Trends and Advances in Electrochemiluminescence Nanobiosensors
Abstract
1. Introduction
1.1. Biosensors
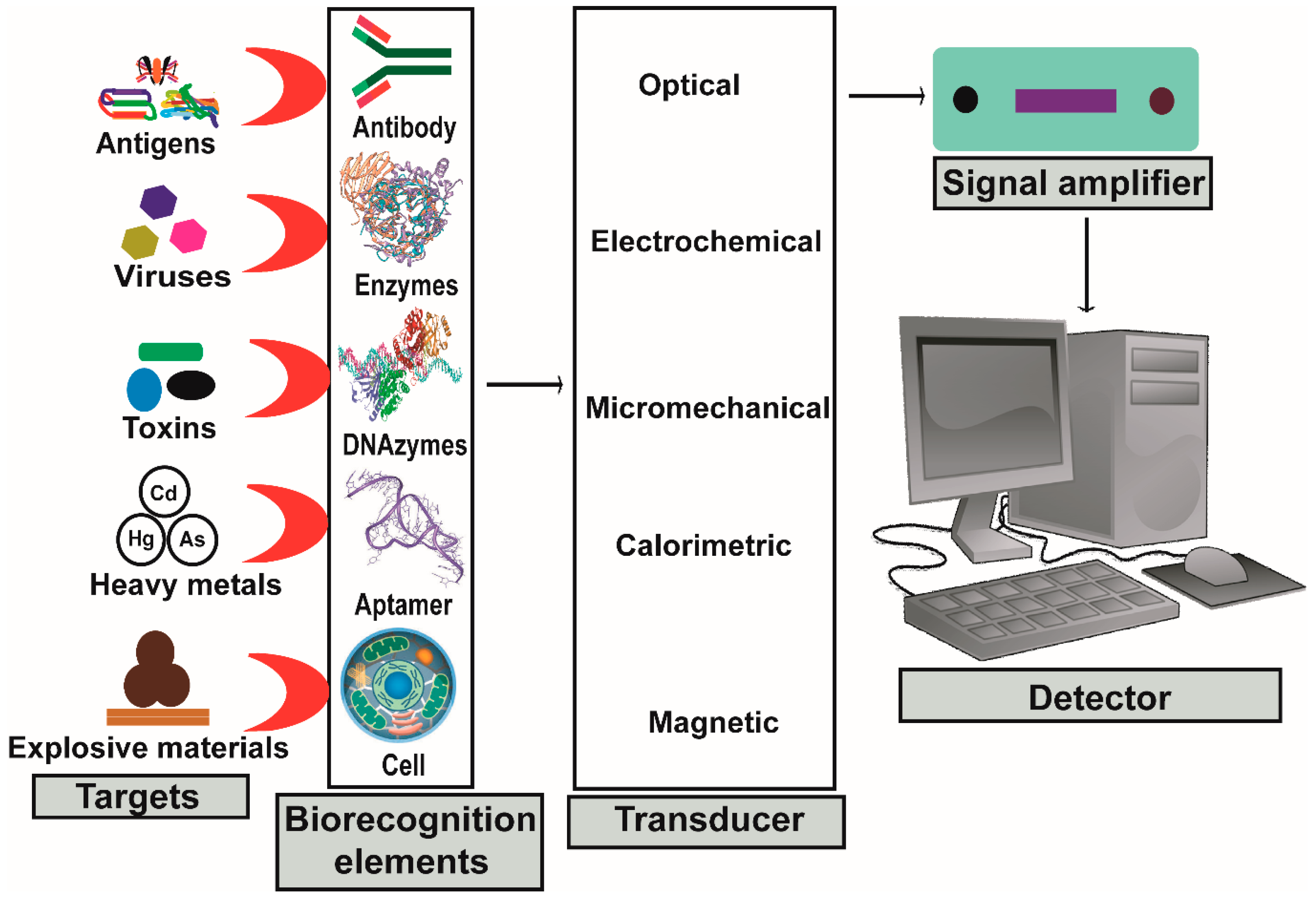
1.2. Biosensor Components
1.3. Construction of Biosensors
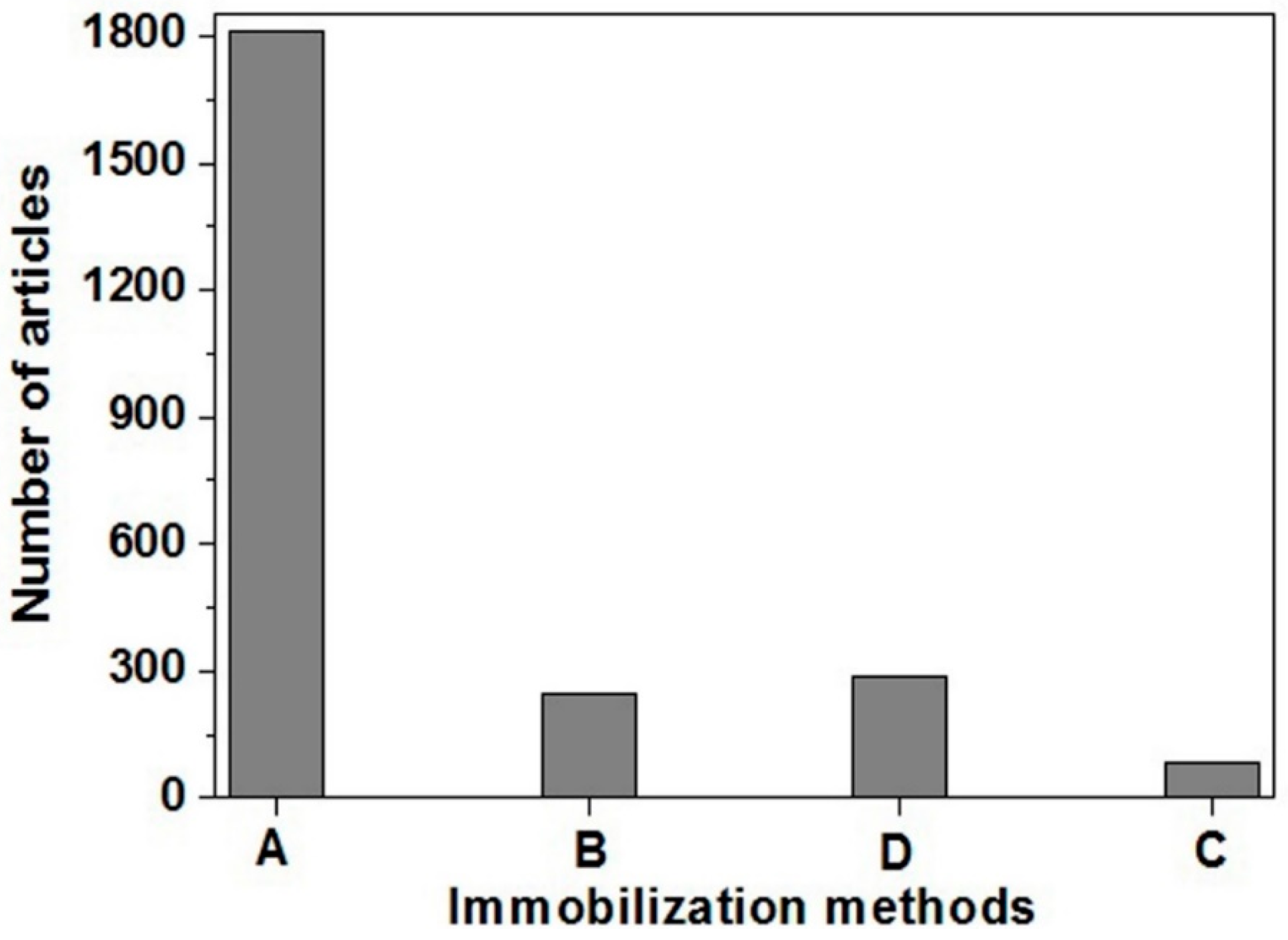
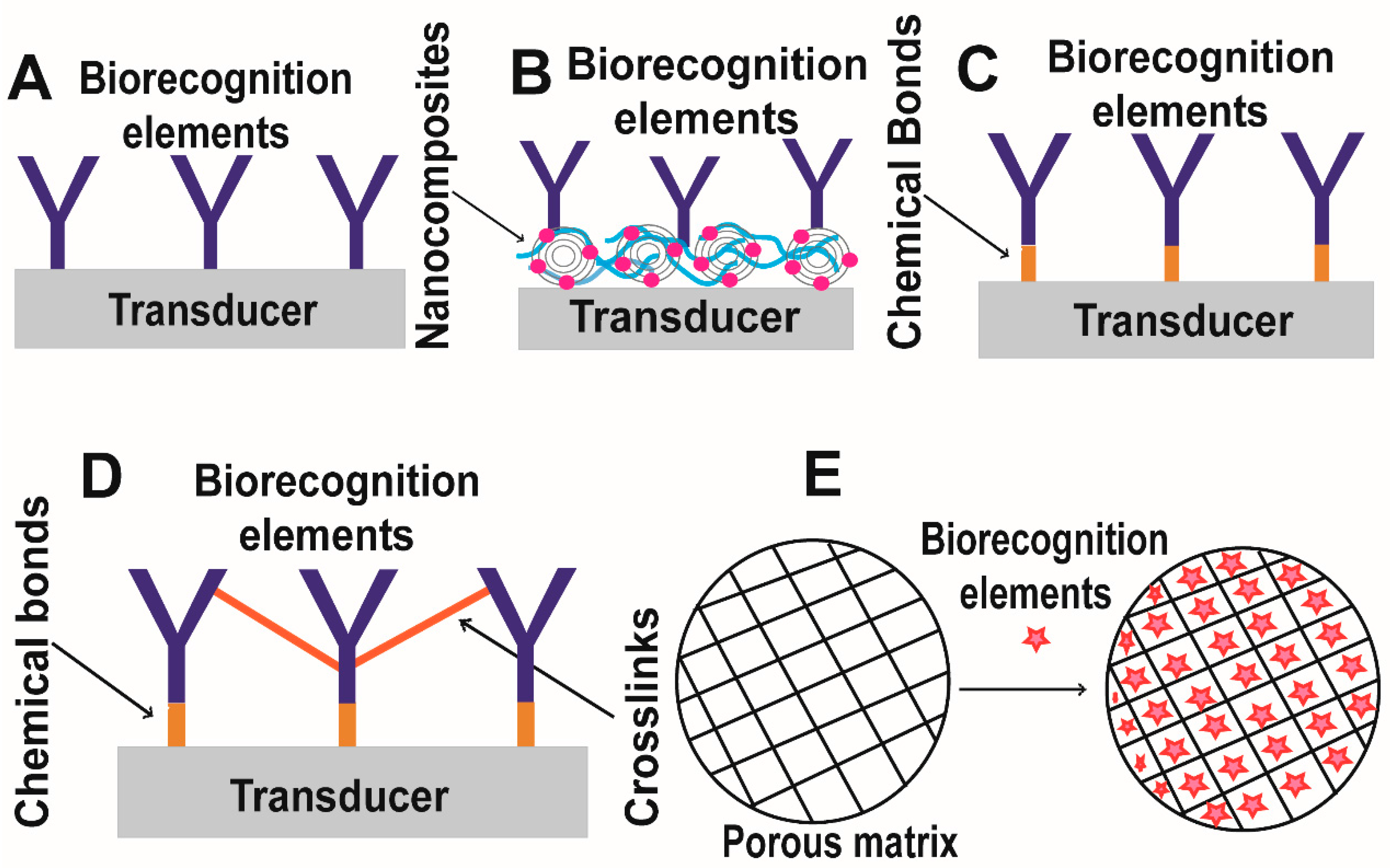
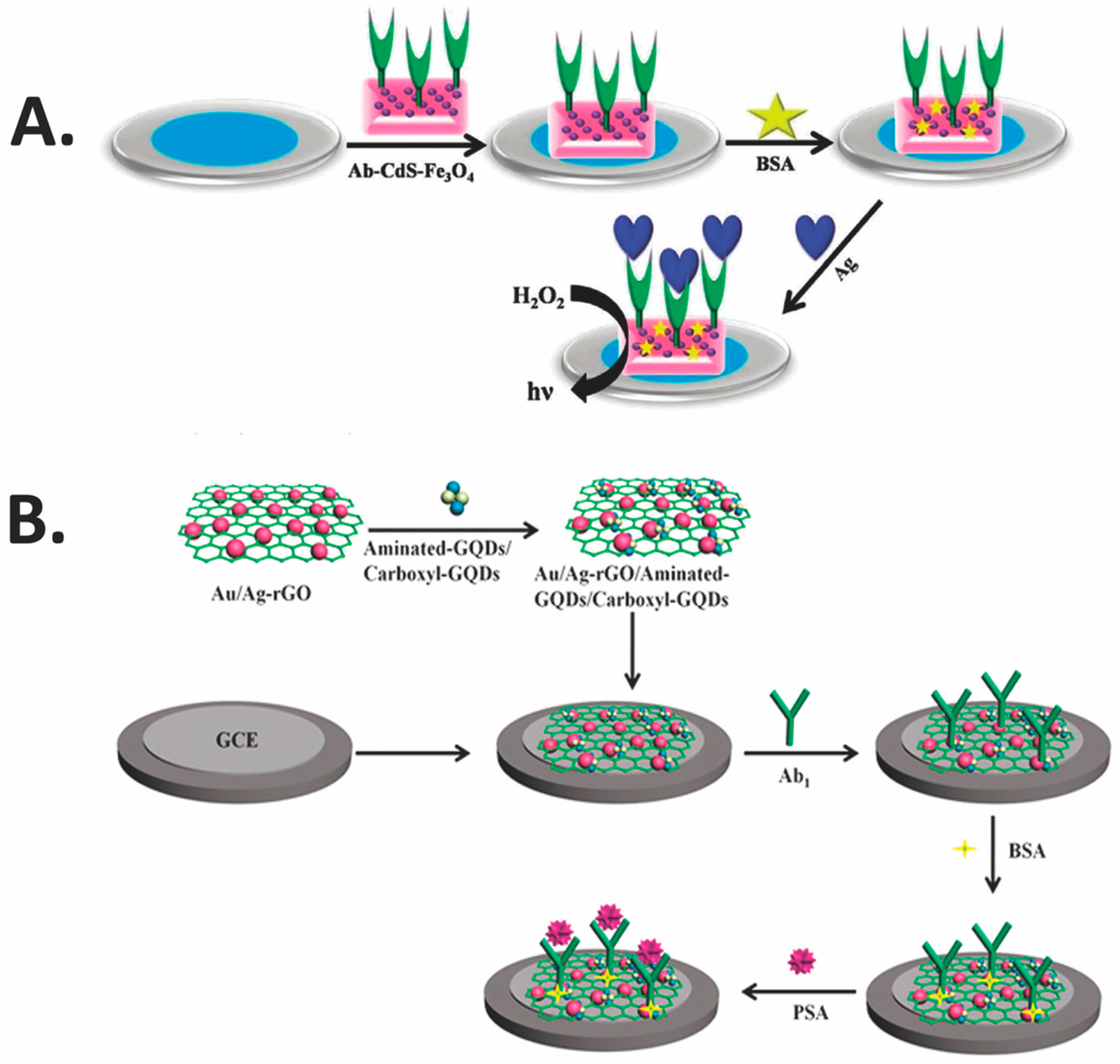
1.3.1. Immobilization of Biomolecules
1.3.2. Transducer
2. Electrochemiluminescence
3. Mechanism of ECL Detection
- (i)
- Oxidative-reduction co-reactant ECL pathway
- (ii)
- Reductive-oxidation co-reactant ECL pathway
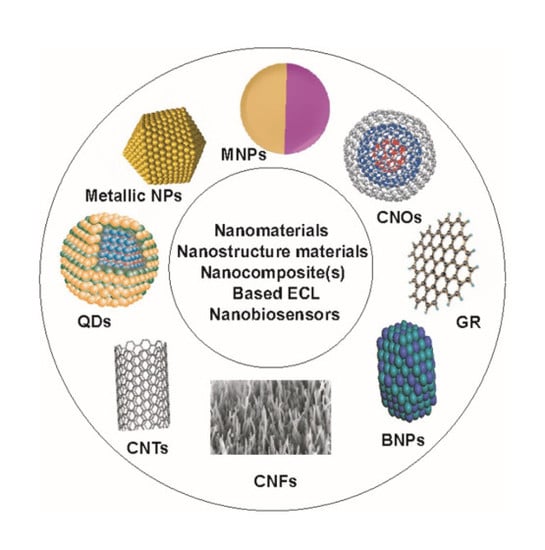
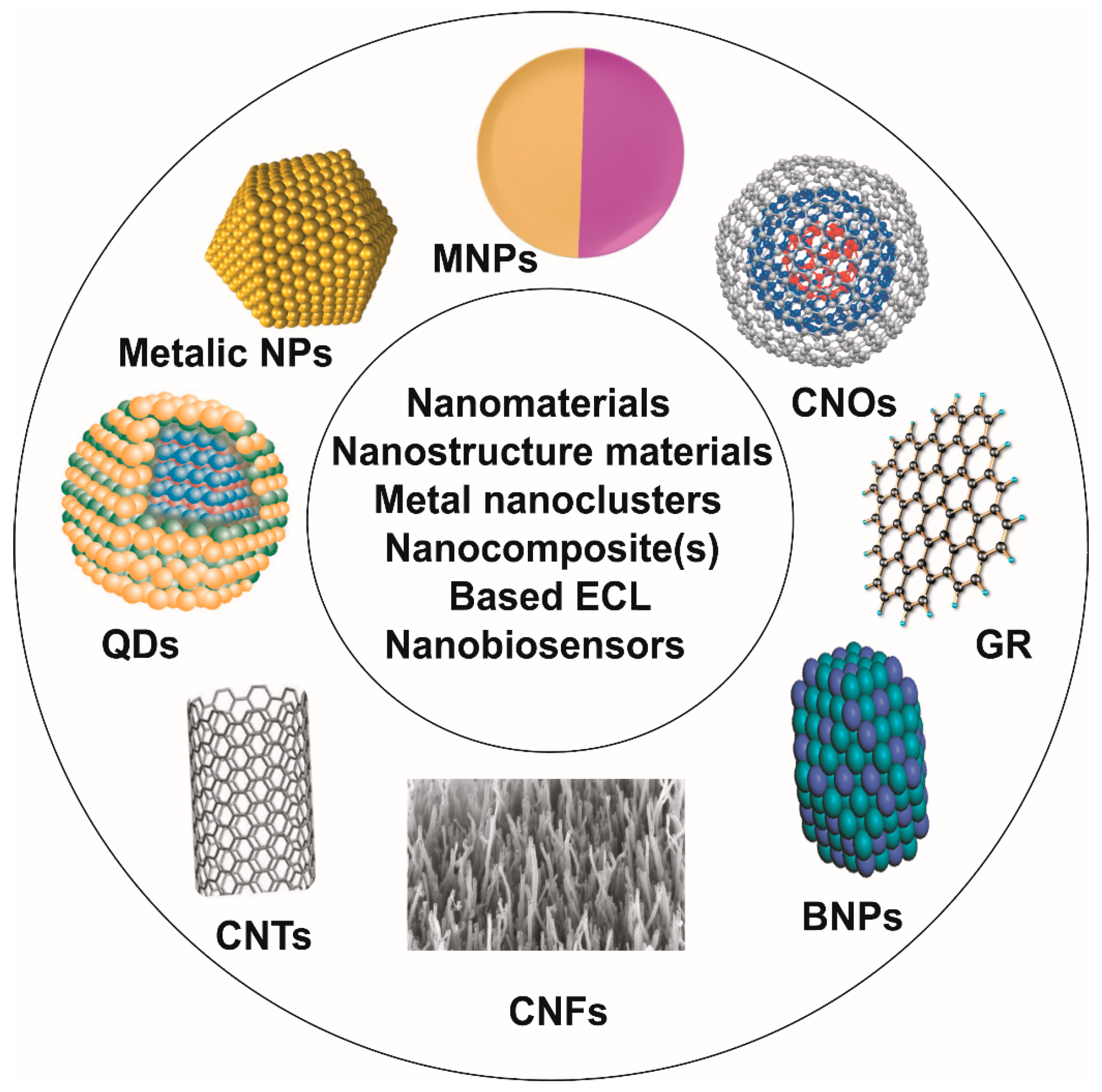
4. Nanostructure Materials, Nanomaterials, Metal Nanoclusters and Nanocomposite(s)
5. Metal- and Magnetic Nanocomposite-Based ECL Nanobiosensors
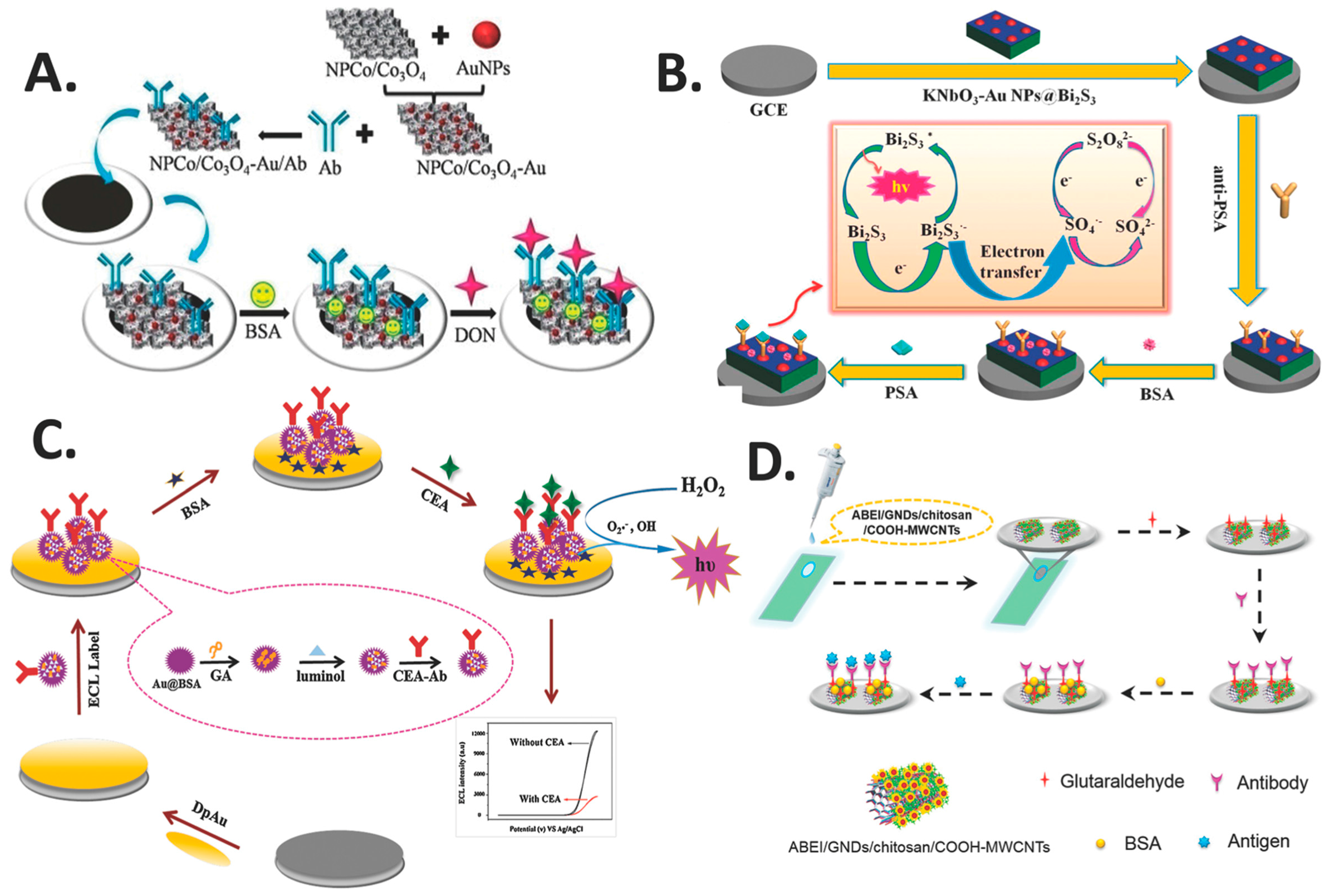
5.1. Gold Nanoparticles (AuNPs)
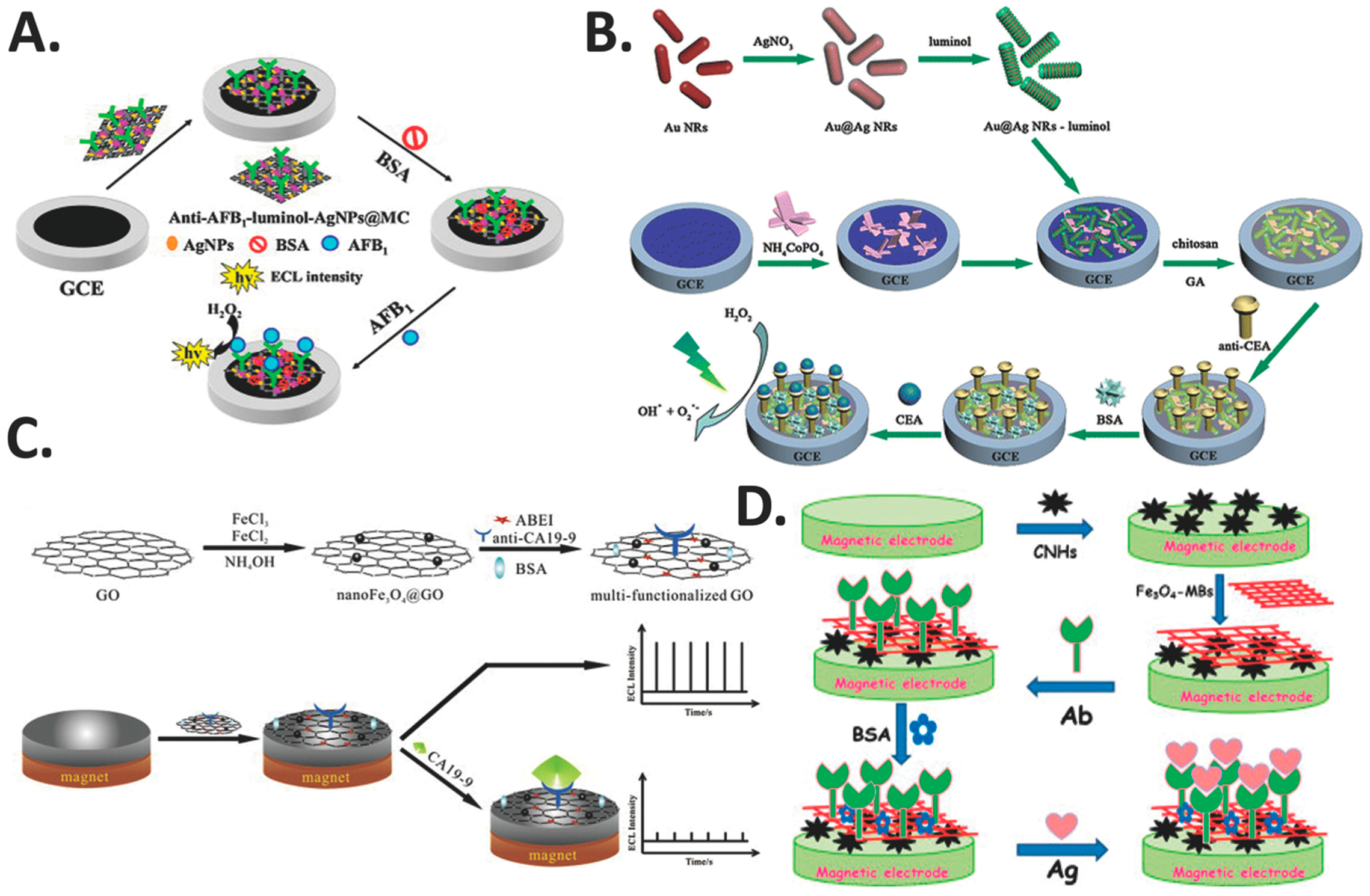
5.2. Silver Nanoparticles (AgNPs)
5.3. Magnetic Nanoparticles (MNPs)
5.4. Metal Nanoclusters (MNCs)
6. Quantum Dots Nanocomposite-Based ECL Nanobiosensors
7. Carbon Nanomaterials (CNMs)- or Carbon Nanostructured Materials (CNSMs)- Nanocomposite-Based ECL Nanobiosensors
7.1. Ordered Mesoporous Carbons (OMCs)
7.2. Graphene
7.3. Carbon Nanotubes and Carbon Nano-Onions
8. Conclusions and Prospects
Acknowledgments
Conflicts of Interest
References
- Fetz, V.; Knauer, S.K.; Bier, C.; Von Kries, J.P.; Stauber, R.H. Translocation biosensors cellular system integrators to dissect CRM1-dependent nuclear export by chemicogenomics. Sensors 2009, 9, 5423–5445. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.A.; Ahmed, M.U. Electrochemical immunosensors and their recent nanomaterial-based signal amplification strategies: A review. RSC Adv. 2016, 6, 24995–25014. [Google Scholar] [CrossRef]
