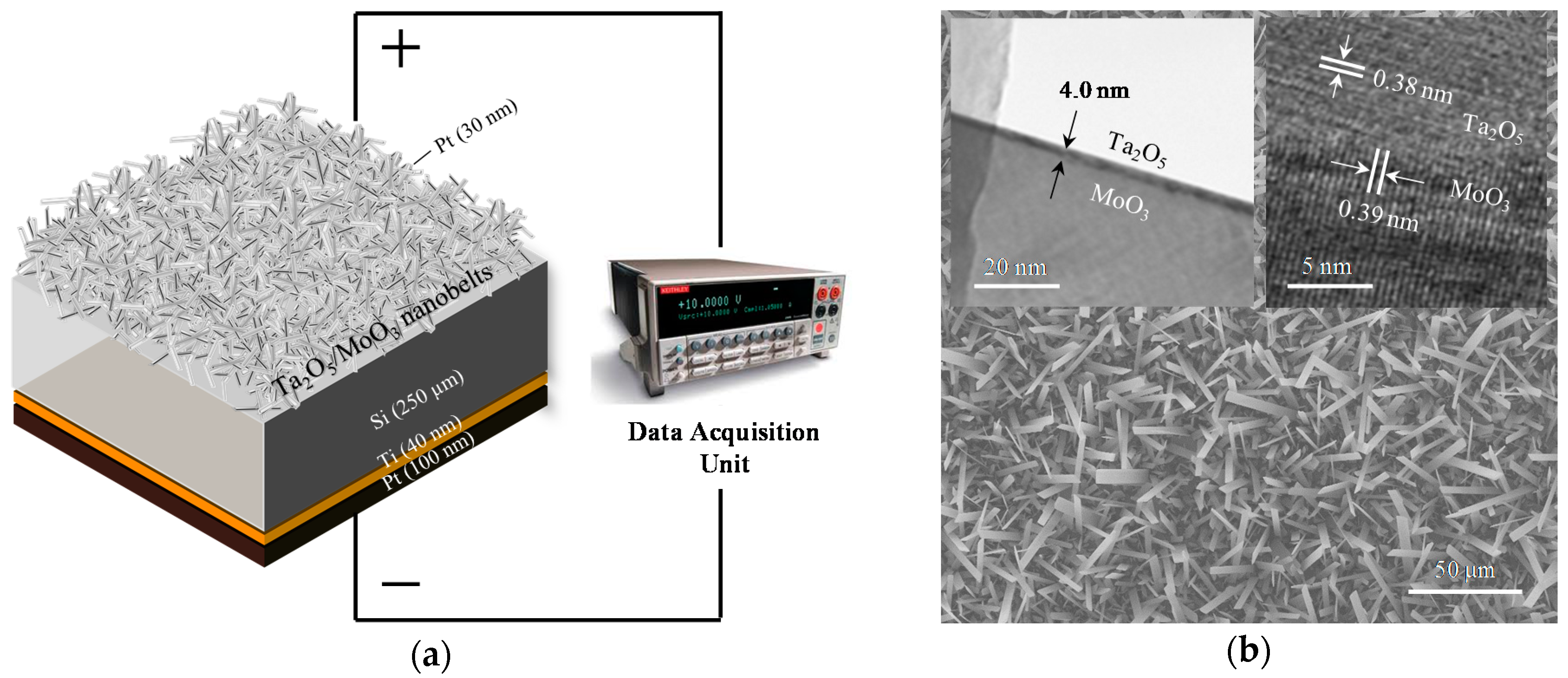
2. Materials and Methods
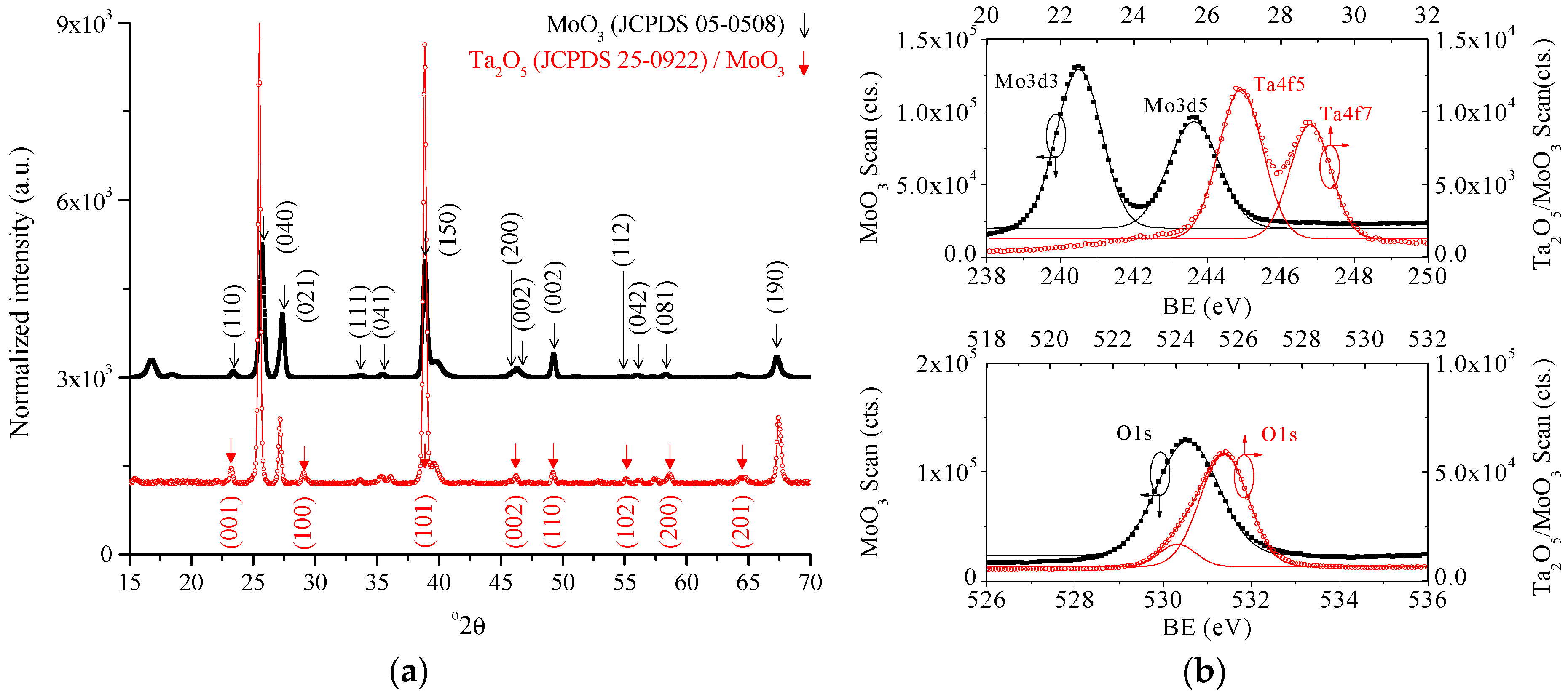
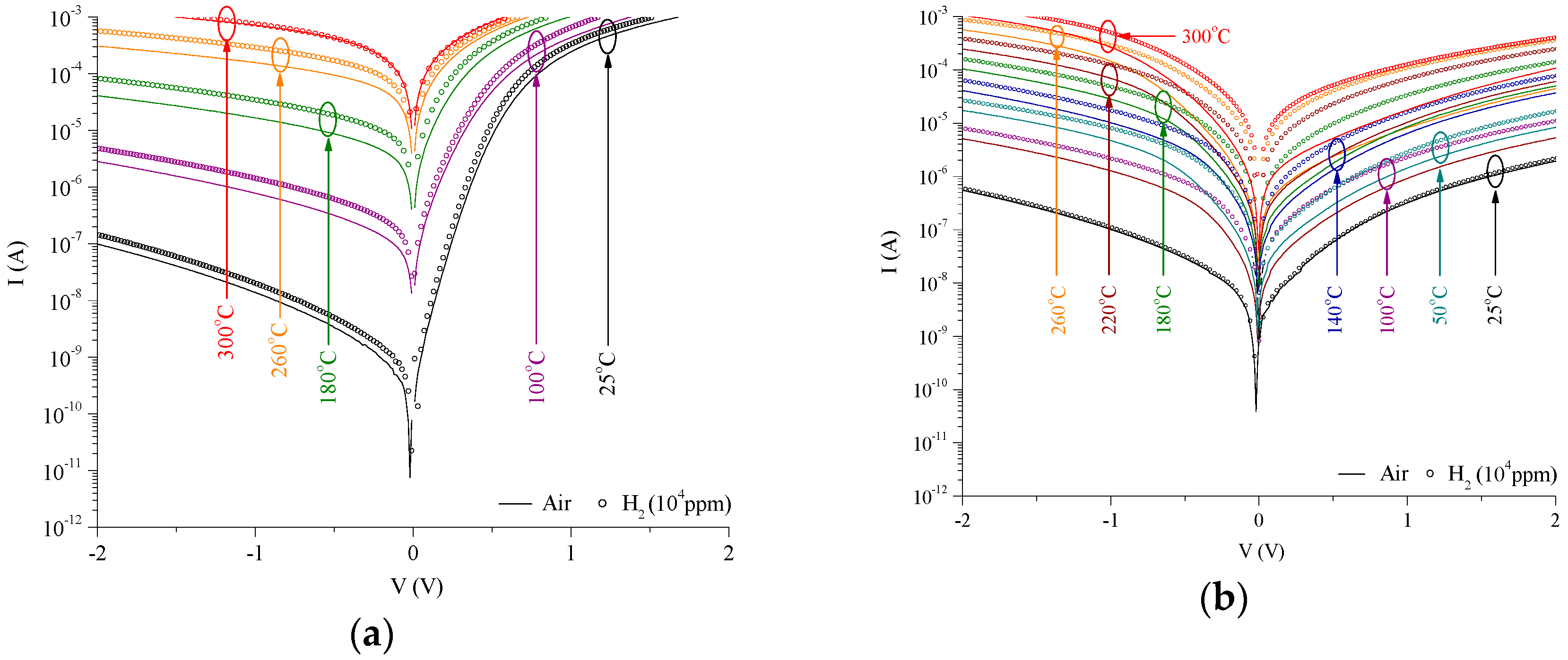
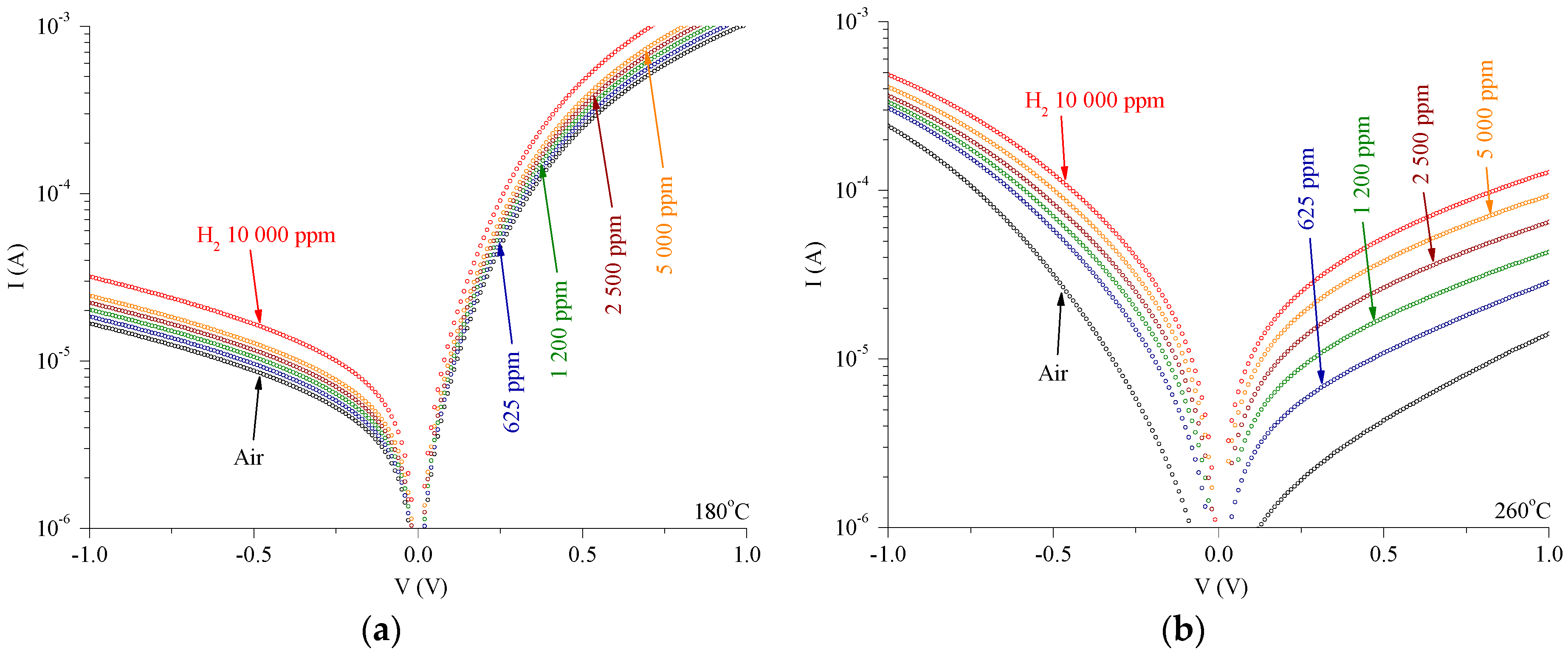
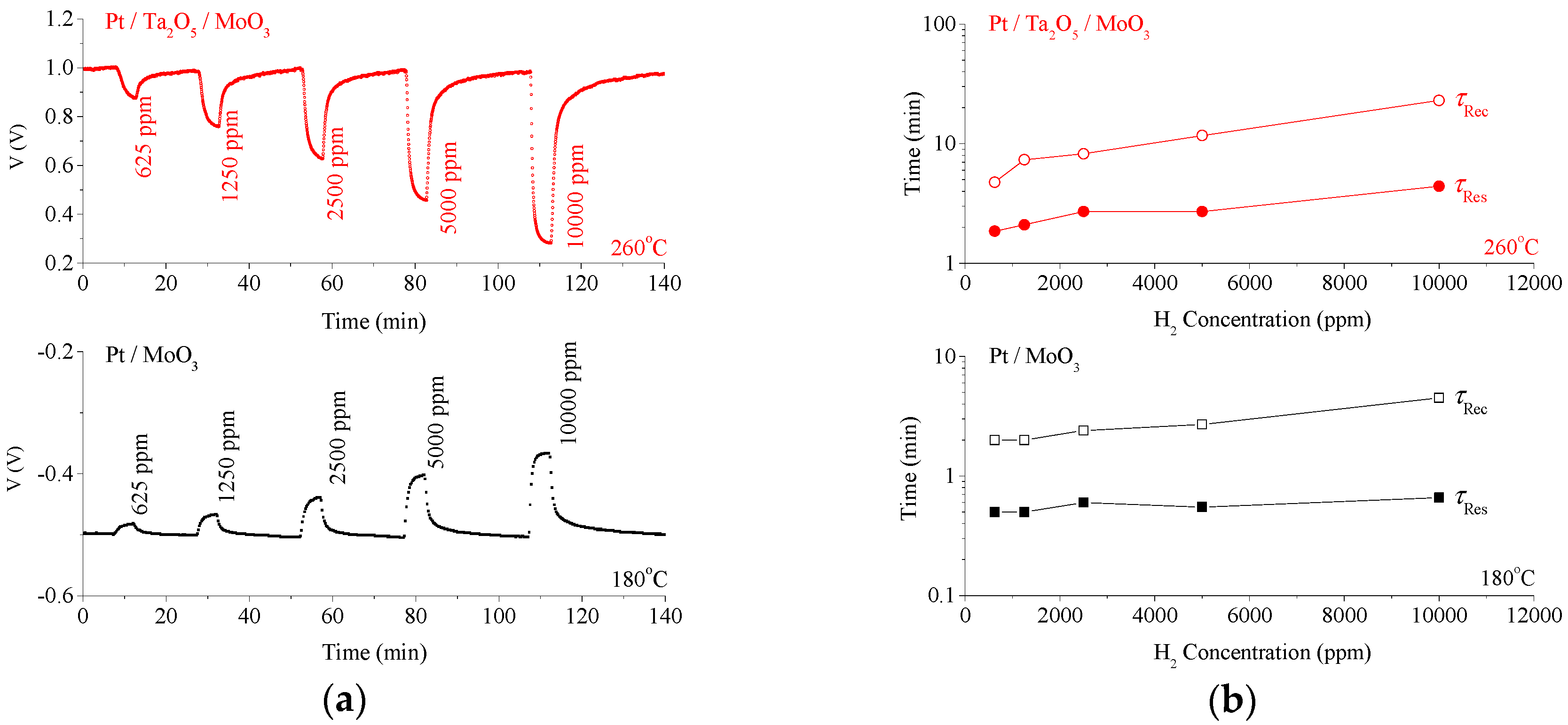
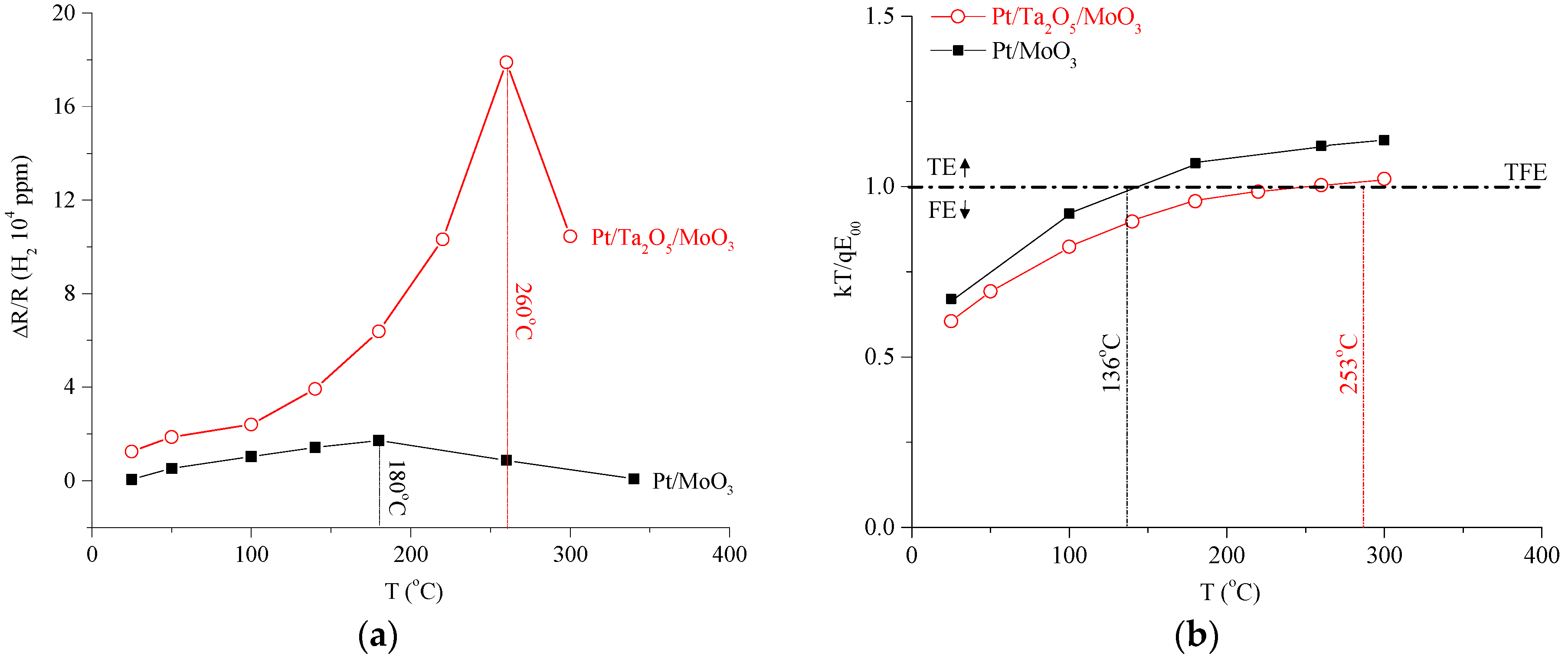
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, I.-D.; Rothschild, A.; Tuller, H.L. Advances and new directions in gas-sensing devices. Acta Mater. 2013, 61, 974–1000. [Google Scholar] [CrossRef]
- Comini, E. Metal oxide nanowire chemical sensors: Innovation and quality of life. Mater. Today 2016, 19, 559–567. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. How shell thickness can affect the gas sensing properties of nanostructured materials: Survey of literature. Sens. Actuators B Chem. 2018, 258, 270–294. [Google Scholar] [CrossRef]
- Farzana, E.; Zhang, Z.; Paul, P.K.; Arehart, A.R.; Ringel, S.A. Influence of metal choice on (010) β-Ga2O3 Schottky diode properties. Appl. Phys. Lett. 2017, 110, 202102. [Google Scholar] [CrossRef]
