Improving the Surface-Enhanced Raman Scattering Performance of Silver Nanodendritic Substrates with Sprayed-On Graphene-Based Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Silver Nanoparticles
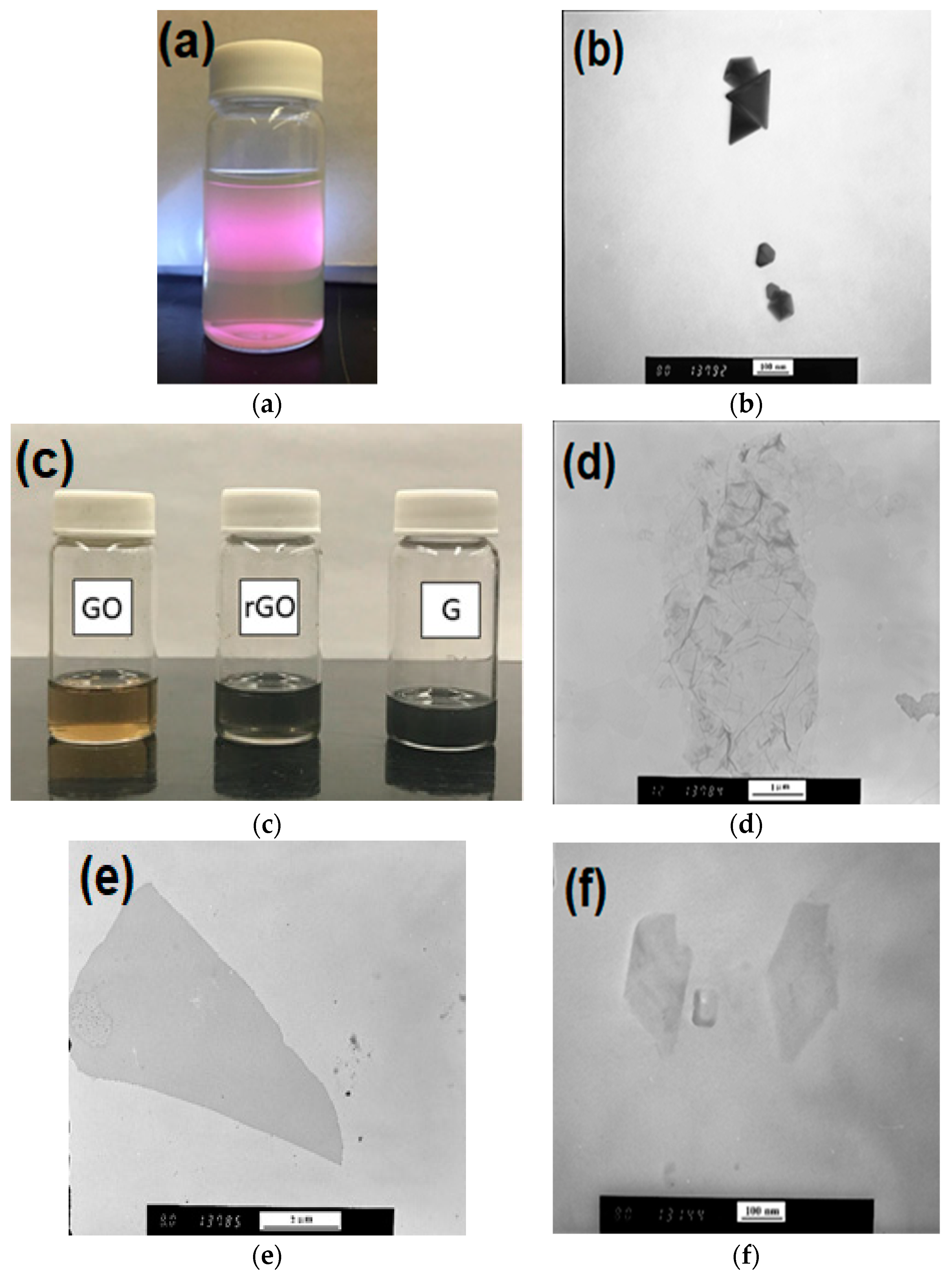
2.2. Graphene Oxide Dispersion
2.3. Reduced Graphene Oxide
2.4. Graphene Nanoplates (GNPs)
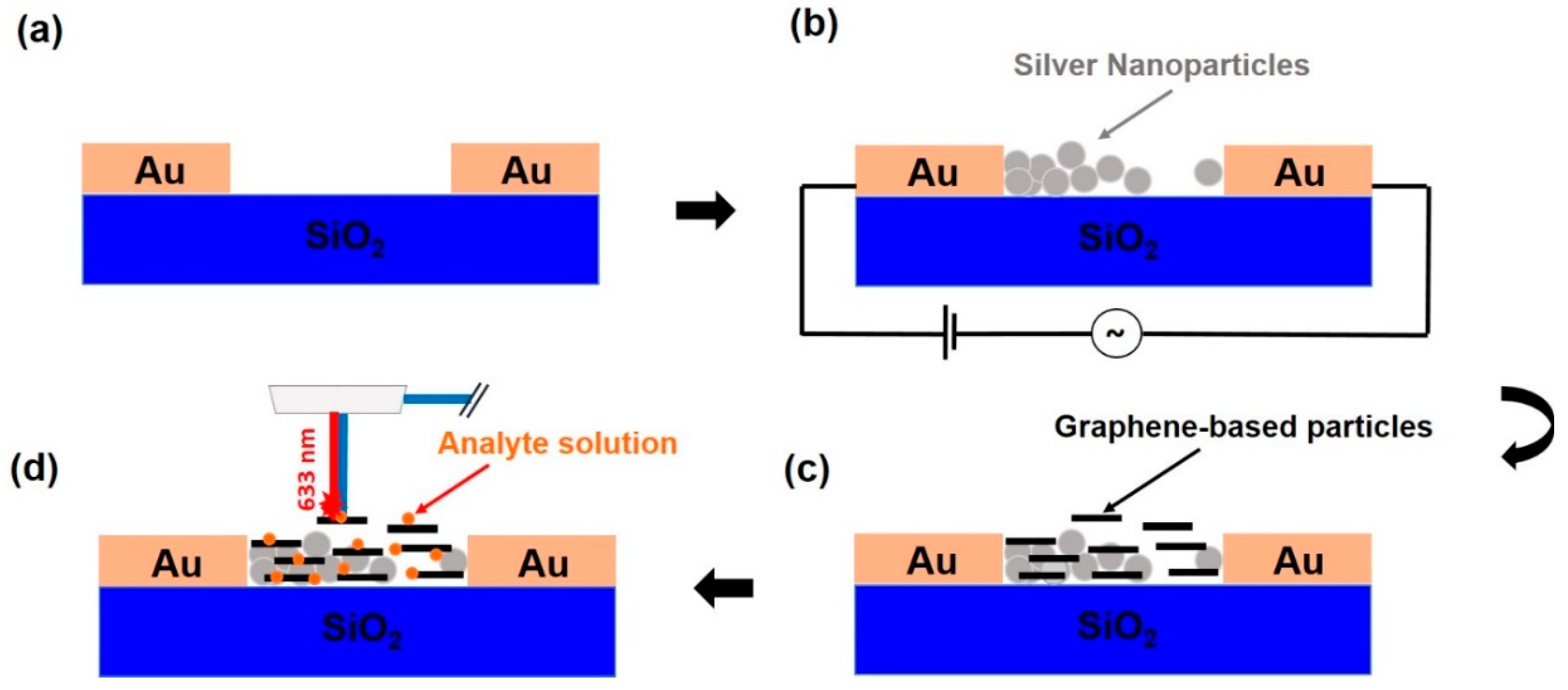
2.5. Microchip Fabrication
2.6. SERS Substrate Preparation
2.7. Sprayed Graphene-Based Coatings
2.8. Characterization
3. Results
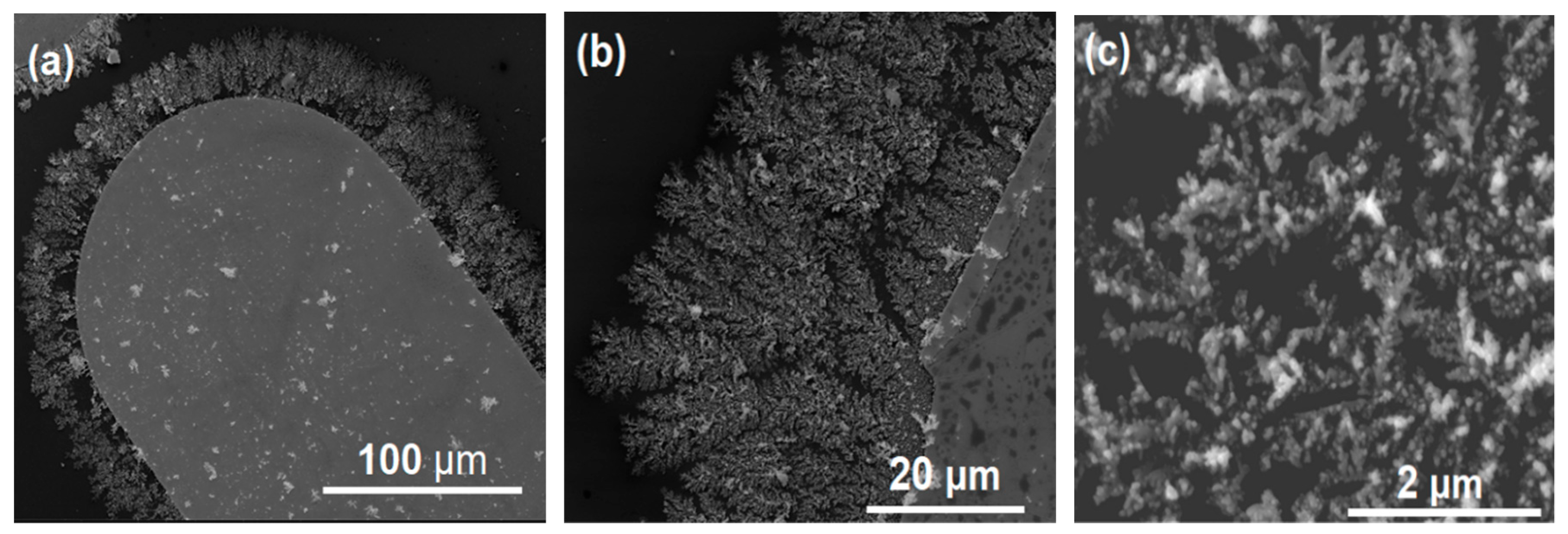
3.1. Electric Field-Assisted Assembly of Dendritic Ag Nanoparticle Structures
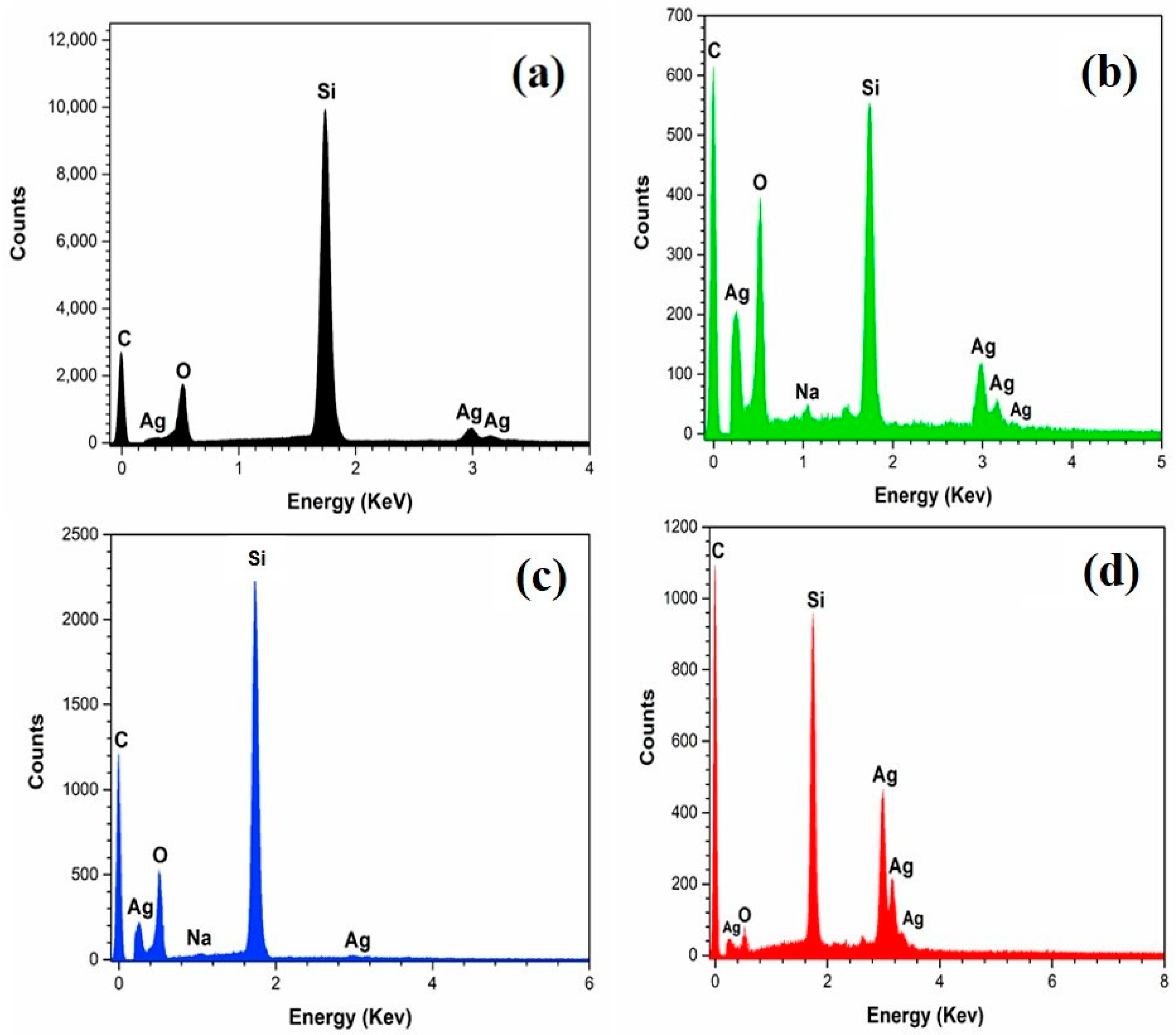
3.2. Dendrites Coated with Graphene-Based Derivatives.
3.3. SERS Performance Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qiu, H.; Wang, M.; Jiang, S.; Zhang, L.; Yang, Z.; Li, L.; Li, J.; Cao, M.; Huang, J. Reliable molecular trace-detection based on flexible SERS substrate of graphene/Ag-nanoflowers/PMMA. Sens. Actuators B Chem. 2017, 249, 439–450. [Google Scholar] [CrossRef]
- Campion, A.; Kambhampati, P. Surface-enhanced Raman scattering. Chem. Soc. Rev. 1998, 27, 241–250. [Google Scholar] [CrossRef]
- Haynes, C.L.; Yonzon, C.R.; Zhang, X.; Van Duyne, R.P. Surface-enhanced Raman sensors: Early history and the development of sensors for quantitative biowarfare agent and glucose detection. J. Raman Spectrosc. 2005, 36, 471–484. [Google Scholar] [CrossRef]
- Baker, G.A.; Moore, D.S. Progress in plasmonic engineering of surface-enhanced Raman-scattering substrates toward ultra-trace analysis. Anal. Bioanal. Chem. 2005, 382, 1751–1770. [Google Scholar] [CrossRef] [PubMed]
