Online Removal of Baseline Shift with a Polynomial Function for Hemodynamic Monitoring Using Near-Infrared Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Detrending Method
2.2. Solid Phantom
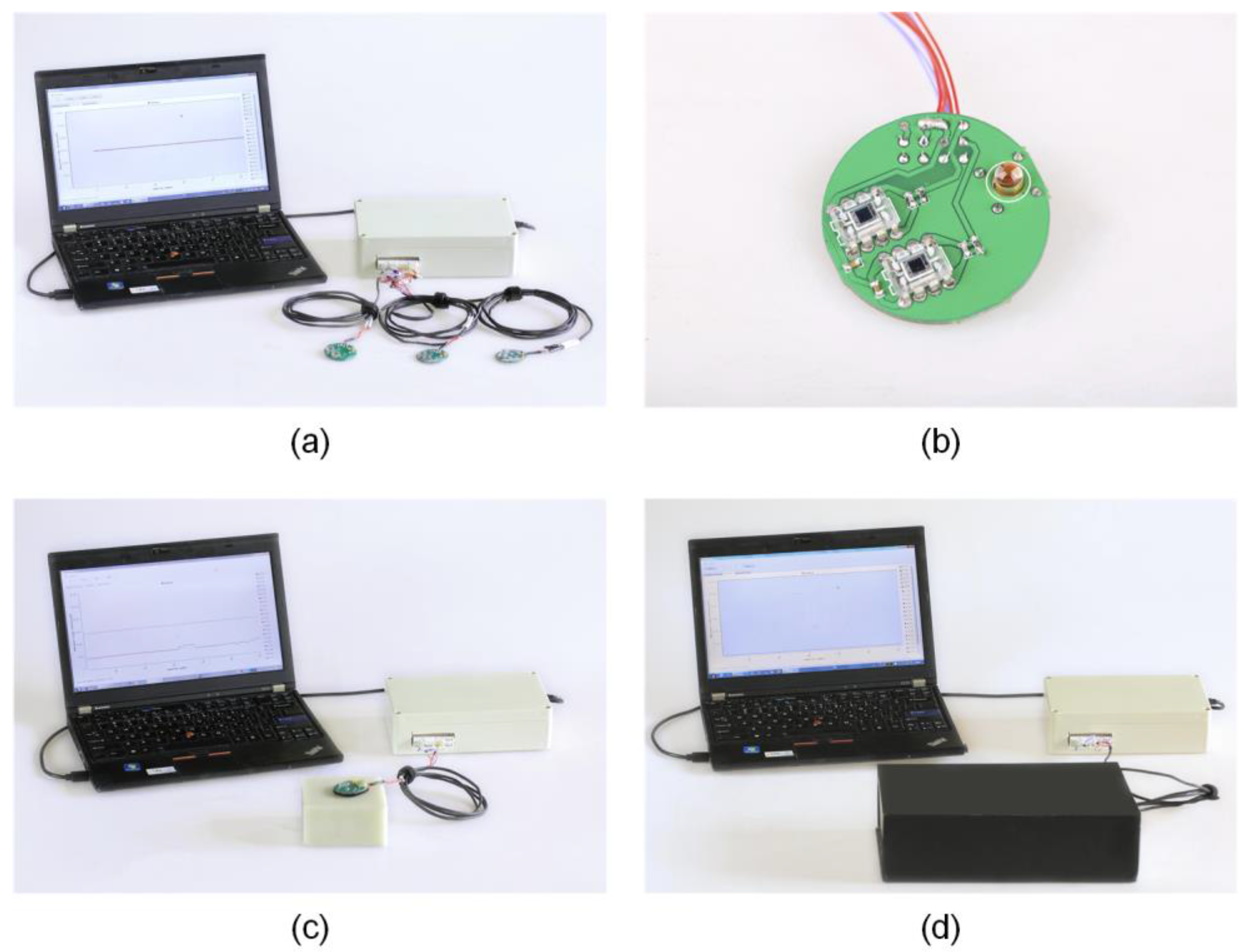
2.3. Data Acquisition System
2.4. Baseline Extraction and Removal
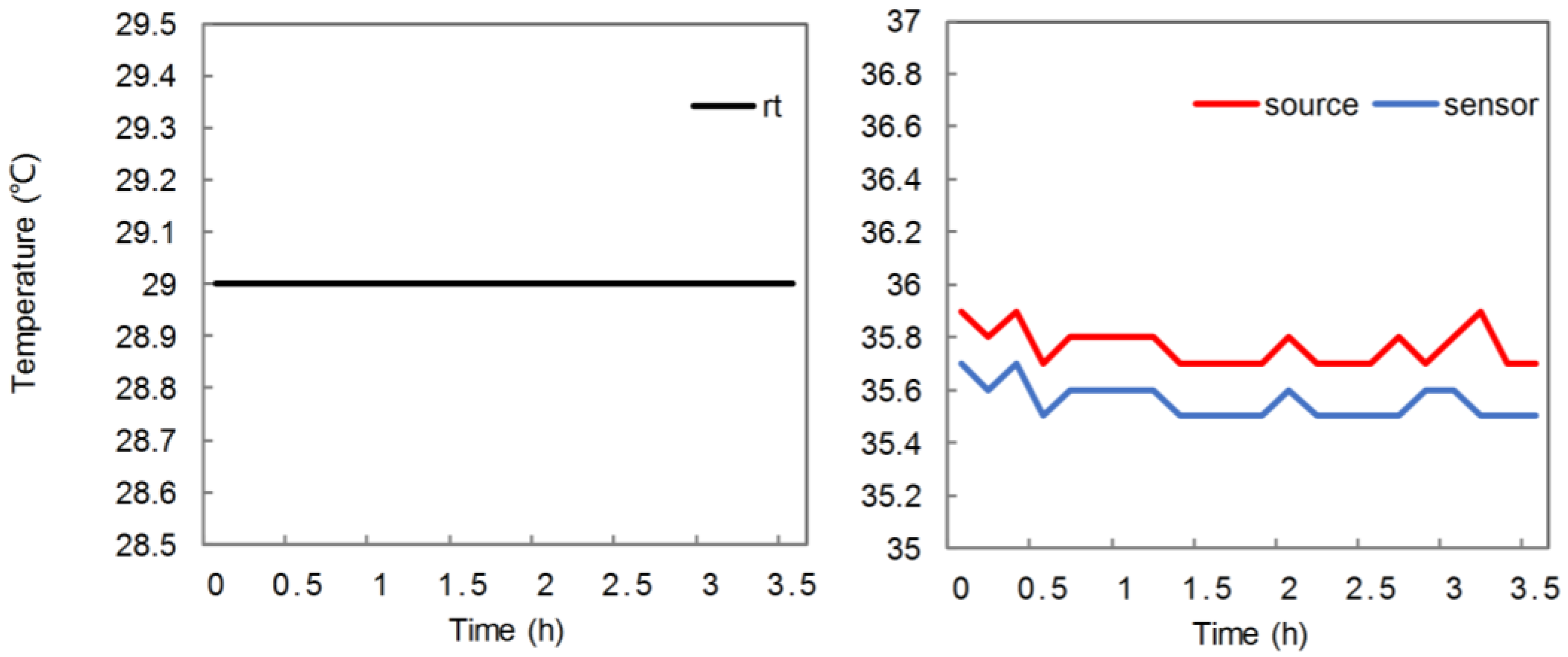
2.5. Experiment on Removal Effect
3. Results
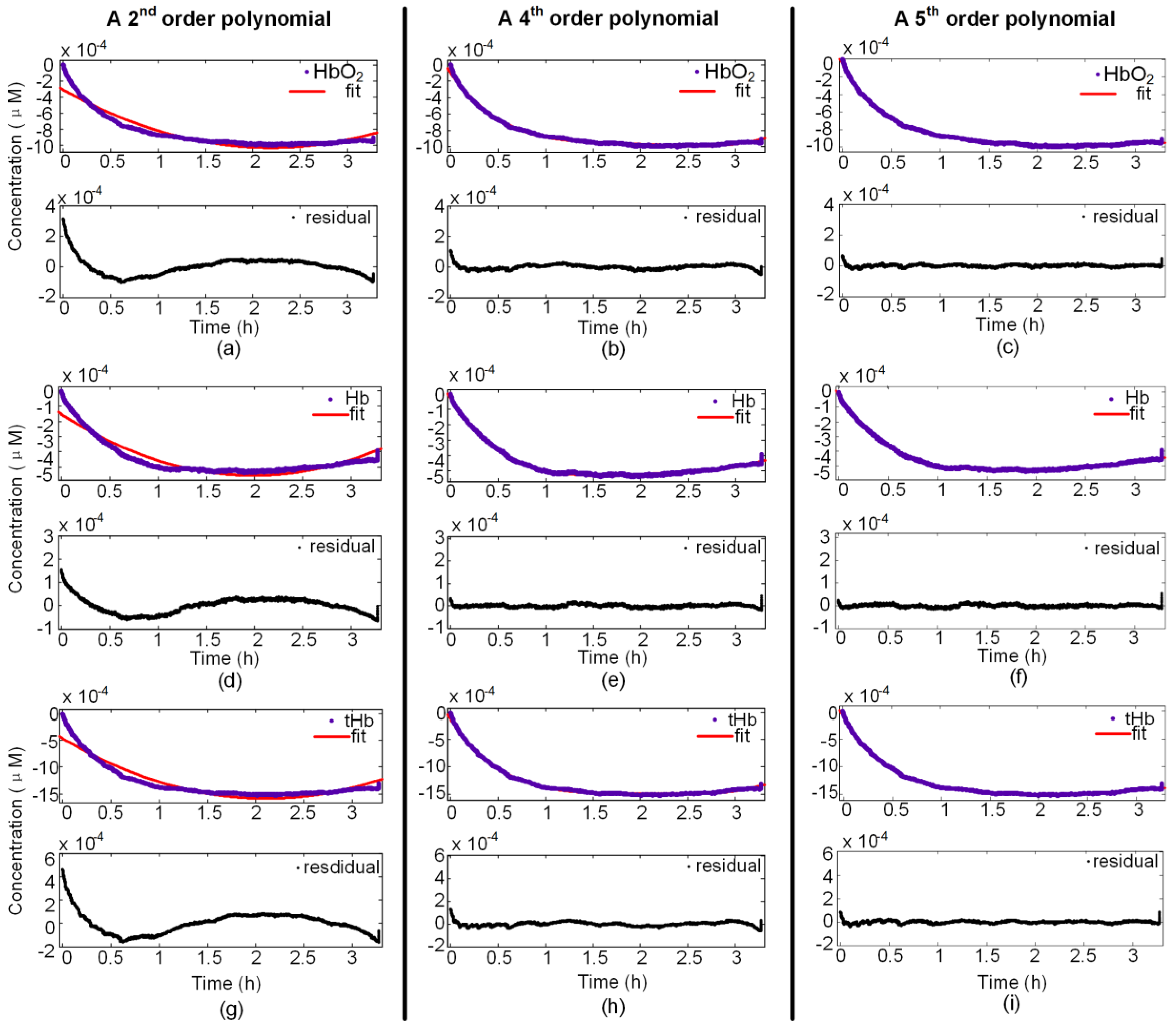
3.1. Fourth-Order Polynomial Function
3.2. Evaluating the Level of Fit
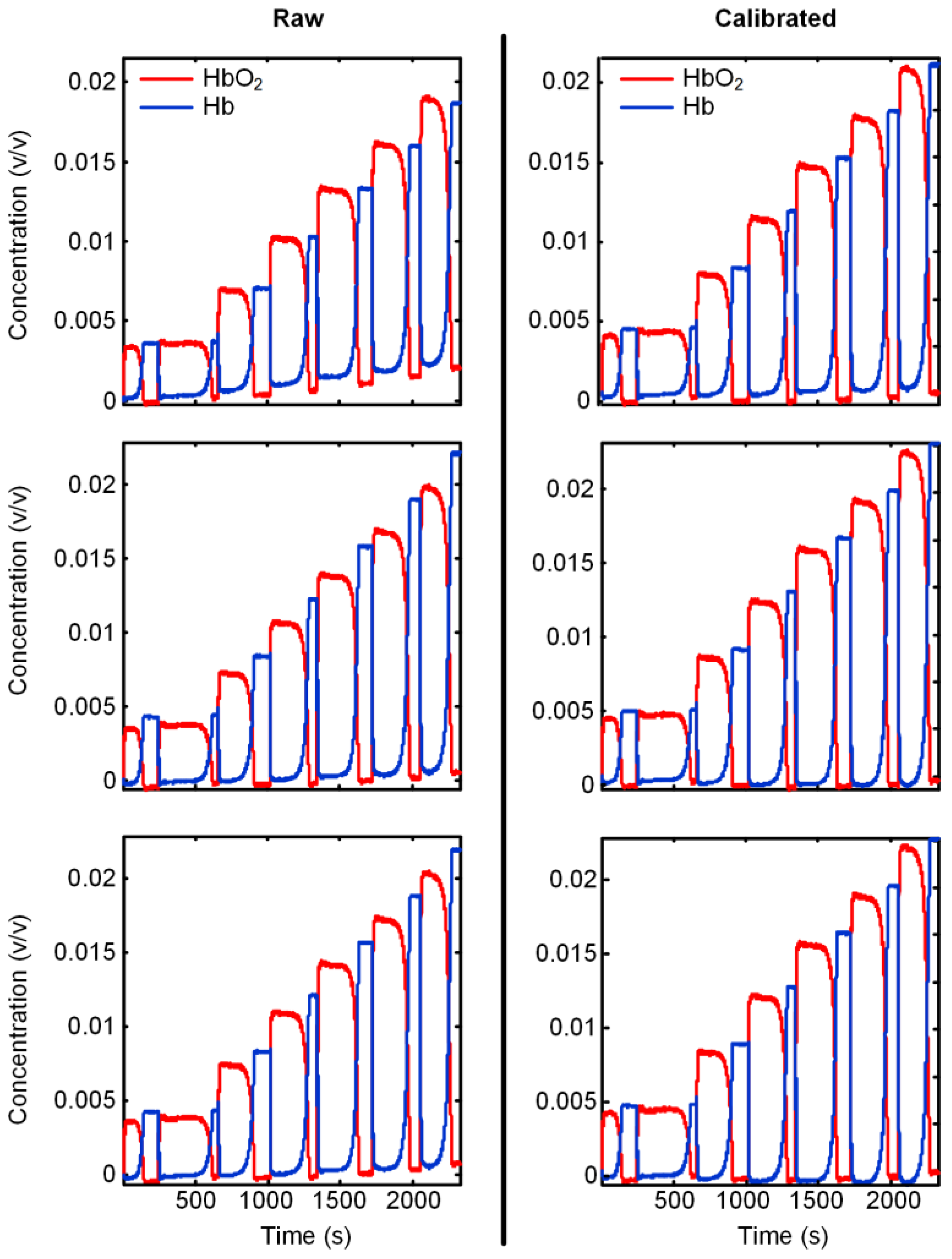
3.3. Verification of the Calibration
4. Conclusions and Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Geladi, P.; Dåbakk, E. An overview of chemometrics applications in near infrared spectrometry. J. Near Infrared Spectrosc. 1995, 3, 119–132. [Google Scholar] [CrossRef]
- Williams, P.; Norris, K. Near-Infrared Technology in the Agricultural and Food Industries; American Association of Cereal Chemists, Inc.: Eagan, MN, USA, 1987. [Google Scholar]
- Jobsis, F.F. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 1977, 198, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Noman, N.; Keum-Shik, H. Corrigendum “fnirs-based brain-computer interfaces: A review”. Front. Hum. Neurosci. 2015, 9, 172. [Google Scholar]
- Naseer, N.; Hong, K.S. Classification of functional near-infrared spectroscopy signals corresponding to the right- and left-wrist motor imagery for development of a brain-computer interface. Neurosci. Lett. 2013, 553, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Scholkmann, F.; Kleiser, S.; Metz, A.J.; Zimmermann, R.; Pavia, J.M.; Wolf, U.; Wolf, M. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage 2014, 85, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fnirs) development and fields of application. Neuroimage 2012, 63, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Lin, Y.; Li, T. Effect of human brain edema on light propagation: A monte carlo modeling based on the visible chinese human dataset. IEEE Photonics J. 2017, 9. [Google Scholar] [CrossRef]
- Li, T.; Yu, L.; Yu, S.; Lian, H.; Chong, H.; Szabunio, M.; Yu, G. Simultaneous measurement of deep tissue blood flow and oxygenation using noncontact diffuse correlation spectroscopy flow-oximeter. Sci. Rep. 2013, 3, 1358. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xue, C.; Wang, P.; Li, Y.; Wu, L. Photon penetration depth in human brain for light stimulation and treatment: A realistic monte carlo simulation study. J. Innov. Opt. Health Sci. 2017, 10. [Google Scholar] [CrossRef]
- Esquerre, C.; Gowen, A.A.; Burger, J.; Downey, G.; O’Donnell, C.P. Suppressing sample morphology effects in near infrared spectral imaging using chemometric data pre-treatments. Chemom. Intell. Lab. Syst. 2012, 117, 129–137. [Google Scholar] [CrossRef]
- Liu, J.M. Photonic Devices; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Rinnan, Å.; Berg, F.V.D.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Wold, S.; Antti, H.; Lindgren, F.; Öhman, J. Orthogonal signal correction of near-infrared spectra. Chemom. Intell. Lab. Syst. 1998, 44, 175–185. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Dhanoa, M.S.; Barnes, R.J.; Lister, S.J. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl. Spectrosc. 2016, 43, 772–777. [Google Scholar]