- Clark, L.C.; Lyons, C. Electrode systems for continuous monitoring cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Fracchiolla, N.S.; Artuso, S.; Cortelezzi, A. Biosensors in clinical practice: Focus on oncohematology. Sensors 2013, 13, 6423–6447. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.P. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Koh, D.; Booth, M.A.; Ahmed, M.U. Combining a gold nanoparticle-polyethylene glycol nanocomposite and carbon nanofiber electrodes to develop a highly sensitive salivary secretory immunoglobulin A immunosensor. Sens. Actuators B Chem. 2018, 255, 557–563. [Google Scholar] [CrossRef]
- Rizwan, M.; Mohd-Naim, N.F.; Keasberry, N.A.; Ahmed, M.U. A highly sensitive and label-free electrochemiluminescence immunosensor for beta 2-microglobulin. Anal. Methods 2017, 9, 2570–2577. [Google Scholar] [CrossRef]
- Inoue, Y.; Inoue, M.; Saito, M.; Yoshikawa, H.; Tamiya, E. Sensitive detection of glycated albumin in human serum albumin using electrochemiluminescence. Anal. Chem. 2017, 89, 5909–5915. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhu, W.; Ren, X.; Khan, M.S.; Zhang, Y.; Du, B.; Wei, Q. Macroporous graphene capped Fe3O4 for amplified electrochemiluminescence immunosensing of carcinoembryonic antigen detection based on CeO2@TiO2. Biosens. Bioelectron. 2017, 15, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Q.; Liu, X.-P.; Liu, P.-Z.; Mao, C.-J.; Niu, H.-L.; Jin, B.-K.; Zhang, S.-Y. Multifunctional reduced graphene oxide (RGO)/Fe3O4/CdSe nanocomposite for electrochemiluminescence immunosensor. Electrochim. Acta 2016, 190, 948–955. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Hossain, M.M.; Safavieh, M.; Wong, Y.L.; Rahman, I.A.; Zourob, M.; Tamiya, E. Toward the development of smart and low cost point-of-care biosensors based on screen printed electrodes. Crit. Rev. Biotechnol. 