- Hiroyoshi, I.; Tomoyoshi, M.; Kenji, S. Electrical characteristics of n-GaN Schottky contacts on cleaved surfaces of free-standing substrates: Metal work function dependence of Schottky barrier height. Jpn. J. Appl. Phys. 2018, 57, 04FG13. [Google Scholar]
- Kumar, M.; Bhati, V.S.; Kumar, M. Effect of Schottky barrier height on hydrogen gas sensitivity of metal/TiO2 nanoplates. Int. J. Hydrog. Energy 2017, 42, 22082–22089. [Google Scholar] [CrossRef]
- Eranna, G. Metal Oxide Nanostructures as Gas Sensing Devices; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Sharma, B.; Sharma, A.; Kim, J.-S. Recent advances on H2 sensor technologies based on MOX and FET devices: A review. Sens. Actuators B Chem. 2018, 262, 758–770. [Google Scholar] [CrossRef]
- Song, Y.G.; Shim, Y.-S.; Kim, S.; Han, S.D.; Moon, H.G.; Noh, M.S.; Lee, K.; Lee, H.R.; Kim, J.-S.; Ju, B.-K.; et al. Downsizing gas sensors based on semiconducting metal oxide: Effects of electrodes on gas sensing properties. Sens. Actuators B Chem. 2017, 248, 949–956. [Google Scholar] [CrossRef]
