Construction of Multiple Switchable Sensors and Logic Gates Based on Carboxylated Multi-Walled Carbon Nanotubes/Poly(N,N-Diethylacrylamide)
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Preparation of Poly(N,N-Diethylacrylamide) Hydrogel Films
2.3. Apparatus and Procedures
3. Results
3.1. Characterization of Poly(N,N-Diethylacrylamide) (PDEA) Films
3.2. pH Responsive Behaviors of Poly(N,N-Diethylacrylamide) Films
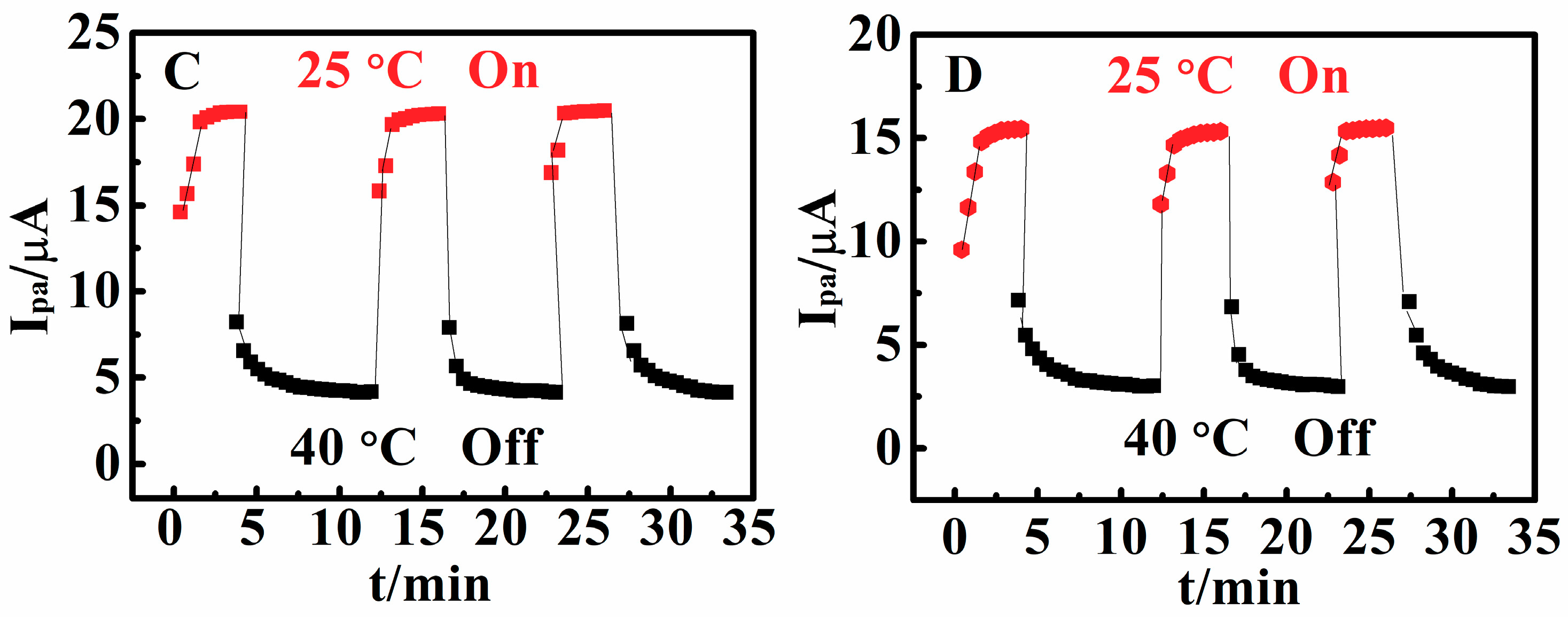
3.3. Temperature-Responsive Behaviors of Poly(N,N-Diethylacrylamide) Films
3.4. Na2SO4-Responsive Behaviors of Poly(N,N-Diethylacrylamide) Films
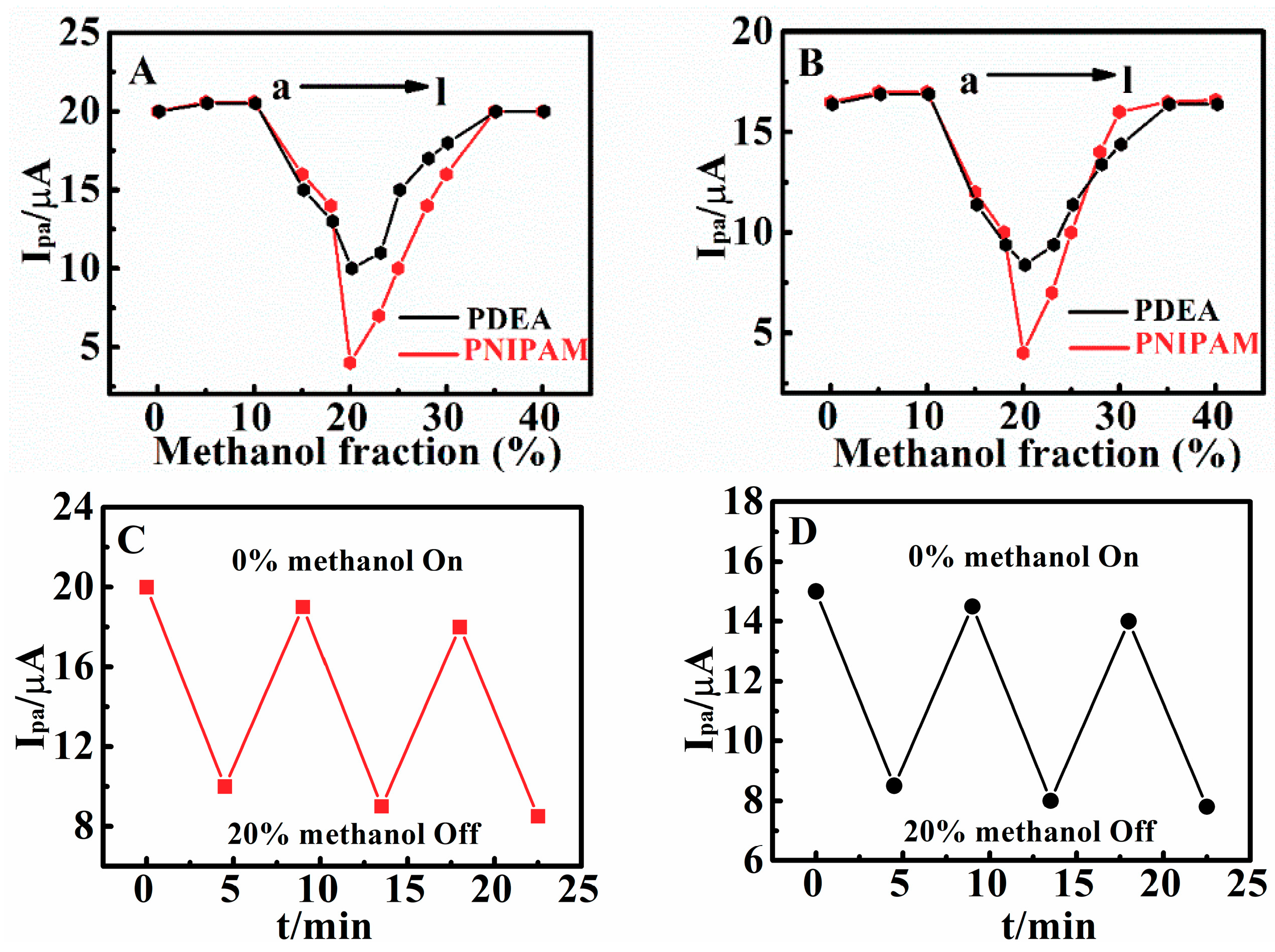
3.5. Methanol-Responsive Behaviors of Poly(N,N-Diethylacrylamide) Films
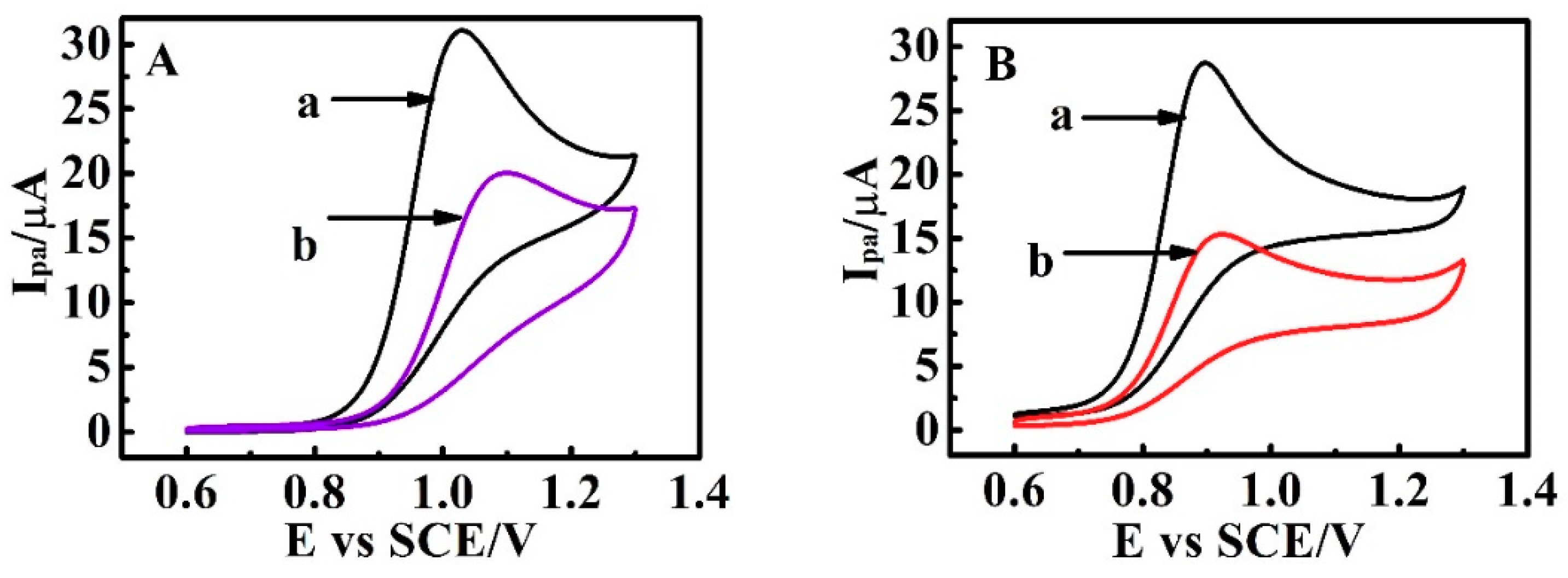
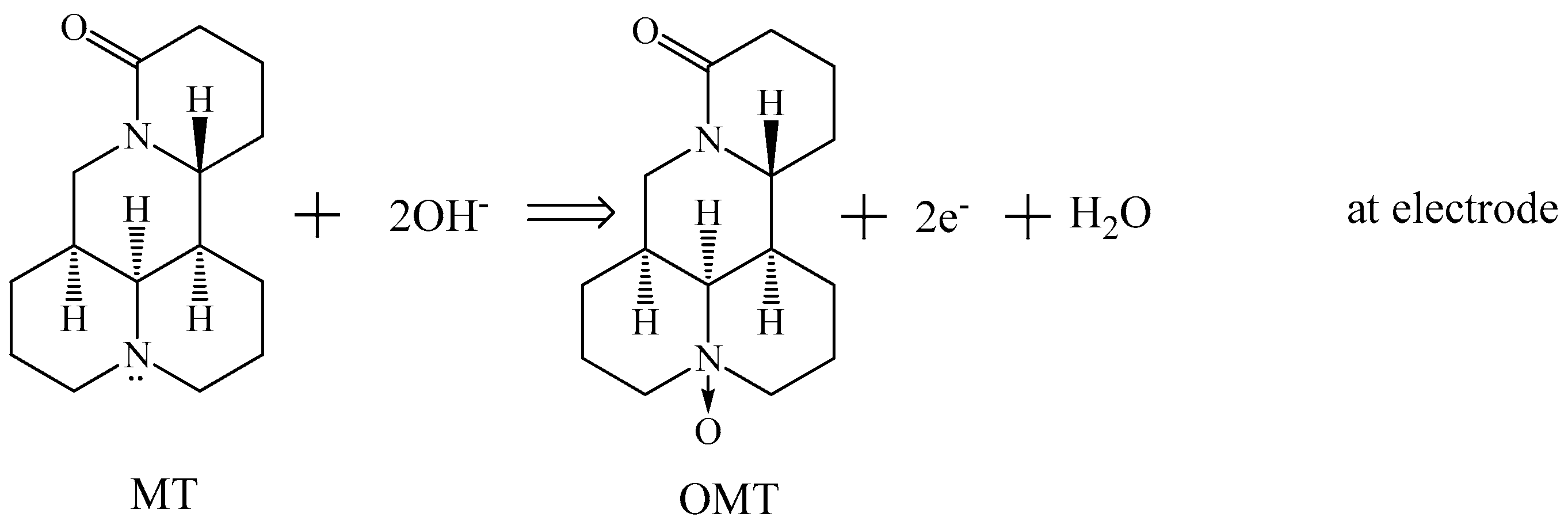
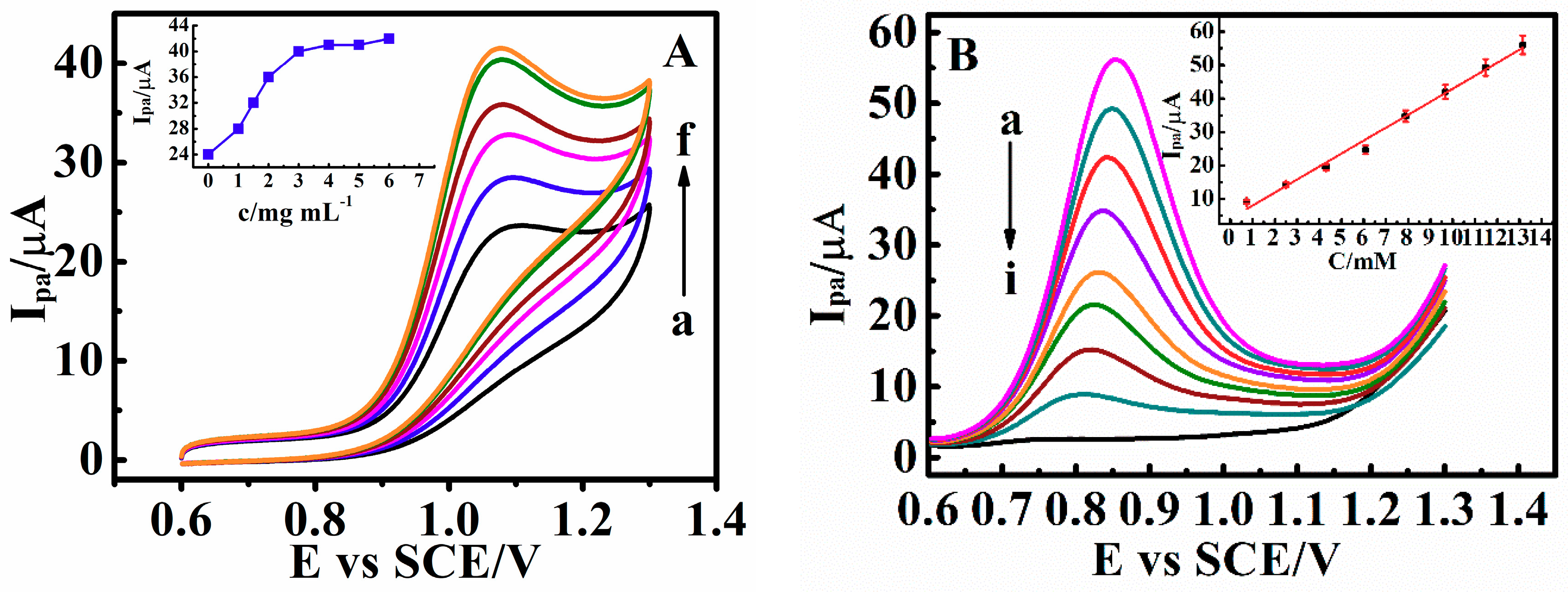
3.6. Electrocatalysis by Carboxylated Multi-Walled Carbon Nanotubes
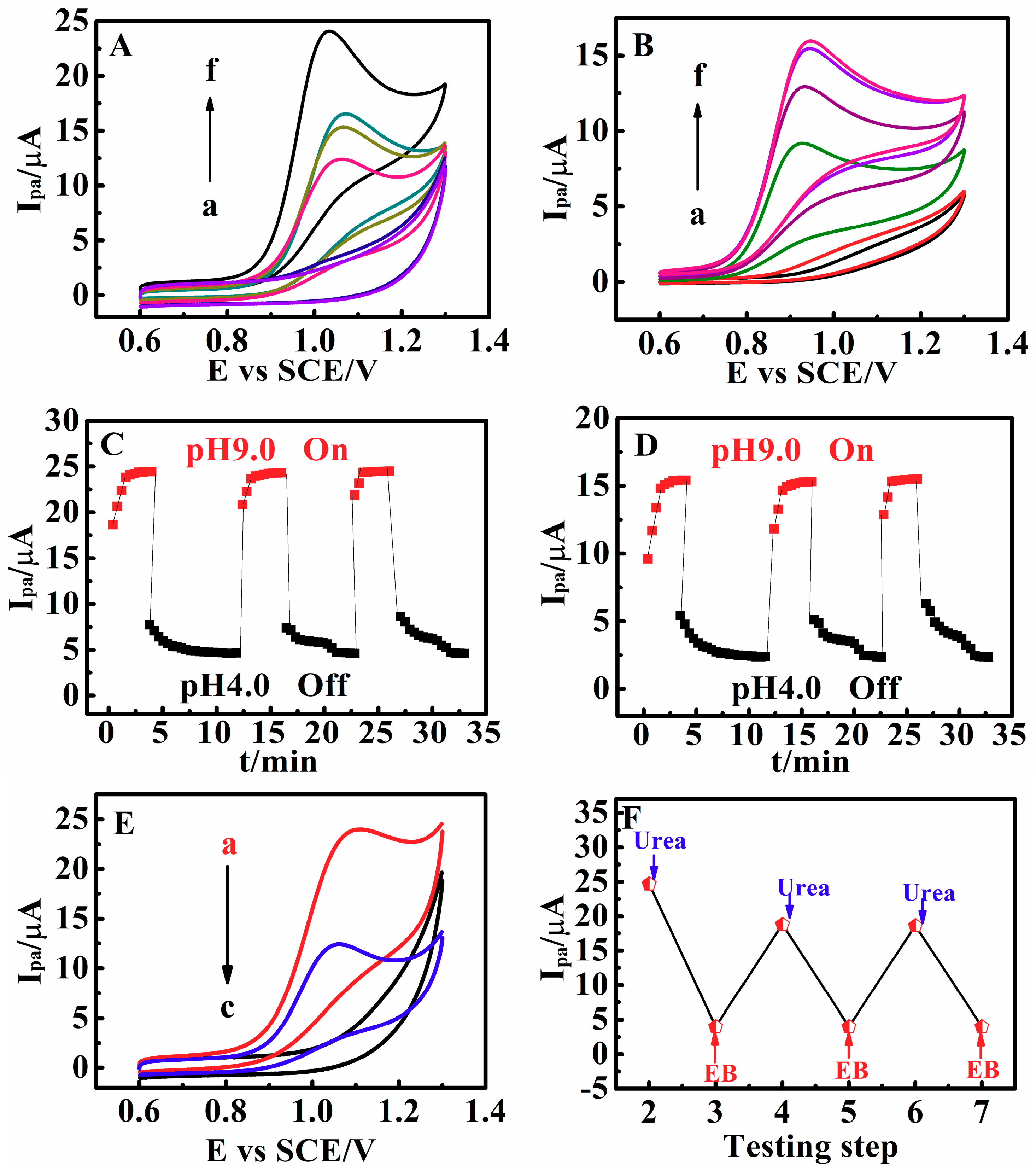
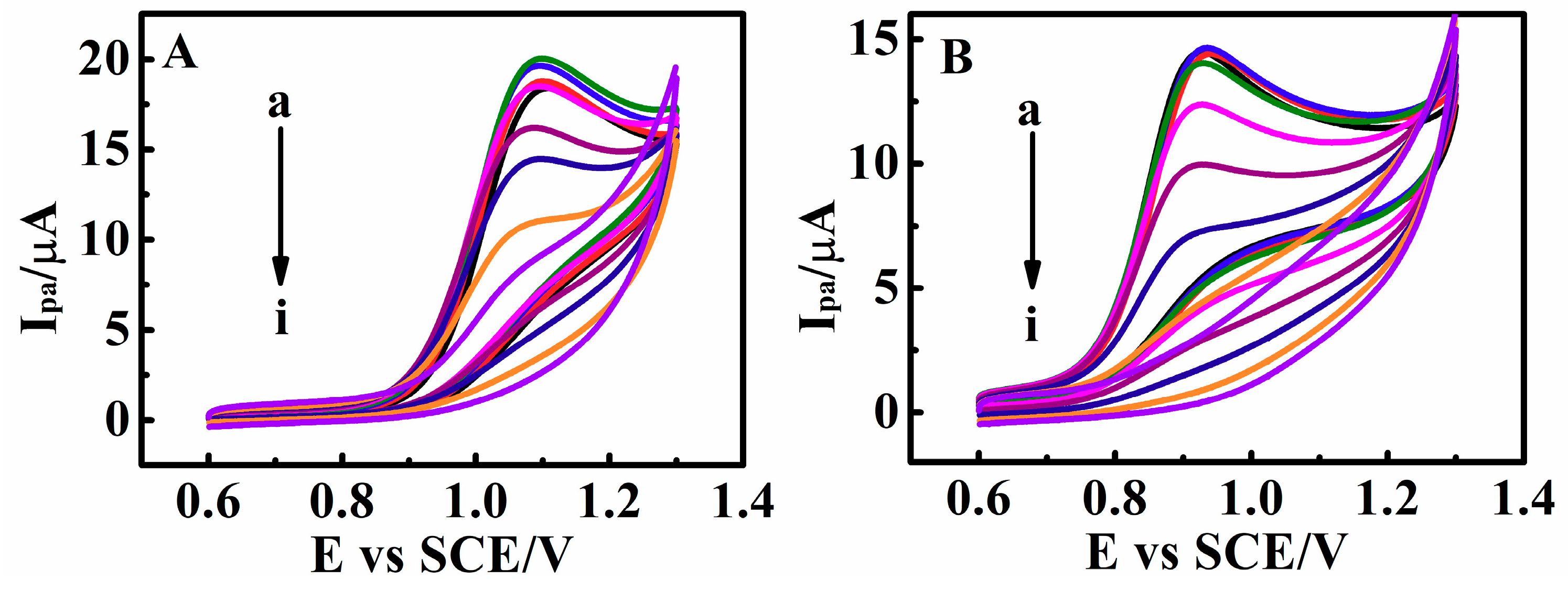
3.7. Switchable Electrocatalysis Controlled by pH, Temperature, SO42− Concentration and Methanol
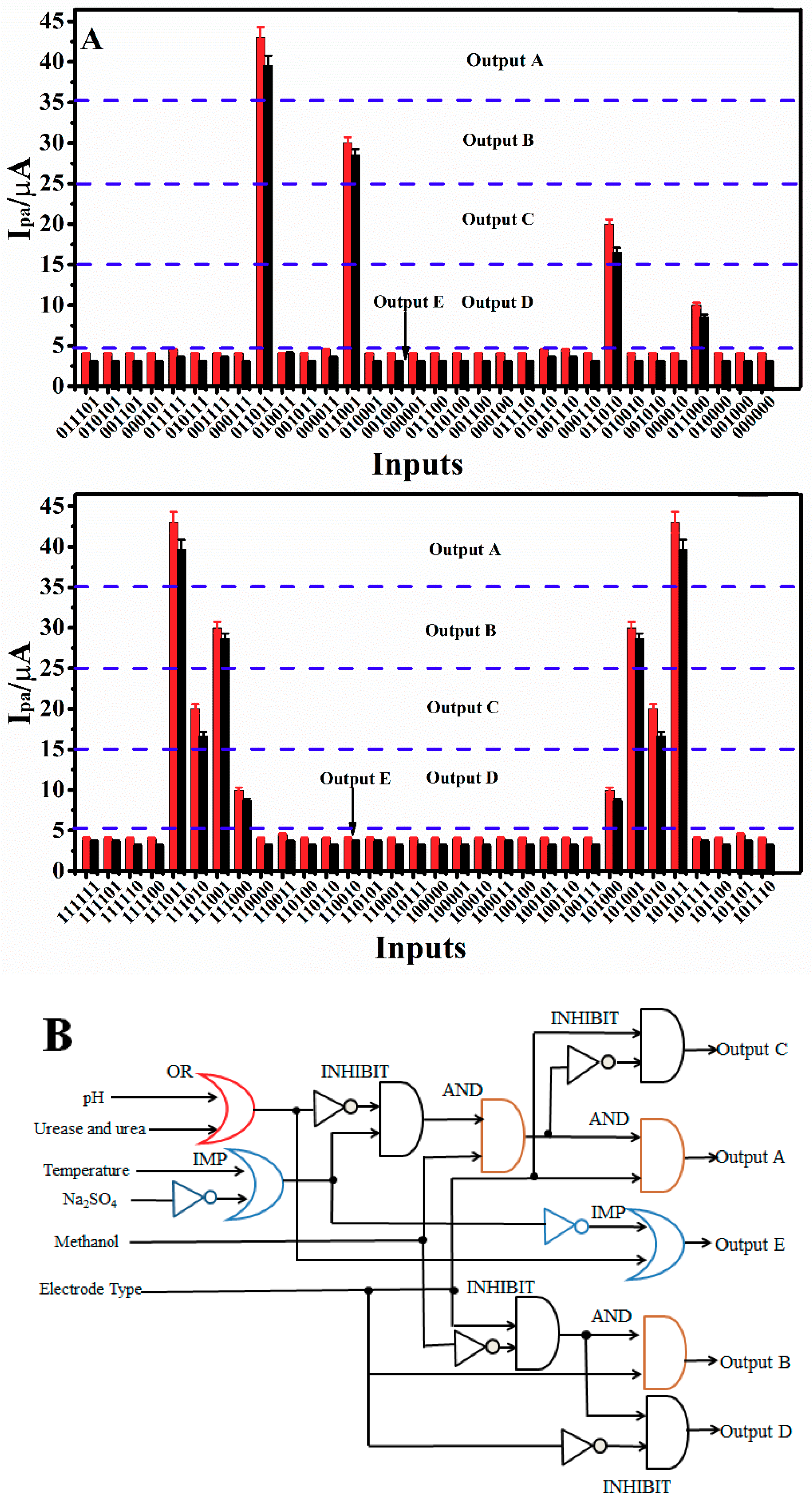
3.8. Establishment of a 6-Input/5-Output Logic Gate
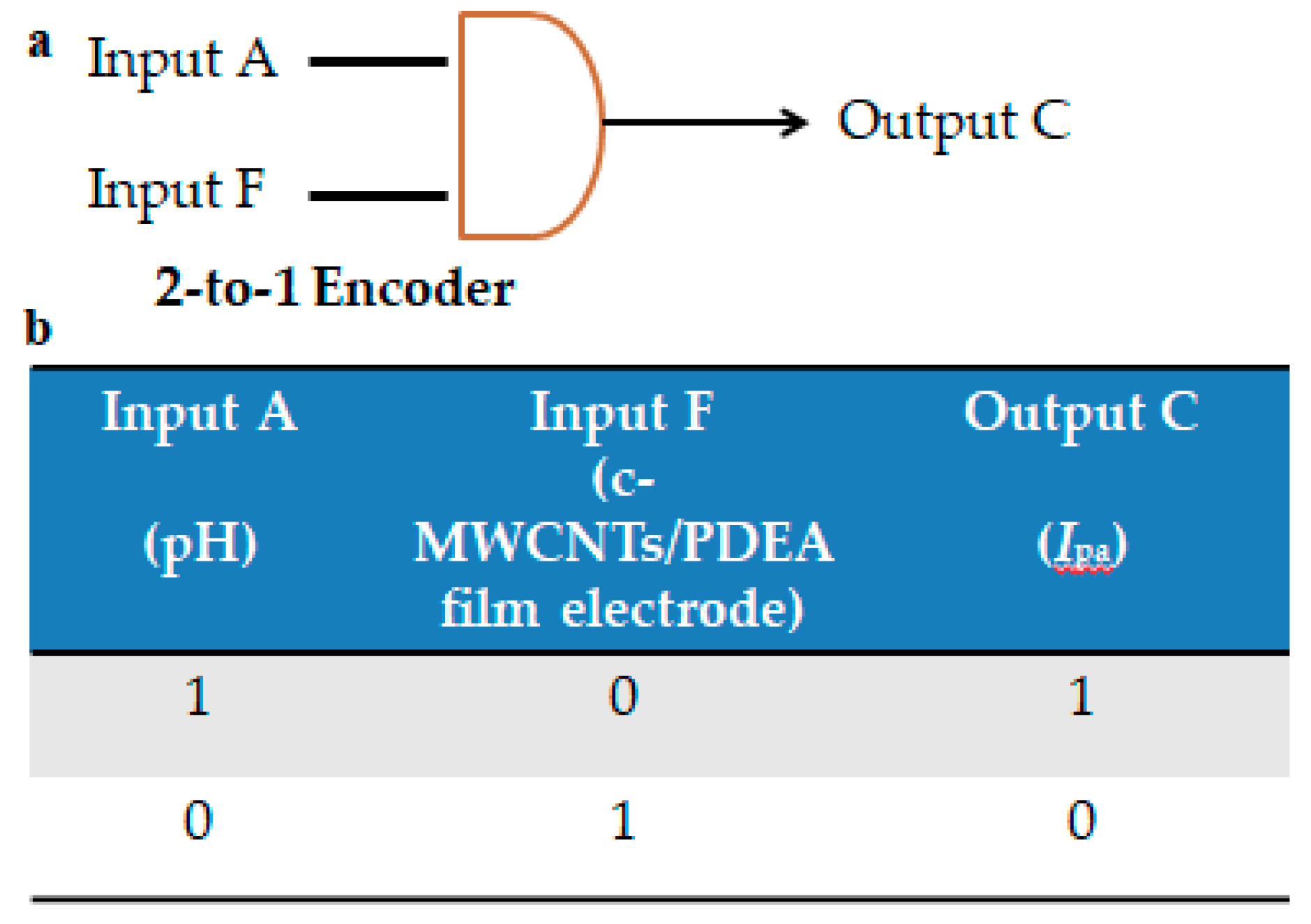
3.9. Fabrication of a 2-to-1 Encoder
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lian, W.; Liang, J.; Shen, L.; Jin, Y.; Liu, H. Enzymatic logic calculation systems based on solid-state electrochemiluminescence and molecularly imprinted polymer film electrodes. Biosens. Bioelectron. 2018, 100, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Erbas-Cakmak, S.; Kolemen, S.; Sedgwick, A.C.; Gunnlaugsson, T.; James, T.D.; Yoon, J.; Akkaya, E.U. Molecular logic gates: The past, present and future. Chem. Soc. Rev. 2018, 47, 2228–2248. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Poghossian, A.; Schoning, M.J. Enzyme-based logic gates and circuits-analytical applications and interfacing with electronics. Anal. Bioanal. Chem. 2017, 409, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, M.; Lu, L.; Yao, H.; Liu, H. An enzymatic calculation system based on electrochemiluminescence and fluorescence of luminol and cyclic voltammetry of ferrocene methanol. Biosens. Bioelectron. 