2016, 36, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Omanovic-Miklicanin, E.; Valzacchi, S. Development of new chemiluminescence biosensors for determination of biogenic amines in meat. Food Chem. 2017, 235, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Li, R.; Lin, C.; Guo, L.; Qiu, B.; Lin, Z.; Chen, G. Electrochemiluminescence biosensor for ultrasensitive determination of ochratoxin A in corn samples based on aptamer and hyperbranched rolling circle amplification. Biosens. Bioelectron. 2015, 70, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Li, Y.; Yan, T.; Pang, X.; Cao, W.; Du, B.; Wu, D.; Wei, Q. Electrochemiluminescence modified electrodes based on RuSi@Ru(bpy)32+ loaded with gold functioned nanoporous CO/Co3O4 for detection of mycotoxin deoxynivalenol. Biosens. Bioelectron. 2015, 70, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Wei, S.X.; Ying, Z.L.J.; Safavieh, M.; Ahmed, M.U. A novel, sensitive and label-free loop-mediated isothermal amplification detection method for nucleic acids using luminophore dyes. Biosens. Bioelectron. 2016, 86, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.S.; Sheng, M.; Ma, L.Y.; He, X.J.; Yang, H. Electrochemiluminescence biosensor for determination of organophosphorous pesticides based on bimetallic Pt-Au/multi-walled carbon nanotubes modified electrode. Talanta 2016, 158, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Kitte, S.A.; Gao, W.; Zholudov, Y.T.; Qi, L.; Nsabimana, A.; Liu, Z.; Xu, G. Stainless steel electrode for sensitive luminol electrochemiluminescence detection of H2O2, glucose, and glucose oxidase activity. Anal. Chem. 2017, 89, 9864–9869. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Tan, X.; Yuan, R.; Chen, S. A dual-potential electrochemiluminescence ratiometric sensor for sensitive detection of dopamine based on graphene-CdTe quantum dots and self-enhanced Ru(II) complex. Biosens. Bioelectron. 