- Matteini, P.; Cottat, M.; Tavanti, F.; Panfilova, E.; Scuderi, M.; Nicotra, G.; Menziani, M.C.; Khlebtsov, N.; Angelis, M.D.; Pini, R. Site-Selective Surface-Enhanced Raman Detection of Proteins. ACS Nano 2017, 11, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Shen, J.; Yan, J.; Zhong, J.; Qin, W.; Liu, R.; Aldalbahi, A.; Zuo, X.; Song, S.; Fan, C.; et al. Highly narrow nanogap-containing Au@Au core-shell SERS nanoparticles: Size-dependent Raman enhancement and applications in cancer cell imaging. Nanoscale 2016, 8, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Frontiera, R.R.; Henry, A.I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Ben-Jaber, S.; Peveler, W.J.; Quesada-Cabrera, R.; Cortes, E.; Sotelo-Vazquez, C.; Abdul-Karim, N.; Mier, S.A.; Parkin, I.P. Photo-induced enhanced Raman spectroscopy for universal ultra-trace detection of explosives, pollutants and biomolecules. Nat. Commun. 2016, 7, 12189. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.; Liu, Y.; Zhang, L.; Wang, F.; Lu, Y. High performance surface-enhanced Raman scattering sensing based on Au nanoparticle-monolayer graphene-Ag nanostar array hybrid system. Sens. Actuators B Chem. 2017, 247, 850–857. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, D.; Ding, M.; Zhao, H.; Zhang, Z.; Li, B.; Shen, D. A white-emitting ZnO–Au nanocomposite and its SERS applications. Appl. Surf. Sci. 2012, 258, 7813–7819. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, K.; Huang, Y.; Zhang, J. Graphene-Ag hybrids on laser-textured Si surface for SERS detection. Sensors 2017, 17, 1462. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Liu, W.; Perlaki, C.M.; Chen, K.; Feng, C.; Liu, Q. Sustained and cost effective silver substrate for surface enhanced Raman spectroscopy based biosensing. Sci. Rep. 2017, 7, 6917. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Lee, Y.H.; Pedireddy, S.; Zhang, Q.; Li, T.; Ling, X.Y. Graphene oxide and shape-controlled silver nanoparticle hybrids for ultrasensitive single-particle surface-enhanced Raman scattering (SERS) sensing. Nanoscale 2014, 6, 4843–4851. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Choy, W.C.H.; Ren, X.; Zhang, D.; Lu, H. Highly intensified surface enhanced Raman scattering by using monolayer graphene as the nanospacer of metal film–metal nanoparticle coupling system. Adv. Funct. Mater. 2014, 24, 3113. [Google Scholar] [CrossRef]
- Gu, H.X.; Xu, L.; Zhang, Y.F.; Li, D.W.; Long, Y.T. Facile Fabrication of a Silver Dendrite-Integrated Chip for Surface Enhanced Raman Scattering. ACS Appl. Mater. Interfaces 2015, 7, 2931–2936. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lin, M.; Li, H.; Kim, N.J. Surface-enhanced Raman spectroscopy coupled with dendritic silver nanosubstrate for detection of restricted antibiotics. J. Raman Spectrosc. 2010, 41, 739–744. [Google Scholar] [CrossRef]
- Gutes, A.; Carraro, C.; Maboudian, R. Silver dendrites from galvanic displacement on commercial aluminum foil as an effective SERS substrate. J. Am. Chem. Soc. 2010, 132, 1476–1477. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhu, D.; Yu, A. Galvanic replacement synthesis of silver dendrites-reduced graphene oxide composites and their surface-enhanced Raman scattering characteristics. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 149, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Liu, Y.J.; Han, Y.; Chen, P.; Zhang, C.; Li, C.; Lu, Z.; Luo, D.; Jiang, S. Graphene oxide-decorated silver dendrites for high-performance surface-enhanced Raman scattering applications. J. Mater. Chem. C 2017, 5, 3908–3915. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, G.; Du, Y.; Xu, J.; Wu, S.; Qu, Y.; Zhu, Y. Plasmonic-enhanced Raman scattering of graphene on growth substrates and its application in SERS. Nanoscale 2014, 6, 13754–13760. [Google Scholar] [CrossRef] [PubMed]
- Dies, H.; Raveendran, J.; Escobedo, C.; Docoslis, A. Rapid identification and quantification of illicit drugs on nanodendritic surface-enhanced Raman scattering substrates. Sens. Actuators B Chem. 2018, 257, 382–388. [Google Scholar] [CrossRef]
- Dies, H.; Raveendran, J.; Escobedo, C.; Docoslis, A. In situ assembly of active surface-enhanced Raman scattering substrates via electric field guided growth of dendritic nanoparticle structures. Nanoscale 2017, 9, 7847–7857. [Google Scholar] [CrossRef] [PubMed]
- Dies, H.; Nosrati, R.; Raveendran, J.; Escobedo, C.; Docoslis, A. SERS-from-scratch: An electric field-guided nanoparticle assembly method for cleanroom-free and low-cost preparation of surface-enhanced Raman scattering substrates. Colloids. Surf. A Physicochem. Eng. Asp. 2018, 553, 695–702. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, L.; Müllen, K. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.C.; Lai, Y.S.; Tsai, C.M.; Kong, Y.T.; Lee, C.I.; Huang, C.L. One-Pot synthesis of monodispersed silver nanodecahedra with optimal SERS activities using seedless photo-assisted citrate reduction method. J. Phys. Chem. C 2012, 116, 24292–24300. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Wang, L.; Sun, X. A method for the production of reduced graphene oxide using benzylamine as a reducing and stabilizing agent and its subsequent decoration with Ag nanoparticles for enzymeless hydrogen peroxide detection. Carbon 2011, 49, 3158–3164. [Google Scholar] [CrossRef]
- Giglio, C. Graphene Nanoplatelet Production through Non-Ionic Surfactant-Assisted Exfoliation of Graphite. Master’s Thesis, Queens University, Kingston, ON, Canada, 2017. [Google Scholar]
- Gallerneault, M.; Truica-Marasescu, F.; Docoslis, A. A route to industrial-scale reduced graphene oxide coatings. Surf. Coat. Technol. 2018, 334, 196–203. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-ray Microanalysis; Springer: Berlin, Germany, 2017. [Google Scholar]
- Guthmuller, J.; Champagne, B. Resonance Raman scattering of rhodamine 6G as calculated by time-dependent density functional Theory: Vibronic and solvent effects. J. Phys. Chem. A 2008, 112, 3215–3223. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Cai, H.; Zhang, W.; Li, X.; Pan, N.; Luo, Y.; Wang, X.; Hou, J.G. Tuning chemical enhancement of SERS by controlling the chemical reduction of graphene oxide nanosheets. ACS Nano 2011, 5, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Novoselov, K.S.; Shin, H.S. Interaction between Metal and Graphene: Dependence on the Layer Number of Graphene. ACS Nano 2011, 5, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Canadian Model for Providing a Safe Workplace: Alcohol and Drug Guidelines and Work Rule, 5th ed.; Construction Owners Association of Alberta: Edmonton, AB, Canada, 2014; pp. 16–17.