- Lipponen, J.A.; Geest, K.B.V.D.; Tarvainen, M.P.; Leinonen, A.; Lahtinen, M.; Karjalainen, P.A. Baseline removal from near infrared spectroscopy measurements for lactate concentration estimation. In IFMBE Proceedings; Springer: Berlin, Germany, 2009; Volume 25, pp. 2042–2045. [Google Scholar]
- Barbin, D.F.; Kaminishikawahara, C.M.; Soares, A.L.; Mizubuti, I.Y.; Grespan, M.; Shimokomaki, M.; Hirooka, E.Y. Prediction of chicken quality attributes by near infrared spectroscopy. Food Chem. 2015, 168, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Laxalde, J.; Ruckebusch, C.; Devos, O.; Caillol, N.; Wahl, F.; Duponchel, L. Characterisation of heavy oils using near-infrared spectroscopy: Optimisation of pre-processing methods and variable selection. Anal. Chim. Acta 2011, 705, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, M.; Wei, G.; Hu, R.; Luo, Z.; Li, G. Improved pls regression based on svm classification for rapid analysis of coal properties by near-infrared reflectance spectroscopy. Sens. Actuators B Chem. 2014, 193, 723–729. [Google Scholar] [CrossRef]
- Pierce, K.M.; Kehimkar, B.; Marney, L.C.; Hoggard, J.C.; Synovec, R.E. Review of chemometric analysis techniques for comprehensive two dimensional separations data. J. Chromatogr. A 2012, 1255, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.S.; Naseer, N. Reduction of delay in detecting initial dips from functional near-infrared spectroscopy signals using vector-based phase analysis. Int. J. Neural Syst. 2016, 26, 1650012. [Google Scholar] [CrossRef] [PubMed]