2017, 90, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, C.; Muzyka, K.; Kitte, S.A.; Li, J.; Zhang, W.; Xu, G. Artemisinin-luminol chemiluminescence for forensic bloodstain detection using a smart phone as a detector. Anal. Chem. 2017, 89, 6160–6165. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.J.; Hendry, J.; Dennany, L. Whole blood electrochemiluminescent detection of dopamine. Anal. Chem. 2015, 87, 11847–11853. [Google Scholar] [CrossRef] [PubMed]
- McGeehan, J.; Dennany, L. Electrochemiluminescent detection of methamphetamine and amphetamine. Forensic Sci. Int. 2016, 264, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, B.; Zhang, X.; You, T. A novel electrochemiluminescence sensor based on [Ru(bpy)2]3+ doped carbon nanodots system for the detection of bisphenol A. Anal. Chim. Acta 2015, 895, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-J.; Kang, T.-F.; Hao, Y.-C.; Lu, L.-P.; Cheng, S.-Y. Electrochemiluminescent immunosensor based on CdS quantum dots for ultrasensitive detection of microcystin-LR. Sens. Actuators B 2015, 214, 117–123. [Google Scholar] [CrossRef]
- Li, G.; Yu, X.; Liu, D.; Liu, X.; Li, F.; Cui, H. Label-free electrochemiluminescence aptasensor for 2,4,6-Trinitrotoluene based on bilayer structure of luminescence functionalized graphene hybrids. Anal. Chem. 2015, 87, 10976–10981. [Google Scholar] [CrossRef] [PubMed]
- Spehar-Délèze, A.M.; Gransee, R.; Martinez-Montequin, S.; Bejarano-Nosas, D.; Dulay, S.; Julich, S.; Tomaso, H.; O’Sullivan, C.K. Electrochemiluminescence DNA sensor array for multiplex detection of biowarfare agents. Anal. Bioanal. Chem. 2015, 407, 6657–6667. [Google Scholar] [CrossRef] [PubMed]
- Scopus. Elsevier’s abstract and citation database. Available online: https://www.scopus.com/search/form.uri?display=basic (accessed on 13 September 2017).