2018, 118, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Lyu, Y.; Liu, H.; Peng, R.; Zhang, X.; Ye, M.; Tan, W. DNA-based dynamic reaction networks. Trends Biochem. Sci. 2018, 43, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Willner, I.; Shlyahovsky, B.; Zayats, M.; Willner, B. DNAzymes for sensing, nanobiotechnology and logic gate applications. Chem. Soc. Rev. 2008, 37, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Katz, E. Digital biosensors with built-in logic for biomedical applications—Biosensors based on a biocomputing concept. Anal. Bioanal. Chem. 2010, 398, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Fu, J.; Zheng, S.; Yao, H.; Jin, Y.; Lu, Y.; Liu, H. Biomolecular logic devices based on stimuli-responsive PNIPAM-DNA film electrodes and bioelectrocatalysis of natural DNA with Ru(bpy)32+ as mediator. Biosens. Bioelectron. 2018, 108, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lian, W.; Zhang, J.; Liu, H. Multi-input and -output logic circuits based on bioelectrocatalysis with horseradish peroxidase and glucose oxidase immobilized in multi-responsive copolymer films on electrodes. Biosens. Bioelectron. 2016, 80, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Pan, J.F.; Chen, S. Label-free and enzyme-free platform with visible output for constructing versatile logic gates using caged G-quadruplex as the signal transducer. Chem. Sci. 2018, 9, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, S.; Halamek, J.; Katz, E. A model system for targeted drug release triggered by biomolecular signals logically processed through enzyme logic networks. Analyst 2014, 139, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Privman, M.; Tam, T.K.; Bocharova, V.; Halamek, J.; Wang, J.; Katz, E. Responsive interface switchable by logically processed physiological signals: toward “smart” actuators for signal amplification and drug delivery. ACS Appl. Mater. Interfaces 2011, 3, 1620–1623. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yu, X.; Yang, T.; Li, M.; Shen, L.; Jin, Y.; Liu, H. A complicated biocomputing system based on multi-responsive P(NIPAM-co-APBA) copolymer film electrodes and electrocatalysis of NADH. Phy. Chem. Chem. Phys. 2017, 19, 22472–22481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, X.; Yang, L.; Yu, P.; Mao, L. Photodecomposition of ferrocenedicarboxylic acid in methanol to form an electroactive infinite coordination polymer and its application in bioelectrochemistry. ACS Appl. Mater. Interfaces 2013, 5, 8120–8124. [Google Scholar] [CrossRef] [PubMed]
