Microcontact Imprinted Plasmonic Nanosensors: Powerful Tools in the Detection of Salmonella paratyphi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bacteria
2.3. Preparation of Microcontact S. paratyphi Imprinted SPR Chips
2.3.1. Preparation and Modification of the Glass Slides
2.3.2. Modification of the SPR Chip Surfaces
2.3.3. Microcontact Imprinting of Salmonella paratyphi onto the SPR Chips
2.4. Characterization of SPR Chips
2.5. Real Time Salmonella paratyphi Detection
2.6. Selectivity of the Microcontact-Salmonella paratyphi Imprinted SPR Chip
2.7. Real Sample Experiments and Reusability
3. Results and Discussion
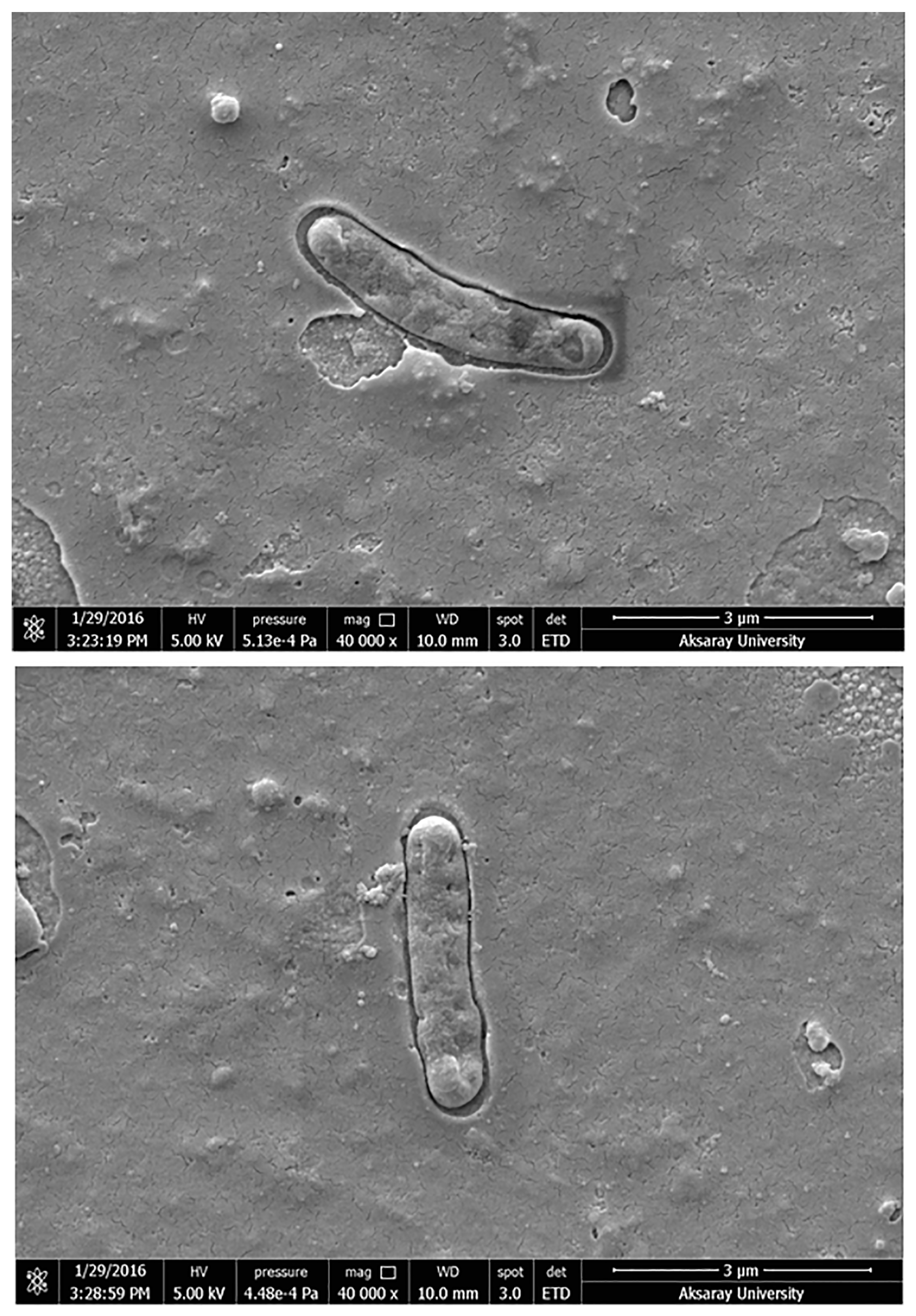