- Newman, J.D.; Tigwell, L.J.; Warner, P.J.; Turner, A.P.F. Biosensors: Boldly going into the new millennium. Sens. Rev. 2001, 21, 268–271. [Google Scholar] [CrossRef]
- Shana, A.; Rogers, K.R. Biosensors. Meas. Sci. Technol. 1994, 5, 461–472. [Google Scholar]
- Yang, L.; Li, Y. AFM and impedance spectroscopy characterization of the immobilization of antibodies on indium-tin oxide electrode through self-assembled monolayer of epoxysilane and their capture of Escherichia coli O157:H7. Biosens. Bioelectron. 2005, 20, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, D.; MacKenzie1, R.; Voros, J.; Reimhult, E. Electrochemical biosensors-Sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.A.; Yoshikawa, H.; Tamiya, E.; Yasin, H.M.; Ahmed, M.U. A highly sensitive gold nanoparticle bioprobe based electrochemical immunosensor using screen printed graphene biochip. RSC Adv. 2014, 4, 58460–58466. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Su, X.; Ai, S. Electrochemical immunosensor with graphene quantum dots and apoferritin-encapsulated Cu nanoparticles double-assisted signal amplification for detection of avian leucosis virus sub group J. Biosens. Bioelectron. 2013, 47, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Lina, C.-C.; Chub, Y.-M.; Chang, H.-C. In situ encapsulation of antibody on TiO2 nanowire immunosensor via electro-polymerization of polypyrrole propylic acid. Sens. Actuators B 2013, 187, 533–539. [Google Scholar] [CrossRef]
- Lim, S.A.; Ahmed, M.U. A carbon nanofiber-based label-free immunosensor for high sensitive detection of recombinant bovine somatotropin. Biosens. Bioelectron. 2015, 70, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.A.; Ahmed, M.U. A label free electrochemical immunosensor for sensitive detection of porcine serum albumin as a marker for pork adulteration in raw meat. Food Chem. 2016, 206, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.; Selvam, A.P.; Craven, J.E.; Prasad, S. Antibody-conjugated gold nanoparticle-based immunosensor for ultra-sensitive detection of Troponin-T. J. Lab. Autom. 2014, 19, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhou, J.; Li, Y.; Han, T.; Zhang, Y.; Hu, L.; Du, B.; Wei, Q. A label-free electrochemiluminescence immunosensor based on EuPO4 nanowire for the ultrasensitive detection of prostate specific antigen. Biosens. Bioelectron. 2016, 80, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Liu, Y.; Wang, Y.; Hu, L.; Ma, H.; Wang, G.; Wei, Q. Label-free electrochemiluminescent immunosensor for detection of prostate specific antigen based on aminated graphene quantum dots and carboxyl graphene quantum dots. Sci. Rep. 2016, 4, 20511. [Google Scholar] [CrossRef] [PubMed]
- Karaseva, N.; Ermolaeva, T. A regenerable piezoelectric immunosensor on the basis of electropolymerized polypyrrole for highly selective detection of Staphylococcal Enterotoxin A in foodstuffs. Microchim. Acta 2015, 182, 1329–1335. [Google Scholar] [CrossRef]
- Caldeira, J.M.L.P.; Rodrigues, J.J.P.C.; Garcia, J.F.R.; Torre, I.D.L. A new wireless biosensor for intra-vaginal temperature monitoring. Sensors 2010, 10, 10314–10327. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Li, L.; Li, P.; Yang, M. Quantum dots: From fluorescence, chemiluminescence, bioluminescence and electrochemiluminescence to electrochemistry. Nanoscale 2017, 9, 13364–13383. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Paolucci, F.; Sojic, N.; Xu, G. Analytical electrochemiluminescence. Anal. Bioanal. Chem. 2016, 408, 7001–7002. [Google Scholar] [CrossRef] [PubMed]
- Bist, I.; Bano, K.; Rusling, J.F. Screening Genotoxicity Chemistry with Microfluidic Electrochemiluminescent Arrays. Sensors 2017, 17, 1008. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, S.; Li, L.; Zhu, J.-J. Nanomaterials-based sensitive electrochemiluminescence biosensing. Nano Today 2017, 12, 98–115. [Google Scholar] [CrossRef]
- Gao, W.; Saqib, M.; Qi, L.; Zhang, W.; Xu, G. Recent advances in electrochemiluminescence devices for point-of-care testing. Curr. Opin. Electrochem. 2017, 3, 4–10. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, W.; Xu, G. Recent advances in electrochemiluminescence. Chem. Soc. Rev. 2015, 44, 3117–3142. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhang, W.; Wang, W.; Chen, Y.; Li, X. Amplification strategies using electrochemiluminescence biosensors for the detection of DNA, bioactive molecules and cancer biomarkers. Trends Anal. Chem. 2015, 65, 137–150. [Google Scholar] [CrossRef]
- Bertoncello, P.; Stewart, A.J.; Dennany, L. Analytical applications of nanomaterials in electrogenerated chemiluminescence. Anal. Bioanal. Chem. 2014, 406, 5573–5587. [Google Scholar] [CrossRef] [PubMed]
- Muzyka, K. Current trends in the development of the electrochemiluminescent immunosensors. Biosens. Bioelectron. 2014, 54, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.M. Electrochemiluminescence (ECL). Chem. Rev. 2004, 104, 3003–3036. [Google Scholar] [CrossRef] [PubMed]
- Miao, W. Electrogenerated Chemiluminescence and Its Biorelated Applications. Chem. Rev. 2008, 108, 2506–2553. [Google Scholar] [CrossRef] [PubMed]
- Bertoncello, P.; Forster, R.J. Nanostructured materials for electrochemiluminescence (ECL)-based detection methods: Recent advances and future perspectives. Biosens. Bioelectron. 2009, 24, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Bertoncello, P. Nanomaterials for biosensing with electrochemiluminescence (ECL) detection. Front. Biosci. 2011, 16, 1084–1108. [Google Scholar] [CrossRef]
- Bertoncello, P.; Ugo, P. Recent Advances in Electrochemiluminescence with Quantum Dots and Arrays of Nanoelectrodes. ChemElectroChem 2017, 4, 1663–1676. [Google Scholar] [CrossRef]
- Ramos, A.P.; Cruz, M.A.E.; Tovani, C.B.; Ciancaglini, P. Biomedical applications of nanotechnology. Biophys. Rev. 2017, 9, 79–89. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hwang, H.M. Nanotechnology in food science: Functionality, applicability, and safety assessment. J. Food Drug Anal. 2016, 24, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.K.; Hayyan, M.; AlSaadi, M.A.; Hayyan, A.; Ibrahim, S. Environmental application of nanotechnology: Air, soil, and water. Environ. Sci. Pollut. Res. Int. 2016, 23, 13754–13788. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Ojha, H.; Bharadwaj, A.; Pathak, D.P.; Sharma, R.K. Preparation and catalytic applications of nanomaterials: A review. RSC Adv. 2015, 5, 53381–53403. [Google Scholar] [CrossRef]
- Peng, H.-S.; Chiu, D.T. Soft fluorescent nanomaterials for biological and biomedical imaging. Chem. Soc. Rev. 2015, 44, 4699–4722. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.K. Applications of nanotechnology in renewable energies-A comprehensive overview and understanding. Renew. Sustain. Energy Rev. 2015, 42, 460–476. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, A.; Zhang, X.; Huang, C.; Yang, D.; Jia, N. Multiwalled carbon nanotubes/gold nanocomposites-based electrochemiluminescent sensor for sensitive determination of bisphenol A. Anal. Bioanal. Chem. 2016, 408, 7173–7180. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Li, Y.; Cao, W.; Yan, T.; Li, Y.; Du, B.; Wei, Q. A label-free electrochemiluminescence immunosensor based on silver nanoparticle hybridized mesoporous carbon for the detection of Aflatoxin B1. Sens. Actuators B 2014, 202, 53–59. [Google Scholar] [CrossRef]