- Núnez-Bajo, E.; Blanco-López, M.C.; Costa-García, A.; FernándezAbedul, M.T. Integration of gold-sputtered electrofluidic paper on wire-included analytical platforms for glucose biosensing. Biosens. Bioelectron. 2017, 91, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Lounasvuori, M.M.; Rosillo-Lopez, M.; Salzmann, C.G.; Caruana, D.J.; Holt, K.B. Electrochemical characterisation of graphene nanoflakes with functionalised edges. Faraday Discuss. 2014, 172, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Song, S.; Yao, H.; Hu, N. Triply switchable bioelectrocatalysis based on poly(N-isopropylacrylamide) hydrogel films with immobilized glucose oxidase. Electrochim. Acta 2011, 56, 5166–5173. [Google Scholar] [CrossRef]
- Wang, L.; Lian, W.; Yao, H.; Liu, H. Multiple-stimuli responsive bioelectrocatalysis based on reduced graphene oxide/poly(N-isopropylacrylamide) composite films and its application in the fabrication of logic gates. ACS Appl. Mater. Interfaces 2015, 7, 5168–5176. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, H.; Yamauchi, T.; Terado, Y.; Odagiri, S.; Sasak, S.; Higashiyama, K. Synthesis of a piperidinone scaffold as an analgesic through kappa-opioid receptor: structure-activity relationship study of matrine alkaloids. Chem. Pharm. Bull. 2016, 64, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, G.; Liu, S.; Wei, J.; Zhang, S.; Li, M.; Zhou, G.; Wang, L. Synthesis and biological evaluation of matrine derivatives containing benzo-alpha-pyrone structure as potent anti-lung cancer agents. Sci. Rep. 2016, 6, 35918–35926. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Li, Q.; Liu, D.; Li, J.; Cai, Q.; Li, C.; Zhao, Q.; Xu, W. Therapeutic effects of matrine derivate MASM in mice with collagen-induced arthritis and on fibroblast-like synoviocytes. Sci. Rep. 2017, 7, 2454–2464. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Zou, Q.; He, Y.; Wang, H.; Li, N.; Zeng, X. Matrine inhibits mouse sperm function by reducing sperm [Ca2+]i and phospho-ERK1/2. Cell. Physiol. Biochem. 2015, 35, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Jiang, J.; Cui, H. Antitumor effects of matrine on cancer stem like cells isolated from the human liver cancer SMMC-7721 cell line. Oncol. Lett. 2018, 15, 1777–1782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Su, K.; Shi, W.; Wang, Y.; Hu, P.; Wang, Y.; Wei, L.; Xiang, J.; Yang, F. Matrine inhibits the adhesion and migration of BCG823 gastric cancer cells by affecting the structure and function of the vasodilator-stimulated phosphoprotein (VASP). Acta Pharmacol. Sin. 2013, 34, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Lin, L.; Wang, P.; Liu, H. Thermo- and sulfate-controllable bioelectrocatalysis of glucose based on horseradish peroxidase and glucose oxidase embedded in poly(N,N-diethylacrylamide) hydrogel films. Appl. Biochem. Biotechnol. 2014, 173, 2005–2018. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, H.; Hu, N. pH-, sugar-, and temperature-sensitive electrochemical switch amplified by enzymatic reaction and controlled by logic gates based on semi-interpenetrating polymer networks. J. Phys. Chem. B 2012, 116, 1700–1708. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, S.; Liu, H. Multiple stimuli-switchable bioelectrocatalysis under physiological conditions based on copolymer films with entrapped enzyme. J. Phys. Chem. B 2014, 118, 6653–6661. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Hu, N. Triply responsive films in bioelectrocatalysis with a binary architecture: Combined layer-by-layer assembly and hydrogel polymerization. J. Phys. Chem. B 2011, 115, 6691–6699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiong, H.; Gao, Y.; Wang, Z.; Li, X.; Ma, X.; Yang, H. Electrochemical behavior and determination of matrine by the reduced graphene oxide-nafion modified glassy carbon electrode. Int. J. Electrochem. Sci. 2015, 10, 6348–6358. [Google Scholar]