- Ma, L.; Fan, H.; Tian, H.; Fang, J.; Qian, X. The n-ZnO/n-In2O3 heterojunction formed by a surface-modification and their potential barrier-control in methanal gas sensing. Sens. Actuators B Chem. 2016, 222, 508–516. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Yin, J.; Li, L.; Zhang, L.-X.; Bie, L.-J. Enhanced ethanol gas-sensing properties of flower-like p-CuO/n-ZnO heterojunction nanorods. Sens. Actuators B Chem. 2014, 202, 500–507. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Chen, W. Fabrication of SnO2–SnO nanocomposites with p–n heterojunctions for the low-temperature sensing of NO2 gas. Nanoscale 2015, 7, 12133–12142. [Google Scholar] [CrossRef] [PubMed]
- Ju, D.; Xu, H.; Xu, Q.; Gong, H.; Qiu, Z.; Guo, J.; Zhang, J.; Cao, B. High triethylamine-sensing properties of NiO/SnO2 hollow sphere P–N heterojunction sensors. Sens. Actuators B Chem. 2015, 215, 39–44. [Google Scholar] [CrossRef]
- Mondal, B.; Basumatari, B.; Das, J.; Roychaudhury, C.; Saha, H.; Mukherjee, N. ZnO–SnO2 based composite type gas sensor for selective hydrogen sensing. Sens. Actuators B Chem. 2014, 194, 389–396. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, L.; Gao, W.; Wu, Z.; Lin, Y.; Li, G.; Guo, W.; Yu, L.; Zeng, H.; Zhu, J. Hydrogen gas sensing properties of MoS2/Si heterojunction. Sens. Actuators B Chem. 2015, 211, 537–543. [Google Scholar] [CrossRef]
- Alev, O.; Şennik, E.; Öztürk, Z.Z. Improved gas sensing performance of p-copper oxide thin film/n-TiO2 nanotubes heterostructure. J. Alloys Compd. 2018, 749, 221–228. [Google Scholar] [CrossRef]
- Lyson-Sypien, B.; Kusior, A.; Rekas, M.; Zukrowski, J.; Gajewska, M.; Michalow-Mauke, K.; Graule, T.; Radecka, M.; Zakrzewska, K. Nanocrystalline TiO2/SnO2 heterostructures for gas sensing. Beilstein J. Nanotechnol. 2017, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Kim, Y.J.; Jung, U.; Lee, B.H. Chemically induced Fermi level pinning effects of high-k dielectrics on graphene. Sci. Rep. 2018, 8, 2992. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.; Battaglia, C.; Azcatl, A.; McDonnell, S.; Kang, J.S.; Yin, X.; Tosun, M.; Kapadia, R.; Fang, H.; Wallace, R.M. MoS2 p-type transistors and diodes enabled by