- Zhang, X.; Ke, H.; Wang, Z.; Guo, W.; Zhang, A.; Huang, C.; Jia, N. An ultrasensitive multi-walled carbon nanotube-platinum-luminol nanocomposite-based electrochemiluminescence immunosensor. Analyst 2017, 142, 2253–2260. [Google Scholar] [CrossRef] [PubMed]
- Doria, G.; Conde, J.; Veigas, B.; Giestas, L.; Almeida, C.; Assunção, M.; Rosa, J.; Baptista, P.V. Noble metal nanoparticles for biosensing applications. Sensors 2012, 12, 1657–1687. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Santos, T.A.P. Sensors and biosensors based on magnetic nanoparticles. Trends Anal. Chem. 2014, 62, 28–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, W.; Foroushani, A.D.; Wang, H.; Li, D.; Liu, J.; Barrow, C.J.; Wang, X.; Yang, W. New gold nanostructures for sensor applications. Materials 2014, 7, 5169–5201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Huang, C.; Shi, H.; Guo, W.; Zhang, X.; Xiang, H.; Jia, T.; Miao, F.; Jia, N. Electrochemiluminescence immunosensor for sensitive determination of tumor biomarker CEA based on multifunctionalized Flower-like Au@BSA nanoparticles. Sens. Actuators B 2017, 238, 24–31. [Google Scholar] [CrossRef]
- Tang, S.; Zhao, Q.; Tua, Y. A sensitive electrochemiluminescent cholesterol biosensor based on Au/hollowed-TiO2 nanocomposite pre-functionalized electrode. Sens. Actuators B 2016, 237, 416–422. [Google Scholar] [CrossRef]
- Yan, P.; Zhang, J.; Tang, Q.; Deng, A.; Li, J. A quantum dot based electrochemiluminescent immunosensor for the detection of pg level phenylethanolamine A using gold nanoparticles as substrates and electron transfer accelerators. Analyst 2014, 139, 4365–4372. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, H.; Wu, D.; Li, X.; Zhao, Y.; Zhang, Y.; Du, B.; Wei, Q. A label-free electrochemiluminescence immunosensor based on KNbO3-Au nanoparticles@Bi2S3 for the detection of prostate specific antigen. Biosens. Bioelectron. 2015, 74, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, Z.; Wang, X.; Li, F.; Cui, H.; Yang, D.; Bian, Z. Sensitive immunosensor for N-Terminal Pro-brain natriuretic peptide based on N-(Aminobutyl)-N-(ethylisoluminol)-functionalized gold nanodots/multiwalled carbon nanotube electrochemiluminescence nanointerface. ACS Appl. Mater. Interfaces 2015, 7, 7599–7604. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Zhang, A.; Xu, Y.; Chen, S.; Ma, F.; Huang, C.; Jia, N. An ultrasensitive electrochemiluminescence sensor for detecting diphenhydramine hydrochloride based on l-cysteine-functionalized multiwalled carbon nanotubes/gold nanoparticles nanocomposites. Sens. Actuators B 2015, 213, 5–11. [Google Scholar] [CrossRef]
- Wu, X.; Chai, Y.; Yuan, R.; Zhong, X.; Zhang, J. Synthesis of multiwall carbon nanotubes-graphene oxide-thionine-Au nanocomposites for electrochemiluminescence detection of cholesterol. Electrochim. Acta 2014, 129, 441–449. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.; Wu, D.; Zhang, Y.; Du, B.; Ma, H.; Wei, Q. Label-free electrochemiluminescent immunosensor for detection of carcinoembryonic antigen based on nanocomposites of GO/MWCNTs-COOH/Au@CeO2. ACS Appl. Mater. Interfaces 2015, 7, 19260–19267. [Google Scholar]
- Yuan, D.; Chen, S.; Yuan, R.; Zhang, J.; Liu, X. An ECL sensor for dopamine using reduced graphene oxide/multiwallcarbon nanotubes/gold nanoparticles. Sens. Actuators B 2014, 191, 415–420. [Google Scholar] [CrossRef]
- Yana, P.; Tanga, Q.; Denga, A.; Li, J. Ultrasensitive detection of clenbuterol by quantum dots based electrochemiluminescent immunosensor using gold nanoparticles as substrate and electron transport accelerator. Sens. Actuators B 2014, 191, 508–515. [Google Scholar] [CrossRef]
- Cao, J.; Wang, H.; Liu, Y. Petal-like CdS nanospheres-based electrochemiluminescence aptasensor for detection of IgE with gold nanoparticles amplification. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 151, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, F.; Yan, Z.; Wu, D.; Ma, H.; Du, B.; Wei, Q. Electrochemiluminescence immunosensing strategy based on the use of Au@Ag nanorods as a peroxidase mimic and NH4CoPO4 as a supercapacitive supporter: Application to the determination of carcinoembryonic antigen. Microchim. Acta 2015, 182, 1421–1429. [Google Scholar] [CrossRef]
- Sha, Y.; Guo, Z.; Chen, B.; Wang, S.; Ge, G.; Qiu, B.; Jiang, X. A one-step electrochemiluminescence immunosensor preparation for ultrasensitive detection of carbohydrate antigen 19-9 based on multifunctionalized graphene oxide. Biosens. Bioelectron. 2015, 66, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Li, Y.; Yan, T.; Pang, X.; Hu, L.; Du, B.; Wei, Q. An electrochemiluminescent immunosensor based on CdS–Fe O nanocomposite electrodes for the detection of Ochratoxin A. New J. Chem. 2015, 39, 4259–4264. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, S.; Zhang, Q.; Gong, L.; Daia, H.; Lin, Y. Magnetic functionalized electrospun nanofibers for magnetically controlled ultrasensitive label-free electrochemiluminescent immunedetection of aflatoxin B1. Sens. Actuators B 2016, 222, 707–713. [Google Scholar] [CrossRef]
- Luo, S.; Xiao, H.; Yang, S.; Liu, C.; Liang, J.; Tang, Y. Ultrasensitive detection of pentachlorophenol based on enhanced electrochemiluminescence of Au nanoclusters/graphene hybrids. Sens. Actuators B 2014, 194, 325–331. [Google Scholar] [CrossRef]
- Nie, F.; Luo, K.; Zheng, X.; Zheng, J.; Song, Z. Novel preparation and electrochemiluminescence application of luminol functional-Au nanoclusters for ALP determination. Sens. Actuators B 2015, 218, 152–159. [Google Scholar] [CrossRef]
- Han, S.; Zhang, Z.; Li, S.; Qi, L.; Xu, G. Chemiluminescence and electrochemiluminescence applications of metal nanoclusters. Sci. China Chem. 2016, 59, 794–801. [Google Scholar] [CrossRef]
- Bard, A.J. Electrogenerated Chemiluminesence; Marcel Dekker, Inc.: New York, NY, USA, 2004. [Google Scholar]
- Nie, G.; Li, C.; Zhang, L.; Wang, L. Fabrication of a simple and sensitive QDs-based electrochemiluminescence immunosensor using a nanostructured composite material for the detection of tumor markers alpha-fetoprotein. J. Mater. Chem. B 2014, 2, 8321–8328. [Google Scholar] [CrossRef]
- Wang, L.; Mei, L.; Liu, X.; Shi, J.; Li, Y.; Gu, N.; Cui, R. A nanocomposite prepared from helical carbon nanotubes, polyallylamine hydrochloride and CdSe quantum dots for electrochemiluminescent determination of dopamine. Microchim. Acta 2015, 182, 1661–1668. [Google Scholar] [CrossRef]
- Liang, H.; Song, D.; Gong, J. Signal-on electrochemiluminescence of biofunctional CdTe quantum dots for biosensing of organophosphate pesticides. Biosens. Bioelectron. 2014, 53, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z. An overview of carbon nanotubes and graphene for biosensing applications. Nano-Micro Lett. 2017, 9, 1–24. [Google Scholar] [CrossRef]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef] [PubMed]
- Bartelmess, J.; Giordani, S. Carbon nano-onions (multi-layer fullerenes): Chemistry and applications. Beilstein J. Nanotechnol. 2014, 5, 1980–1998. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, B.; Tavakoli, A.; Bozorgzadeh, S. Improved electrogenerated chemiluminescence of luminol by cobalt nanoparticles decorated multi-walled carbon nanotubes. J. Electroanal. Chem. 2016, 762, 80–86. [Google Scholar] [CrossRef]