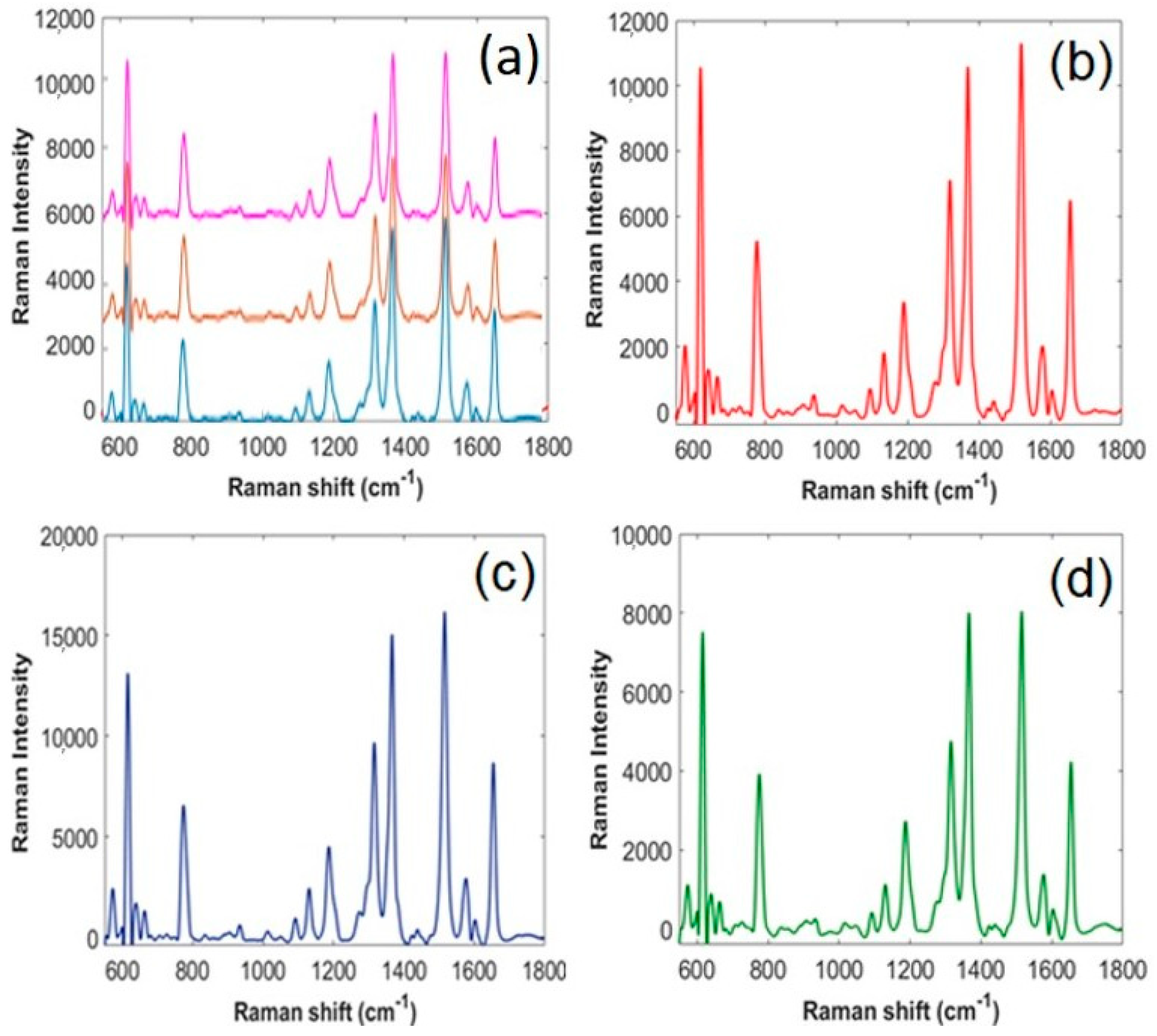
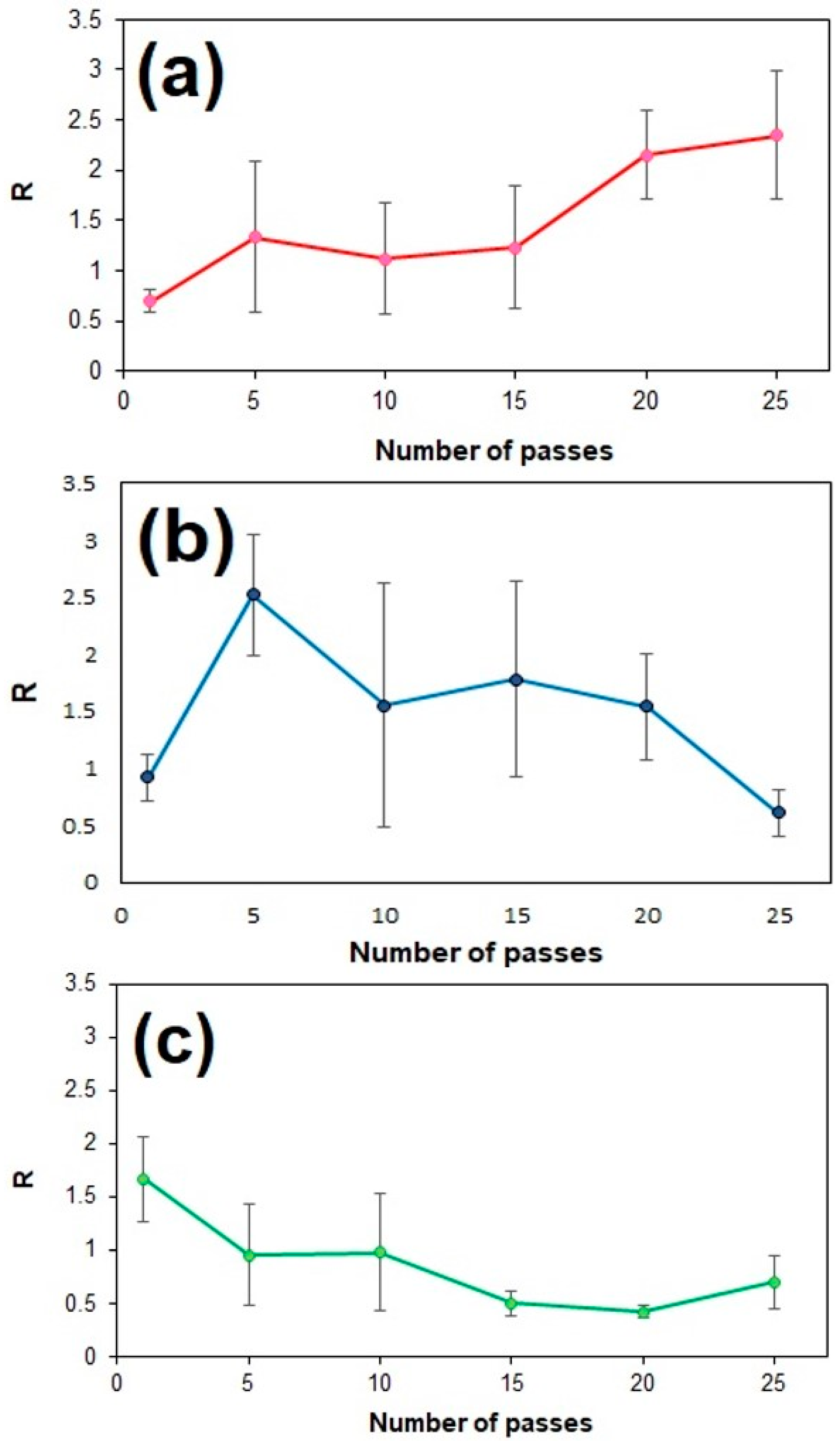
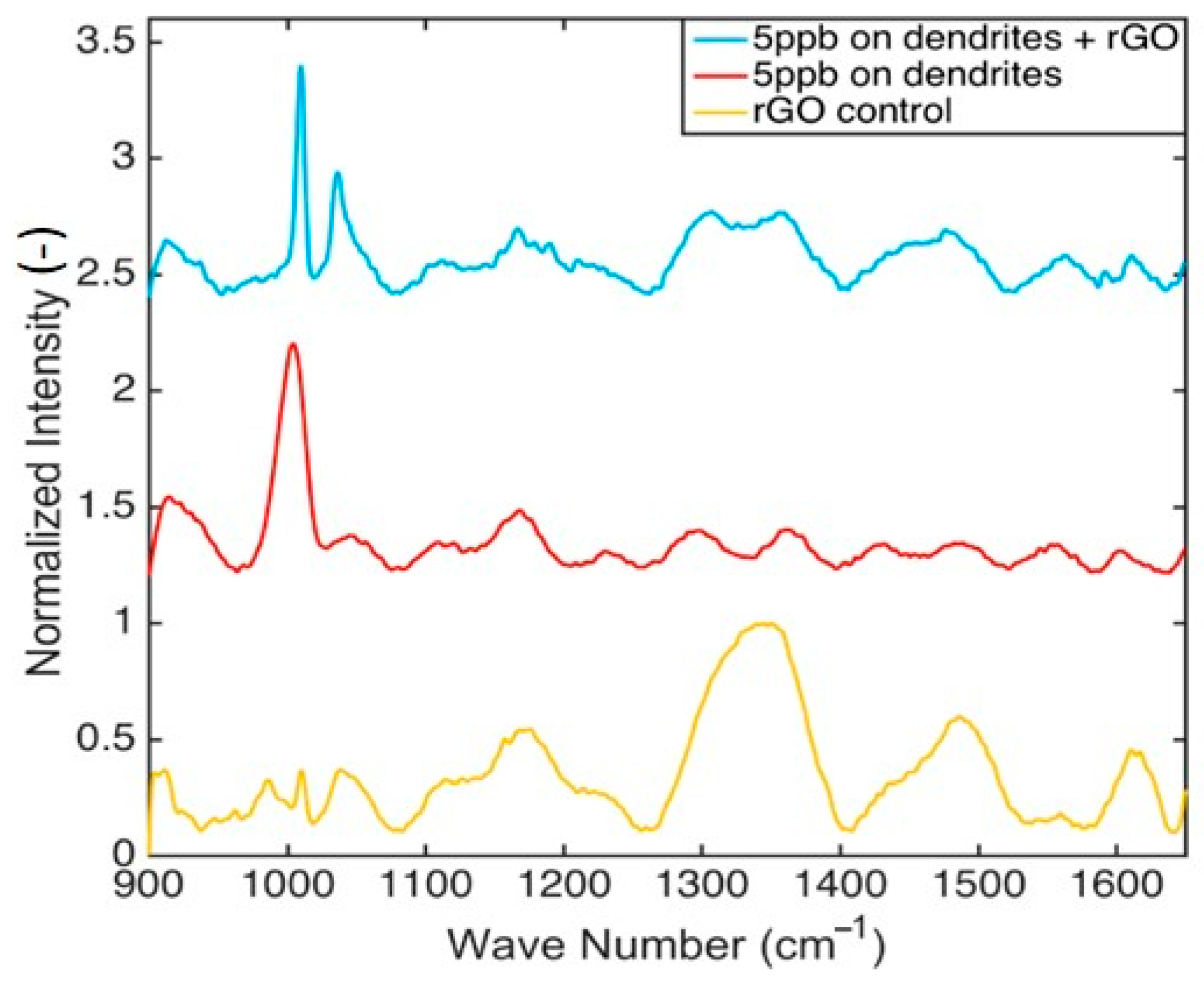
| Coating | Solvent | Optimal Passes, N | Standard Deviation (S.D.) | |
|---|---|---|---|---|
| GO | Ethanol | 25 | 2.3 | 0.64 |
| rGO | NMP | 5 | 2.5 | 0.53 |
| GNP | NMP | 1 | 1.6 | 0.45 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadi, A.; Nicholls, D.L.; Docoslis, A. Improving the Surface-Enhanced Raman Scattering Performance of Silver Nanodendritic Substrates with Sprayed-On Graphene-Based Coatings. Sensors 2018, 18, 3404. https://doi.org/10.3390/s18103404
Mohammadi A, Nicholls DL, Docoslis A. Improving the Surface-Enhanced Raman Scattering Performance of Silver Nanodendritic Substrates with Sprayed-On Graphene-Based Coatings. Sensors. 2018; 18(10):3404. https://doi.org/10.3390/s18103404
Chicago/Turabian StyleMohammadi, Aida, Danielle Lilly Nicholls, and Aristides Docoslis. 2018. "Improving the Surface-Enhanced Raman Scattering Performance of Silver Nanodendritic Substrates with Sprayed-On Graphene-Based Coatings" Sensors 18, no. 10: 3404. https://doi.org/10.3390/s18103404
APA StyleMohammadi, A., Nicholls, D. L., & Docoslis, A. (2018). Improving the Surface-Enhanced Raman Scattering Performance of Silver Nanodendritic Substrates with Sprayed-On Graphene-Based Coatings. Sensors, 18(10), 3404. https://doi.org/10.3390/s18103404