high work function MoOx contacts. Nano Lett. 2014, 14, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Downing, C.A.; Ahmady, B.; Catlow, C.R.A.; de Leeuw, N.H. The interaction of hydrogen with the {010} surfaces of Mg and Fe olivine as models for interstellar dust grains: A density functional theory study. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2013, 371, 20110592. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Matsuda, K.; Takahashi, K.; Sawada, K. CO2 Sensing Characteristics of a La2O3/SnO2 Stacked Structure with Micromachined Hotplates. Sensors 2017, 17, 2156. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, Y.; Cai, F.X.; Shafiei, M.; Chen, G.; Motta, N.; Wlodarski, W.; Kalantar-Zadeh, K.; Lai, P.T. A comparison study on hydrogen sensing performance of MoO3 nanoplatelets coated with a thin layer of Ta2O5 or La2O3. J. Appl. Sci. Eng. 2014, 17, 31–38. [Google Scholar]
- Yu, J.; Wen, H.; Shafiei, M.; Field, M.R.; Liu, Z.F.; Wlodarski, W.; Motta, N.; Li, Y.X.; Kalantar-Zadeh, K.; Lai, P.T. A hydrogen/methane sensor based on niobium tungsten oxide nanorods synthesised by hydrothermal method. Sens. Actuators B Chem. 2013, 184, 118–129. [Google Scholar] [CrossRef]