- Yeganeh, H.Z.; Toronov, V.; Elliott, J.T.; Diop, M.; Lee, T.Y.; Lawrence, K.S. Broadband continuous-wave technique to measure baseline values and changes in thetissue chromophore concentrations. Biomed. Opt. Express 2012, 3, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Lapborisuth, P.; Zhang, X.; Noah, A.; Hirsch, J. Neurofeedback-based functional near-infrared spectroscopy upregulates motor cortex activity in imagined motor tasks. Neurophotonics 2017, 4, 021107. [Google Scholar] [CrossRef] [PubMed]
- Huppert, T.J.; Diamond, S.G.; Boas, D.A. Quantitative estimation of cerebral hemodynamic changes through the multimodality fusion of bold and diffuse optical tomography. In Proceedings of the Biomedical Topical Meeting, Fort Lauderdale, FL, USA, 19–22 March 2006. [Google Scholar]
- Scholkmann, F.; Spichtig, S.; Muehlemann, T.; Wolf, M. How to detect and reduce movement artifacts in near-infrared imaging using moving standard deviation and spline interpolation. Physiol. Meas. 2010, 31, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Hebden, J.C.; Price, B.D.; Gibson, A.P.; Royle, G. A soft deformable tissue-equivalent phantom for diffuse optical tomography. Phys. Med. Biol. 2006, 51, 5581–5590. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Duan, M.; Li, K.; Yu, G.; Ruan, Z. Bedside monitoring of patients with shock using a portable spatially-resolved near-infrared spectroscopy. Biomed. Opt. Express 2015, 6, 3431–3436. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, Y.; Lin, Y.; Li, K. Significant and sustaining elevation of blood oxygen induced by Chinese cupping therapy as assessed by near-infrared spectroscopy. Biomed. Opt. Express 2017, 8, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhong, F.; Pan, B.; Li, Z.; Chong, H.; Deng, Z. A brief review of opt101 sensor application in near-infrared spectroscopy instrumentation for intensive care unit clinics. Sensors 2017, 17, 1701. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, G.; Li, T. A portable high-density absolute-measure nirs imager for detecting prefrontal lobe response to driving fatigue. Microelectron. Reliab. 2017, in press. [Google Scholar]
- Afseth, N.K.; Kohler, A. Extended multiplicative signal correction in vibrational spectroscopy, a tutorial. Chemom. Intell. Lab. Syst. 2012, 117, 92–99. [Google Scholar] [CrossRef]
- Jiang, S.; Pogue, B.W.; Mcbride, T.O.; Paulsen, K.D. Quantitative analysis of near-infrared tomography: Sensitivity to the tissue-simulating precalibration phantom. J. Biomed. Opt. 2003, 8, 308–315. [Google Scholar] [CrossRef] [PubMed]
- MathWorks. Evaluating Goodness of Fit. Available online: http://cn.mathworks.com/help/curvefit/evaluating-goodness-of-fit.html?requestedDomain=www.mathworks.com (accessed on 7 October 2017).
- Zhao, Y.; Qiu, L.; Sun, Y.; Huang, C.; Li, T. Optimal hemoglobin extinction coefficient data set for near-infrared spectroscopy. Biomed. Opt. Express 2017, 8, 5151–5159. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Sun, Y.; Chen, X.; Zhao, Y.; Ren, R. Noninvasive diagnosis and therapeutic effect evaluation of deep vein thrombosis in clinics by near-infrared spectroscopy. J. Biomed. Opt. 2015, 20, 010502. [Google Scholar] [CrossRef] [PubMed]