- Liu, Z.; He, D.; Zhang, X.; Li, Y.; Zhu, C.; Dong, L.; Zhang, X.; Xing, Y.; Wang, C.; Qiao, H.; et al. Neuroprotective effect of early and short-time applying sophoridine in pMCAO rat brain: Down-regulated TRAF6 and up-regulated p-ERK1/2 expression, ameliorated brain infaction and edema. Brain Res. Bull. 2012, 88, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Su, X.; Bo, T.; Zhang, X.; Liu, H.; Li, K. Determination of dissociation constants of ten alkaloids by capillary zone electrophoresis. J. Sep. Sci. 2003, 26, 549–554. [Google Scholar] [CrossRef]
- Huo, Z.; Wan, Q.; Chen, L. Synthesis and evaluation of porous polymethylsilsesquioxane microspheres as low silanol activity chromatographic stationary phase for basic compound separation. J. Chromatogr. A 2018, 1553, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Amir, L.; Tam, T.K.; Pita, M.; Meijler, M.M.; Alfonta, L.; Katz, E. Biofuel cell controlled by enzyme logic systems. J. Am. Chem. Soc. 2008, 131, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Pita, M. Biofuel cells controlled by logically processed biochemical signals: towards physiologically regulated bioelectronic devices. Chemistry 2009, 15, 12554–12564. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tam, T.K.; Pita, M.; Ornatska, M.; Minko, S.; Katz, E. Bioelectrocatalytic system coupled with enzyme-based biocomputing ensembles performing boolean logic operations: approaching “smart” physiologically controlled biointerfaces. ACS Appl. Mater. Interfaces 2008, 1, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Termuhlen, F.; Kuckling, D.; Schonhoff, M. Isothermal titration calorimetry to probe the coil-to-globule transition of thermoresponsive polymers. J. Phys. Chem. B 2017, 121, 8611–8618. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, M.; Bian, F.; Wang, B.; Chen, S.; Jin, S. The effect of NaCl on the conformational behavior of acenaphthylene labeled poly(N,N-diethylacrylamide) in dilute aqueous solution. Macromol. Chem. Phys. 2006, 207, 104–110. [Google Scholar] [CrossRef]
- Panayiotou, M.; Freitag, R. Influence of the synthesis conditions and ionic additives on the swelling behaviour of thermo-responsive polyalkylacrylamide hydrogels. Polymer 2005, 46, 6777–6785. [Google Scholar] [CrossRef]
- Liu, B.; Wang, J.; Ru, G.; Liu, C.; Feng, J. Phase transition and preferential alcohol adsorption of poly(N,N-diethylacrylamide) gel in water/alcohol mixtures. Macromolecules 2015, 48, 1126–1133. [Google Scholar] [CrossRef]