- Yu, J.; Yuan, L.; Wen, H.; Shafiei, M.; Field, M.R.; Liang, J.; Yang, J.; Liu, Z.F.; Wlodarski, W.; Motta, N.; et al. Hydrothermally formed functional niobium oxide doped tungsten nanorods. Nanotechnology 2013, 24, 495501. [Google Scholar] [CrossRef] [PubMed]
- Smit, G.; Rogge, S.; Klapwijk, T. Scaling of nano-Schottky-diodes. Appl. Phys. Lett. 2002, 81, 3852–3854. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Understanding the fundamental principles of metal oxide based gas sensors; the example of CO sensing with SnO2 sensors in the presence of humidity. J. Phys. Condens. Matter 2003, 15, 813. [Google Scholar] [CrossRef]
- Pokhrel, S.; Simion, C.; Quemener, V.; Barsan, N.; Weimar, U. Investigations of conduction mechanism in Cr2O3 gas sensing thick films by ac impedance spectroscopy and work function changes measurements. Sens. Actuators B Chem. 2008, 133, 78–83. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Rhodes, D.; Feng, S.; Nguyen, M.A.T.; Watanabe, K.; Taniguchi, T.; Mallouk, T.E.; Terrones, M.; Balicas, L.; Zhu, J. Gate-modulated conductance of few-layer WSe2 field-effect transistors in the subgap regime. Appl. Phys. Lett. 2016, 108, 69901. [Google Scholar] [CrossRef]
- Shiwakoti, N.; Bobby, A.; Asokan, K.; Antony, B. Transport properties of Gallium Phosphide based Schottky contact with thin insulating layer. Mater. Sci. Semicond. Process. 2017, 61, 145–149. [Google Scholar] [CrossRef]
- Yamada, H.; Chonan, H.; Takahashi, T.; Shimizu, M. Electrical properties of Ni/n-GaN Schottky diodes on freestanding m-plane GaN substrates. Appl. Phys. Express 2017, 10, 041001. [Google Scholar] [CrossRef]
- Rhoderick, E.H.; Williams, R. Metal-Semiconductor Contacts; Clarendon Press Oxford: New York, NY, USA, 1988. [Google Scholar]
- Umezawa, H.; Shikata, S.I. Leakage current analysis of diamond Schottky barrier diodes operated at high temperature. Jpn. J. Appl. Phys. 2014, 53, 4S. [Google Scholar] [CrossRef]