| P | C | HP | Second Order | Third Order | Fourth Order | Fifth Order | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| SSE | R-Square | SSE | R-Square | SSE | R-Square | SSE | R-Square | |||
| 1 | 1 | HbO2 | 4.67 × 10−4 | 0.977 | 4.25 × 10−4 | 0.98 | 2.07 × 10−4 | 0.99 | 8.3 × 10−5 | 0.996 |
| Hb | 1.65 × 10−4 | 0.979 | 1.27 × 10−4 | 0.981 | 7.81 × 10−5 | 0.99 | 3.05 × 10−5 | 0.996 | ||
| tHb | 1.17 × 10−3 | 0.978 | 9.63 × 10−4 | 0.979 | 5.34 × 10−4 | 0.99 | 2.08 × 10−4 | 0.996 | ||
| 2 | HbO2 | 1.66 × 10−3 | 0.979 | 1.54 × 10−4 | 0.983 | 5.90 × 10−4 | 0.993 | 2.26 × 10−4 | 0.997 | |
| Hb | 1.87 × 10−4 | 0.975 | 1.59 × 10−4 | 0.981 | 3.26 × 10−5 | 0.996 | 1.71 × 10−5 | 0.998 | ||
| tHb | 8.01 × 10−4 | 0.979 | 7.29 × 10−4 | 0.982 | 3.77 × 10−4 | 0.99 | 1.47 × 10−4 | 0.996 | ||
| 2 | 1 | HbO2 | 3.87 × 10−4 | 0.957 | 3.38 × 10−4 | 0.958 | 3.52 × 10−5 | 0.996 | 8.08 × 10–6 | 0.999 |
| Hb | 6.97 × 10−4 | 0.957 | 6.37 × 10−4 | 0.961 | 5.60 × 10−5 | 0.997 | 1.26 × 10−5 | 0.999 | ||
| tHb | 4.57 × 10−5 | 0.955 | 3.34 × 10−5 | 0.963 | 4.38 × 10−7 | 0.998 | 5.43 × 10−7 | 0.999 | ||
| 2 | HbO2 | 1.60 × 10−3 | 0.977 | 1.29 × 10−3 | 0.979 | 1.61 × 10−4 | 0.998 | 3.78 × 10−5 | 0.999 | |
| Hb | 2.96 × 10−4 | 0.961 | 2.43 × 10−4 | 0.967 | 1.87 × 10−5 | 0.998 | 5.52 × 10–6 | 0.999 | ||
| tHb | 5.43 × 10−4 | 0.982 | 4.03 × 10−4 | 0.986 | 8.97 × 10−5 | 0.997 | 3.45 × 10−5 | 0.999 | ||