- Maeda, Y.; Nakamura, T.; Ikeda, I. Change in solvation of poly(N,N-diethylacrylamide) during phase transition in aqueous solutions as observed by IR spectroscop. Macromolecules 2002, 35, 10172–10177. [Google Scholar] [CrossRef]
- Maeda, Y.; Yamabe, M. A unique phase behavior of random copolymer of N-isopropylacrylamide and N,N-diethylacrylamide in water. Polymer 2009, 50, 519–523. [Google Scholar] [CrossRef]
- Sipa, K.; Brycht, M.; Leniart, A.; Urbaniak, P.; Nosal-Wiercinska, A.; Palecz, B.; Skrzypek, S. β-Cyclodextrins incorporated multi-walled carbon nanotubes modified electrode for the voltammetric determination of the pesticide dichlorophen. Talanta 2018, 176, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Dukovic, G.; Brus, L.E.; Heinz, T.F. The optical resonances in carbon nanotubes arise from excitons. Science 2005, 308, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Capek, I. Dispersions, novel nanomaterial sensors and nanoconjugates based on carbon nanotubes. Adv. Colloid Interface Sci. 2009, 150, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, M.; Ramaprabhu, S. Enhanced electrochemical performance by unfolding a few wings of graphene nanoribbons of multiwalled carbon nanotubes as an anode material for Li ion battery applications. Nanoscale 2015, 7, 13379–13386. [Google Scholar] [CrossRef] [PubMed]
- Perets, Y.; Aleksandrovych, L.; Melnychenko, M.; Lazarenko, O.; Vovchenko, L.; Matzui, L. The electrical properties of hybrid composites based on multiwall carbon nanotubes with graphite nanoplatelets. Nanoscale Res. Lett. 2017, 12, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, K.; Dai, X.; Li, Y.; Guo, J.; Liu, H.; Li, G.; Tan, Y.; Zeng, J.; Guo, Z. Enhanced electrical conductivity and piezoresistive sensing in multi-wall carbon nanotubes/polydimethylsiloxane nanocomposites via the construction of a self-segregated structure. Nanoscale 2017, 9, 11017–11026. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Liu, J.; Cao, A.; Song, W.; Wang, D. Interfacial synthesis of ordered and stable covalent organic framework on amino-functionalized carbon nanotube with enhanced electrochemical performance. Chem. Comm. 2017, 53, 6303–6306. [Google Scholar] [CrossRef] [PubMed]