- Umezawa, H.; Saito, T.; Tokuda, N.; Ogura, M.; Ri, S.G.; Yoshikawa, H.; Shikata, S.I. Leakage current analysis of diamond Schottky barrier diode. Appl. Phys. Lett. 2007, 90, 073506. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheung, K.W.; Yu, J.; Ho, D. Determination of the Optimal Sensing Temperature in Pt/Ta2O5/MoO3 Schottky Contacted Nanobelt Straddling Heterojunction. Sensors 2018, 18, 3770. https://doi.org/10.3390/s18113770
Cheung KW, Yu J, Ho D. Determination of the Optimal Sensing Temperature in Pt/Ta2O5/MoO3 Schottky Contacted Nanobelt Straddling Heterojunction. Sensors. 2018; 18(11):3770. https://doi.org/10.3390/s18113770
Chicago/Turabian StyleCheung, Ka Wai, Jerry Yu, and Derek Ho. 2018. "Determination of the Optimal Sensing Temperature in Pt/Ta2O5/MoO3 Schottky Contacted Nanobelt Straddling Heterojunction" Sensors 18, no. 11: 3770. https://doi.org/10.3390/s18113770
APA StyleCheung, K. W., Yu, J., & Ho, D. (2018). Determination of the Optimal Sensing Temperature in Pt/Ta2O5/MoO3 Schottky Contacted Nanobelt Straddling Heterojunction. Sensors, 18(11), 3770. https://doi.org/10.3390/s18113770