- Walcarius, A. Recent Trends on Electrochemical Sensors Based on Ordered Mesoporous Carbon. Sensors 2017, 17, 1863. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Lian, S.; Zhao, L.; Chen, X.; Huang, Z.; Chen, X. A novel electrochemiluminescence glucose biosensor based on platinum nanoflowers/graphene oxide/glucose oxidase modified glassy carbon electrode. J. Solid State Electrochem. 2014, 18, 2375–2382. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, W.; Qi, W.; Gao, W.; Hanif, S.; Saqib, M.; Xu, G. Label-free signal-on ATP aptasensor based on the remarkable quenching of tris(2,2′-bipyridine)-ruthenium(II) electrochemiluminescence by single-walled carbon nanohorn. Chem. Commun. 2015, 51, 4256–4258. [Google Scholar]
- Choudhary, N.; Hwang, S.; Choi, W. Carbon Nanomaterials: A Review. In Handbook of Nanomaterials Properties; Bhushan, B., Luo, D., Schricker, S., Sigmund, W., Zauscher, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Guo, Z.; Dong, S. Electrogenerated Chemiluminescence from Ru(bpy)32+ ion-exchange in carbon nanotube/perfluorosulfonated ionomer composite films. Anal. Chem. 2004, 76, 2683–2688. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.Y.; Chen, J.H.; Chen, G.N. An ECL biosensor for glucose based on carbon-nanotube/Nafion film modified glass carbon electrode. Electrochim. Acta 2008, 53, 2396–2401. [Google Scholar] [CrossRef]
- Chen, J.H.; Lin, Z.Y.; Chen, G.N. Enhancement of electrochemiluminesence of lucigenin by ascorbic acid at single-wall carbon nanotube film-modified glassy carbon electrode. Electrochim. Acta 2007, 52, 4457–4462. [Google Scholar] [CrossRef]

| R − e− |  | R˙+ | (oxidation at the electrode surface) |
| R + e− |  | R˙− | (reduction at the electrode surface) |
| R˙+ + R˙− |  | R + R* | (annihilation process/formation of excited state) |
| R* |  | R + hv | (emission of light) |
| R − e− |  | R˙+ | (oxidation at the electrode surface) |
| C − e− |  | C˙+ | (reduction at the electrode surface) |
| C + C |  | R + C˙ | (homogenous chemical reactions) |
| C˙+ |  | *Cr | (homogenous chemical reactions) |
| *Cr + R |  | R˙− + P | (homogenous chemical reactions) |
| R˙− + R˙+ |  | R + R* | Or R˙+ + *Cr  R* + P (excited state formation species) R* + P (excited state formation species) |
| R* |  | hv | (emission of light) |
| R + e− |  | R˙− | (reduction at the electrode surface) |
| C + e− |  | C˙− | (reduction at the electrode surface) |
| R˙− + C |  | R + C˙− | (homogenous chemical reactions) |
| C˙− |  | *Co | (homogenous chemical reactions) |
| *Co + R |  | R˙+ + P | (homogenous chemical reactions) |
| R˙− + R˙+ |  | R + R* | Or R˙+ + *Co  R* + P (excited state species formation) R* + P (excited state species formation) |
| R* |  | hv | (emission of light) |

| Signal Type on Transducer | Signal Transduced | Type of Biosensors | Reference |
|---|---|---|---|
| Chemical | Electrical | Electrochemical | [6] |
| Optical | Electrical | Optical | [7] |
| Mass change | Electrical | Piezoelectric | [38] |
| Temperature change | Electrical | Thermal | [39] |
| Electrode | NMs/NSMs | NCs | Function | Assay type | Bioanalyte | LOD | Range | Ref. |
|---|---|---|---|---|---|---|---|---|
| GCE | AuNPs | MWCNTs-Cys-AuNPs | Signal amplification, reproducibility, high sensitivity, stability and cost-effective | Direct | Diphen-hydramine hydrochloride (DPH) | 6.7 × 10−9 M | 2 × 10−8–7.5 × 10−4 M | [72] |
| QDs-SPE | AuNPs | AuNPs@CNOs/Chitosan | High ECL, increased effective surface area, high anti-β2M binding, enhanced photons capture, high detection limit, wide linear range, long-term stability and good selectivity | Label-free | Beta-2 microglobulin (β2M) | 1 fg mL−1 | 1 fg mL−1–100 ng mL−1 | [7] |
| GCE | AuNPs | MWCNTs-Au | Signal amplification, sensitivity, selectivity, reproducibility, stability and low cost | Direct | Bisphenol A | 0.083 μM | 0.25–100 μM | [61] |
| GCE | AuNPs | Au@BSA nanoparticles | Large surface area, biocompatibility, specificity, stability, reproducibility, favorable selectivity, wide dynamic range and an ultralow detection limit | Label-free | CEACEA) | 0.0003 ng mL−1 | 0.001–200 ng mL−1 | [67] |
| GCE | AuNPs | AuNPs/ion liquid/hollowed TiO2 nano-shell | Specificity, repeatability, stability, storage, reproducibility, recovery for real sample test, ultralow detection limit, wide linear range, reliability, low-cost and on-site monitoring | Enzymatic | Cholesterol | 6.30 × 10−9 M | 8.33 × 10−9–4.17 × 10−7 M | [68] |
| GCE | AuNPs | NPCo/Co3O4–Au | Stability, reproducibility and immobilization of large amount of biomolecules | Label-free | DON | 1 pg mL−1 | 5 pg mL−1–100 ng mL−1 | [15] |
| GCE | AuNPs | MWCNTs-GO-Thi-Au | Enhanced ECL signal, high sensitivity, good selectivity and stability | Enzymatic | Cholesterol | 50 nM | 0.15–828 µM | [73] |
| GCE | AuNPs | KNbO3-AuNPs@Bi2S3 | Strong ECL signal, sensitivity, stability, long-term stability, acceptable selectivity, precision and accuracy | Label-free | PSA | 3 pg mL−1 | 0.0055 ng mL−1 | [70] |
| GCE | AuNPs | GO/MWCNTs-COOH/Au@CeO2 | High sensitivity, repeatability, selectivity, long-term stability, wide linear range | Label-free | CEA | 0.02 ng mL−1 | 0.05–100 ng mL−1 | [74] |
| GCE | AuNPs | AuNPs-CdSe QDs | Sensitivity, large surface area, rapid, acceptable precision, good stability, bioactivity and low-cost | Competitive | Phenylethanolamine A (PA) | 0.0047 ng mL−1 | 0.02 –50 ng mL−1 | [69] |
| GCE | AuNP | rGO/MWCNTs/AuNPs | Enhanced ECL signal intensity, high sensitivity, good reproducibility, stability and repeatability | Direct | Dopamine (DA) | 0.067 µM | 0.20–70 µM | [75] |
| GCE | AuNPs | AuNPs-CdSe QDs | Rapid and ultrasensitive, good stability, specificity and fabrication reproducibility | Competitive | Clenbuterol (CLB) | 0.0084 ng mL−1 | 0.02–50 ng mL−1 | [76] |
| GCE | AuNPs | Fe3O4 (Au-FrGO) | Large ECL signal, surface area, biocompatibility, sensitive response, excellent stability, repeatability and selectivity | Label-free | CEA | 3.28 fg mL−1 | 0.01 pg mL−1–10 ng mL−1 | [9] |
| GCE | AuNPs | petal-like CdS/AuNPs | high sensitivity, stability, good selectivity and wide linear range | Label-free | Immunoglobulin E | 8.0 × 10−14 M | 5.0 × 10−13–1.0 × 10−9 M | [77] |
| ITO | Gold nanodots | ABEI/GNDs/chitosan/COOH-MWCNTs | Strong and stable ECL signal, selectivity, stable and reliable response, extremely high sensitivity, satisfactory recovery | Label-free | N-Terminal Pro-brain natriuretic peptide (NT-proBNP) | 3.86 fg mL−1 | 0.01–100 pg mL−1 | [71] |
| GCE | AgNPs | luminol-AgNPs@OMC | Fast, sensitive, specific, stable and reliable | Label-free | Aflatoxin B1 (AFB1) | 50 fg mL−1 | 0.1 pg mL−1–50 ng mL−1 | [62] |
| GCE | Au@Ag nanorods | Au and Ag Bimetallic | Catalytic, sensitive, stable, specific and reproducible | Label-free | CEA | 30 fg mL−1 | 0.1 pg mL−1–380 ng mL−1 | [78] |
| GCE | nanoFe3O4 | nanoFe3O4@GO | Good conductivity, magnetism, stability, reproducibility and regeneration | Label-free | Carbohydrate antigen 19-9 (CA19-9) | 0.0005 U mL−1 | 0.001–5 U mL−1 | [79] |
| GCE | Fe3O4 | Fe3O4 (Au-FrGO) | Large ECL signal, specific surface area, biocompatibility, sensitive response, excellent stability, repeatability and selectivity | Label-free | CEA | 3.28 fg mL−1 | 0.01 pg mL−1–10 ng mL−1 | [9] |
| GCE | Fe3O4 | (RGO)/Fe3O4/CdSe | Sensitivity, excellent reproducibility and stability, wide linear range and high selectivity | Label-free | Interleukin-6 (IL-6) | 0.65 pg mL−1 | 0.002–20 ng mL−1 | [10] |