- Puertolas, J.A.; Kurtz, S.M. Evaluation of carbon nanotubes and graphene as reinforcements for UHMWPE-based composites in arthroplastic applications: A review. J. Mech. Behav. Biomed. Mater. 2014, 39, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Heidarimoghadam, R.; Farmany, A. Rapid determination of furosemide in drug and blood plasma of wrestlers by a carboxyl-MWCNT sensor. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 1242–1265. [Google Scholar] [CrossRef] [PubMed]
- Batra, B.; Pundir, C.S. An amperometric glutamate biosensor based on immobilization of glutamate oxidase onto carboxylated multiwalled carbon nanotubes/gold nanoparticles/chitosan composite film modified Au electrode. Biosens. Bioelectron. 2013, 47, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Badeau, B.A.; Comerford, M.P.; Arakawa, C.K.; Shadish, J.A.; DeForest, C.A. Engineered modular biomaterial logic gates for environmentally triggered therapeutic delivery. Nat. Chem. 2018, 10, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, M.; Yu, X.; Li, C.Z.; Liu, H. Biomacromolecular logic gate, encoder/decoder and keypad lock based on DNA damage with electrochemiluminescence and electrochemical signals as outputs. Chem. Commun. 2015, 51, 13185–13188. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Hu, N. pH-controllable bioelectrocatalysis based on “on−off” switching redox property of electroactive probes for spin-assembled layer-by-layer films containing branched poly(ethyleneimine). J. Phys. Chem. B 2010, 114, 3648–3654. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Hu, N. Dual-switchable bioelectrocatalysis synergistically controlled by pH and perchlorate concentration based on poly(4-vinylpyridine) films. J. Phys. Chem. B 2010, 114, 11689–11695. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, G.; Hu, J.; Lu, X.; Li, J. Temperature, ionic strength and pH induced electrochemical switching of smart polymer interfaces. Chem. Commun. 2006, 4820–4822. [Google Scholar] [CrossRef]
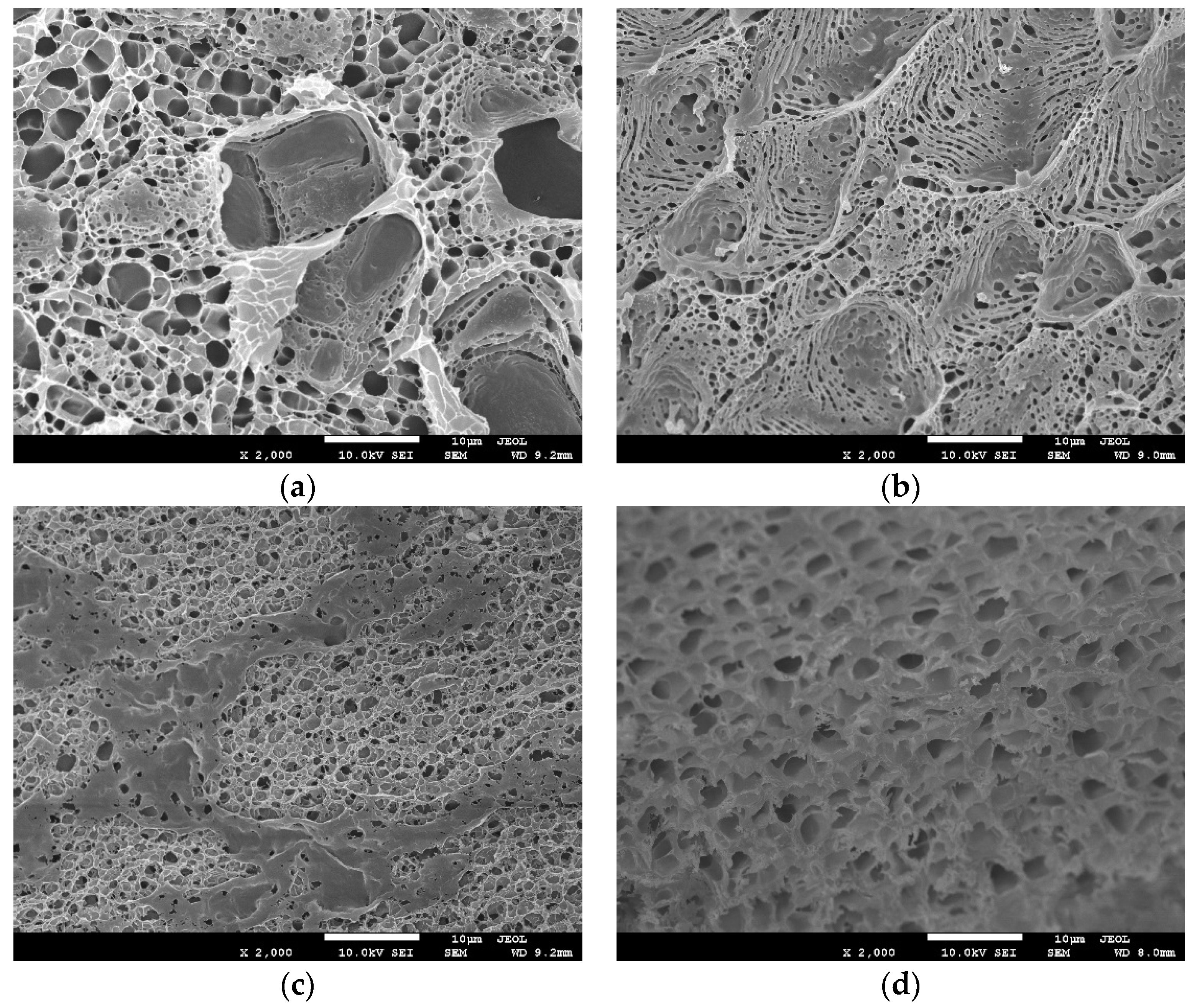


| Measurement Conditions | Average Thickness/µm |
|---|---|
| pH 9.0 and 25 °C | 410 ± 30 |
| pH 4.0 and 25 °C | 405 ± 8 |