| 3 | 1 | HbO2 | 4.67 × 10−4 | 0.977 | 2.21 × 10−4 | 0.927 | 2.07 × 10−4 | 0.99 | 8.3 × 10−5 | 0.996 |
| Hb | 1.65 × 10−4 | 0.979 | 6.29 × 10−5 | 0.913 | 7.81 × 10−5 | 0.99 | 3.05 × 10−5 | 0.996 | ||
| tHb | 1.17 × 10−3 | 0.978 | 5.23 × 10−4 | 0.917 | 5.34 × 10−4 | 0.99 | 2.08 × 10−4 | 0.996 | ||
| 2 | HbO2 | 1.66 × 10−3 | 0.979 | 3.34 × 10−4 | 0.959 | 5.90 × 10−4 | 0.993 | 2.26 × 10−4 | 0.997 | |
| Hb | 1.87 × 10−4 | 0.975 | 1.43 × 10−4 | 0.968 | 3.26 × 10−5 | 0.996 | 1.71 × 10−5 | 0.998 | ||
| tHb | 8.01 × 10−4 | 0.979 | 4.03 × 10−4 | 0.95 | 3.77 × 10−4 | 0.99 | 1.47 × 10−4 | 0.996 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Ji, Y.; Li, Y.; Li, T. Online Removal of Baseline Shift with a Polynomial Function for Hemodynamic Monitoring Using Near-Infrared Spectroscopy. Sensors 2018, 18, 312. https://doi.org/10.3390/s18010312
Zhao K, Ji Y, Li Y, Li T. Online Removal of Baseline Shift with a Polynomial Function for Hemodynamic Monitoring Using Near-Infrared Spectroscopy. Sensors. 2018; 18(1):312. https://doi.org/10.3390/s18010312
Chicago/Turabian StyleZhao, Ke, Yaoyao Ji, Yan Li, and Ting Li. 2018. "Online Removal of Baseline Shift with a Polynomial Function for Hemodynamic Monitoring Using Near-Infrared Spectroscopy" Sensors 18, no. 1: 312. https://doi.org/10.3390/s18010312
APA StyleZhao, K., Ji, Y., Li, Y., & Li, T. (2018). Online Removal of Baseline Shift with a Polynomial Function for Hemodynamic Monitoring Using Near-Infrared Spectroscopy. Sensors, 18(1), 312. https://doi.org/10.3390/s18010312