| GCE | Fe3O4 | CdS-Fe3O4 | Sensitive response, wide linear range, low detection limit, rapid, specific, stable, reliable and ultrasensitive ECL | Label-free | Ochratoxin A (OTA) | 2 pg mL−1 | 0.01–100 ng mL−1 | [80] |
| GCE | Magnetic nanofibers/CNHs | Magnetic nanofibers-Fe3O4 | Excellent electrical conductivity, large surface areas, variable porosity, amplified ECL, acceptable precision, reproducibility, biocompatibility, sensitivity, low detection limit and stability | Label-free | AFB1 | 0.02 ng mL−1 | 0.05–200 ng mL−1 | [81] |
| Modified titanium ribbon | AuNCs | Au NCs/GR | High sensitivity, low detection limit, wide dynamic range, low toxicity, good regeneration and can be used with real samples | Direct | Pentachlorophenol (PCP) | 0.1 fM | 0.1 fM–0.1 nM | [82] |
| GCE | AuNCs | lum-AuNCs | 100-folds enhancement of ECL, high specificity, good stability and potential clinical application | Direct | Alkaline phosphatase (ALP) | 0.1 nM | 0.3–12 nM | [83] |
| Electrode | NMs/NSMs | Nanocomposites | Function | Assay Type | Bioanalyte | LOD | Range | Ref |
|---|---|---|---|---|---|---|---|---|
| GCE | CdS QDs | CdS-Fe3O4 | Ultrasensitive ECL detection, wide linear range, low detection limit, rapid, specific, stable and reliable | Label-free | OTA | 2 pg mL−1 | 0.01–100 ng mL−1 | [80] |
| GCE | CdSe QDs | CdSe QDs/PICA-MWNT | Good biocompatibility, high ECL intensity, synergistic improvement of sensitivity, good selectivity and reproducibility | Label-free | Alpha-fetoprotein (AFP) | 0.4 pg mL−1 | 0.002–2000 ng mL−1 | [86] |
| GCE | CdSe QDs | HCNTs-Polyallylamine hydrochloride (PAH)-CdSe QDs | Signal amplification, increased surface area | Direct | DA | 0.2 × 10−9 M | 1.0 × 10−9–2.0 × 10−5 M | [87] |
| GCE | CdSe QDs | AuNPs-CdSe QDs | Electron transport accelerators, rapid, acceptable, precision, good stability, bioactivity, low-cost and lower detection limit | Competitive | PA | 0.0047 ng mL−1 | 0.02–50 ng mL−1 | [69] |
| GCE | CdSe QDs | AuNPs-CdSe QDs | Rapid, ultrasensitive, stability, specificity, fabrication, reproducibility and sensitivity | Competitive | CLB | 0.0084 ng mL−1 | 0.02–50 ng mL−1 | [76] |
| GCE | CdSe QDs | (RGO)/Fe3O4/CdSe | Sensitivity, stability, wide linear range, reproducibility and selectivity | Label-free | IL-6 | 0.65 pg mL−1 | 0.002–20 ng mL−1 | [10] |
| GCE | CdTe QDs | Graphene nanosheets (GNs)/CdTe QDs | Sensitivity, selectivity, reproducibility and ideal stability | Enzymatic | organophosphate pesticides (OPs) | 0.06 ng mL−1 | 0.2–10 ng mL−1 | [88] |
| Au/AgrGO | Graphene QDs | Graphene QDs | Ultrasensitive ECL, excellent electron conductivity, stability, sensitivity and repeatability | Label-free | PSA | 0.29 pg mL−1 | 1 pg mL−1–10 ng mL−1 | [38] |
| Electrode | NMs/NSMs | NCs | Function | Assay type | Bioanalyte | LOD | Range | Ref |
|---|---|---|---|---|---|---|---|---|
| GCE | MWCNTs | MWCNTs-Cys-AuNPs | Signal amplification, reproducibility, stability, sensitivity and cost-effective | Direct | DPH | 6.7 × 10−9 M | 2 × 10−8–7.5 × 10−4 M | [72] |
| GCE | MWCNTs | MWCNT-Pt-luminol | ECL signal, sensitivity, fast analysis, stability and specificity | Label-free | CA19-9 | 0.00004 U mL−1 | 0.0001–10.0 U mL−1 | [63] |
| GCW | MWCNTs | MWCNTs-Au | Signal amplification, selectivity, stability and low-cost | Direct | Bisphenol A | 0.083 μM | 0.25–100 μM | [61] |
| GCE | HCNTs | HCNTs-Polyallylamine hydrochloride (PAH)-CdSe QDs | Signal amplification, increased surface area, stability, sensitivity and excellent specificity | Direct | DA | 0.2 × 10−9 M | 1.0 × 10−9–2.0 × 10−5 M | [87] |
| GCE | Magnetic nanofibers/CNHs | CNHs | Excellent electrical conductivity, biocompatibility, large surface areas, variable porosity, amplified ECL, large antibody, acceptable precision, low detection limit, reproducibility and stability | Label-free | AFB1 | 0.02 ng mL−1 | 0.05–200 ng mL−1 | [81] |
| GCE | MWCNTs | MWCNT-Pt-luminol | ECL signal, fast analysis, stability, reproducibility and specificity | Label-free | CA19-9 | 0.00004 U mL−1 | 0.0001–10.0 U mL−1 | [85] |
| GCE | MWCNTs | nanoCo-MWCNTs | Sensitivity, economical and practical applications | Direct | Glucose | 50 nM | 0.5–600 μM | [86] |
| GCE | MWCNTs | MWCNTs-GO-Thi-Au | Enhancing ECL, high sensitivity and good selectivity | Enzymatic | Cholesterol | 50 nM | 0.15–828 µM | [73] |
| GCE | MWCNTs | GO/MWCNTs-COOH/Au@CeO2 | High sensitivity, repeatability, accelerated electrons transfer, long-term stability and wide linear range | Label-free | CEA | 0.02 ng mL−1 | 0.05–100 ng mL−1 | [74] |
| ITO | MWCNTs | ABEI/GNDs/chitosan/COOH-MWCNTs | Strong and stable ECL signal, selectivity, stability and extremely high sensitivity | Label-free | NT-proBNP | 3.86 fg mL−1 | 0.01–100 pg mL−1 | [71] |
| GCE | MWCNTs | rGO/MWCNTs/AuNPs | Enhanced ECL signal intensity, high sensitivity, good reproducibility and repeatability | Direct | DA | 0.067 µM | 0.20–70 µM | [75] |
| GCE | GO | MWCNTs-GO-Thi-Au | Enhanced ECL, high sensitivity and excellent stability | Enzymatic | Cholesterol | 50 nM | 0.15–828 µM | [73] |
| GCE | GO | GO/MWCNTs-COOH/Au@CeO2 | High sensitivity, accelerated electron transfer, selectivity and wide linear range | Label-free | CEA | 0.02 ng mL−1 | 0.05–100 ng mL−1 | [74] |
| GCE | GO | nanoFe3O4@GO | Good conductivity, magnetism, reproducibility and regeneration | Label-free | CA19-9 | 0.0005 U mL−1 | 0.001–5 U mL−1 | [79] |
| GCE | rGO | rGO/MWCNTs/AuNPs | Enhanced ECL signal intensity, high sensitivity and repeatability | Direct | DA | 0.067 µM | 0.20–70 µM | [75] |
| GCE | rGO | Fe3O4 (Au-FrGO) | Increased ECL, specific surface area, biocompatibility, sensitive response, excellent stability and selectivity | Label-free | CEA | 3.28 fg mL−1 | 0.01 pg mL−1–10 ng mL−1 | [9] |
| GCE | rGO | (RGO)/Fe3O4/CdSe | Sensitivity, stability, excellent reproducibility and selectivity | Label-free | IL-6 | 0.65 pg mL−1 | 0.002–20 ng mL−1 | [10] |
| GCE | GO | PtNFs/GO/GODx | High electrocatalytic activity, enhanced luminol ECL, reliability, high surface area, linear range and low detection limit | Direct | Glucose | 2.8 μM | 5–80 μM | [94] |
| QDs-SPE | CNOs | AuNPs@CNOs | High ECL, increased effective surface area for capturing and binding of antibodies, improved electron transmission rate, enhanced photon capture and highly sensitive | Label-free | β2M | 1 fg mL−1 | 1 fg mL−1–100 ng mL−1 | [7] |
| GCE | SWCNH | Aptamer/SWCNH | Quenching of ECL, sensitive, selective, simple, time-saving and cost-effective | Label-free | Adenosine triphosphate (ATP) | 1 nM | 5 nM–500 μM | [95] |
| GCE | OMCs | luminol-AgNPs@OMC | Fast, sensitive, specific, stable and reliable | Label-free | AFB1 | 50 fg mL−1 | 0.1 pg mL−1–50 ng mL−1 | [62] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizwan, M.; Mohd-Naim, N.F.; Ahmed, M.U. Trends and Advances in Electrochemiluminescence Nanobiosensors. Sensors 2018, 18, 166. https://doi.org/10.3390/s18010166
Rizwan M, Mohd-Naim NF, Ahmed MU. Trends and Advances in Electrochemiluminescence Nanobiosensors. Sensors. 2018; 18(1):166. https://doi.org/10.3390/s18010166
Chicago/Turabian StyleRizwan, Mohammad, Noor Faizah Mohd-Naim, and Minhaz Uddin Ahmed. 2018. "Trends and Advances in Electrochemiluminescence Nanobiosensors" Sensors 18, no. 1: 166. https://doi.org/10.3390/s18010166
APA StyleRizwan, M., Mohd-Naim, N. F., & Ahmed, M. U. (2018). Trends and Advances in Electrochemiluminescence Nanobiosensors. Sensors, 18(1), 166. https://doi.org/10.3390/s18010166