| pH 9.0 and 40 °C | 276 ± 15 |
| pH 9.0 and 25 °C containing 0.35 M Na2SO4 | 207 ± 20 |
| pH 9.0 and 25 °C containing 20% methanol | 328 ± 18 |
| Input | Definition | The “1” State | The “0” State |
|---|---|---|---|
| A | pH | 9.0 | 5.0 |
| B | Urease and urea | 6 mM urea | 0 mM urea |
| C | Temperature | 25 °C | 40 °C |
| D | Na2SO4 | 0.35 M | 0 M |
| E | Methanol | 0% | 20% |
| F | Electrode Type | c-MWCNTs/PDEA | PDEA |
| Outputs | PDEA States | Drug States | Methanol Fraction | Electrode Type |
|---|---|---|---|---|
| Output A (Ipa ≥ 35 µA) | No phase transition | molecule | 0% | c-MWCNTs/PDEA electrode |
| Output B (35 µA < Ipa ≤ 25 µA) | No phase transition | molecule | 20% | c-MWCNTs/PDEA electrode |
| Output C (25 µA < Ipa ≤ 15 µA) | No phase transition | molecule | 0% | PDEA electrode |
| Output D (15 µA < Ipa ≤ 5 µA) | No phase transition | molecule | 20% | PDEA electrode |
| Output E (Ipa < 5 µA) | The phase transition of PDEA was appeared or drugs were present in the form of nitrogen cationic radical or both of these things happen at the same time | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Bai, X.; Ma, Y.; Wei, J.; Peng, J.; Shi, K.; Yao, H. Construction of Multiple Switchable Sensors and Logic Gates Based on Carboxylated Multi-Walled Carbon Nanotubes/Poly(N,N-Diethylacrylamide). Sensors 2018, 18, 3358. https://doi.org/10.3390/s18103358
Wu X, Bai X, Ma Y, Wei J, Peng J, Shi K, Yao H. Construction of Multiple Switchable Sensors and Logic Gates Based on Carboxylated Multi-Walled Carbon Nanotubes/Poly(N,N-Diethylacrylamide). Sensors. 2018; 18(10):3358. https://doi.org/10.3390/s18103358
Chicago/Turabian StyleWu, Xuemei, Xiaoqing Bai, Yang Ma, Jie Wei, Juan Peng, Keren Shi, and Huiqin Yao. 2018. "Construction of Multiple Switchable Sensors and Logic Gates Based on Carboxylated Multi-Walled Carbon Nanotubes/Poly(N,N-Diethylacrylamide)" Sensors 18, no. 10: 3358. https://doi.org/10.3390/s18103358
APA StyleWu, X., Bai, X., Ma, Y., Wei, J., Peng, J., Shi, K., & Yao, H. (2018). Construction of Multiple Switchable Sensors and Logic Gates Based on Carboxylated Multi-Walled Carbon Nanotubes/Poly(N,N-Diethylacrylamide). Sensors, 18(10), 3358. https://doi.org/10.3390/s18103358