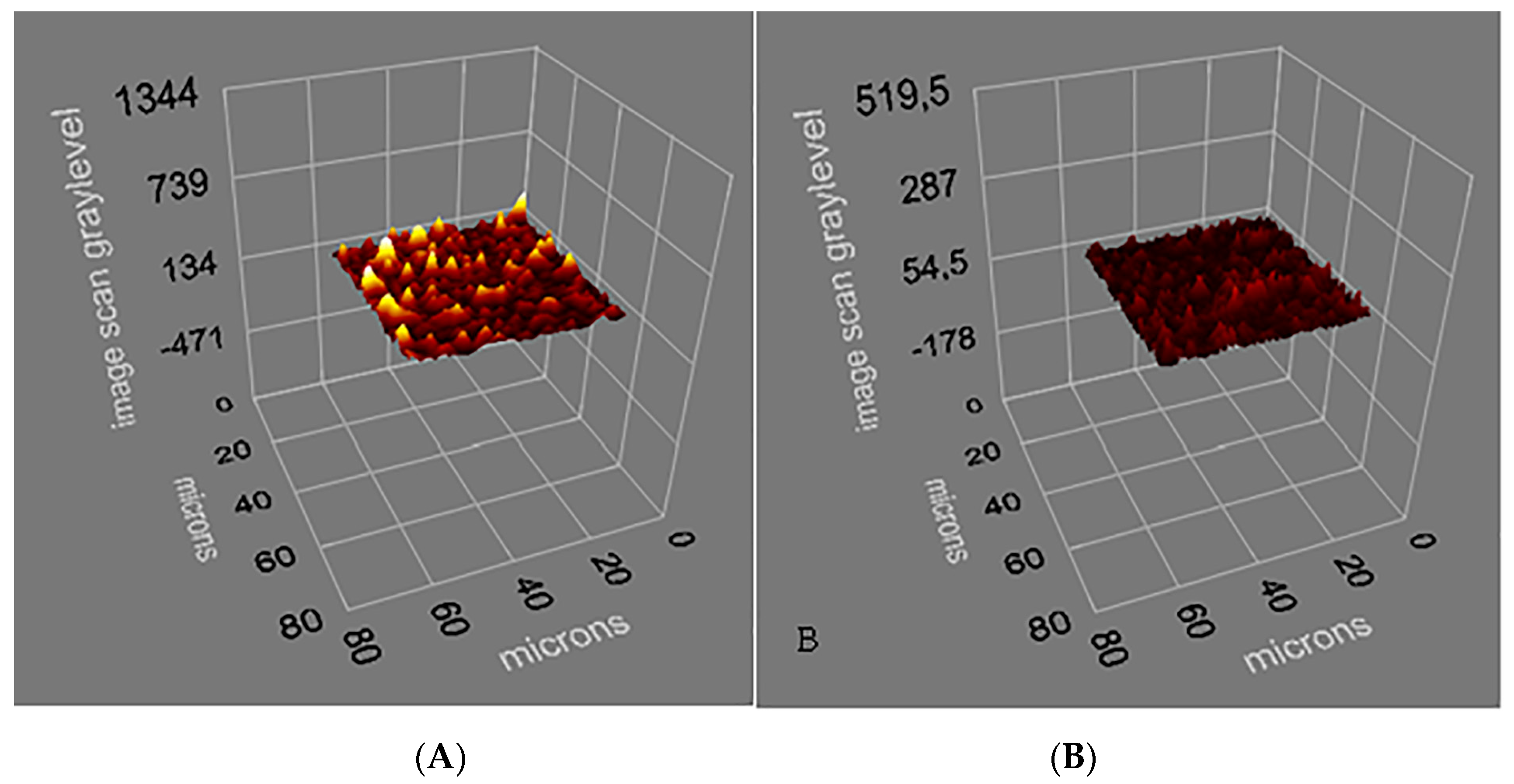
3.1. Characterization of Salmonella paratyphi-Imprinted SPR Chips
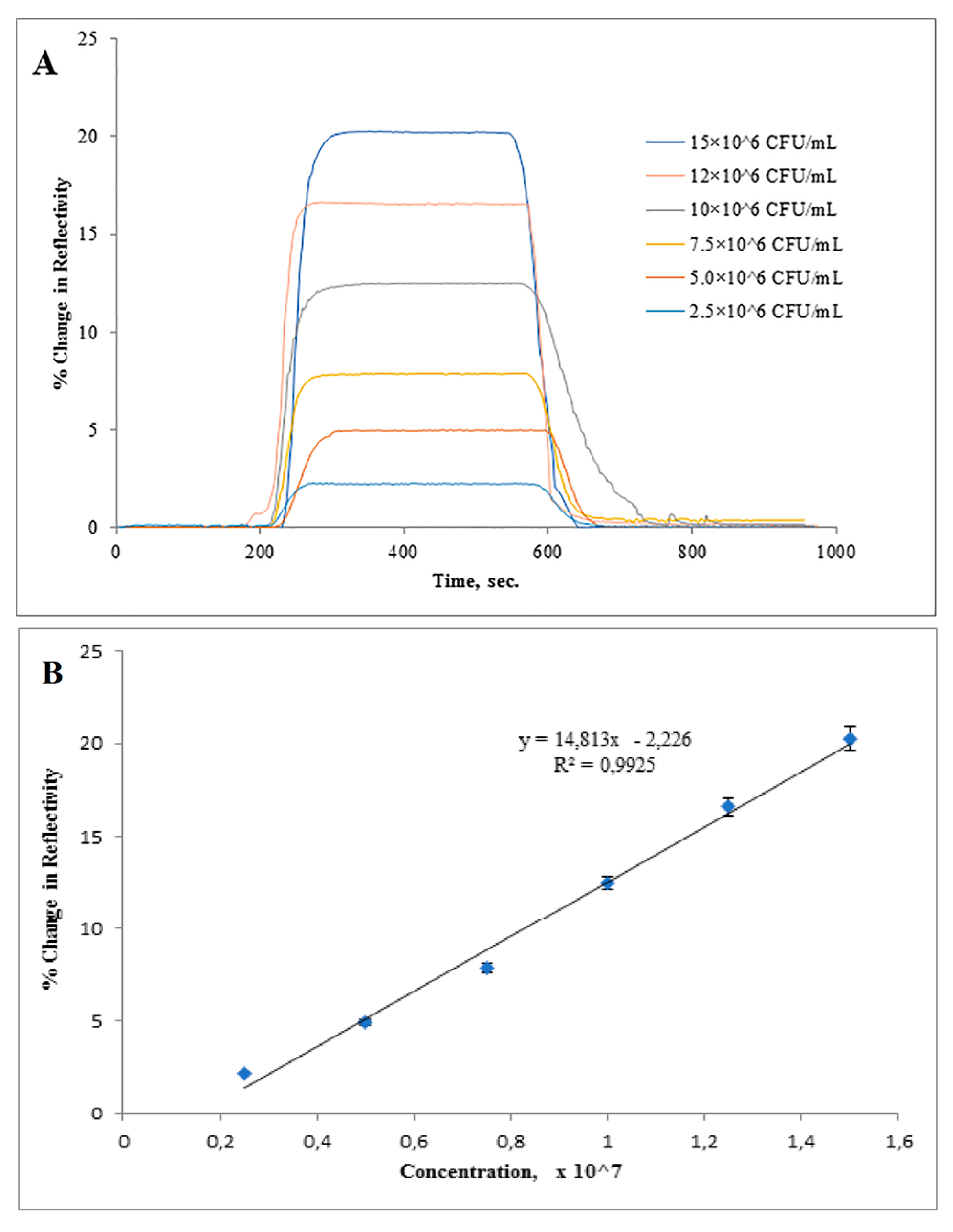
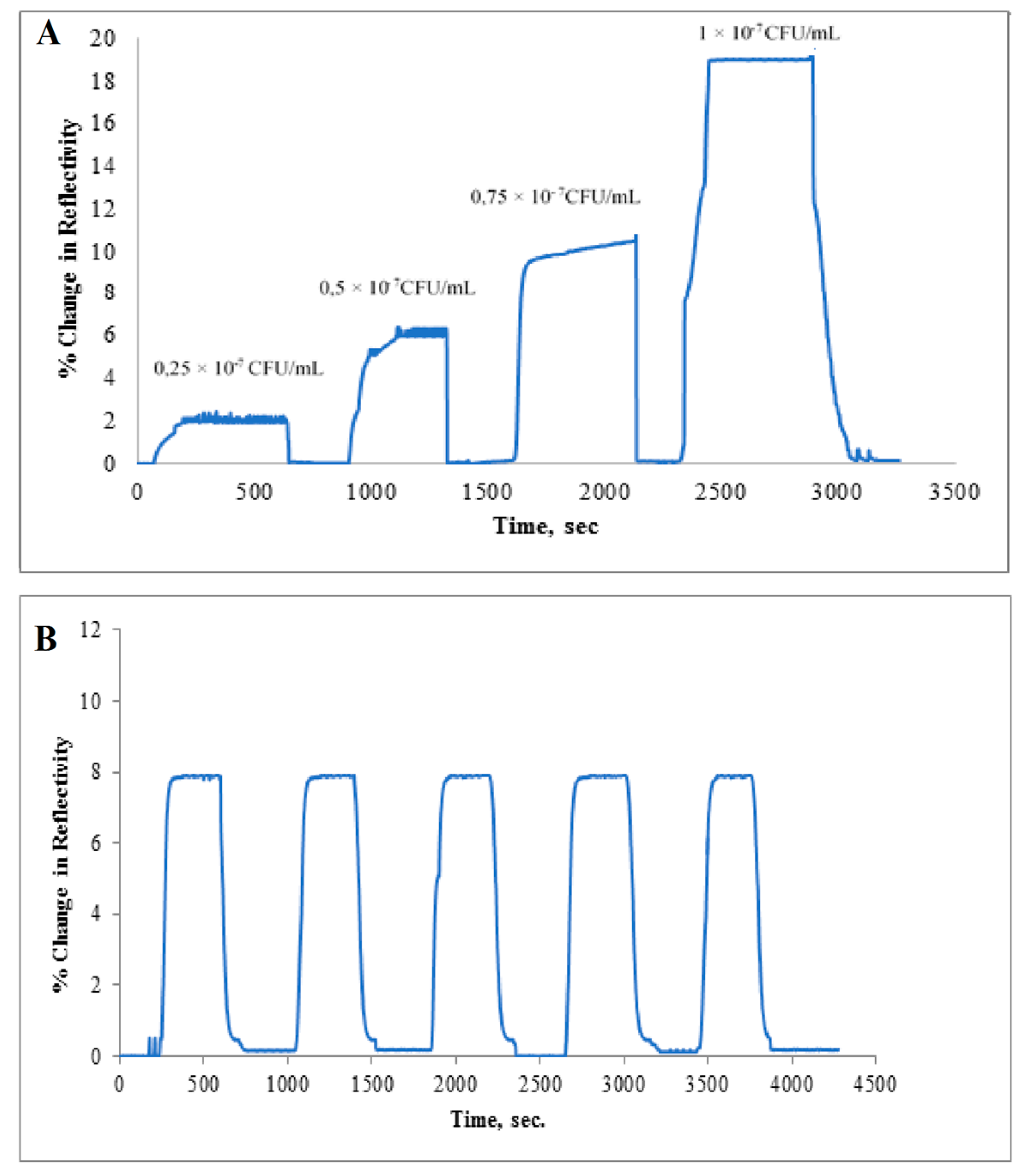
3.2. Real Time Detection of Salmonella paratyphi
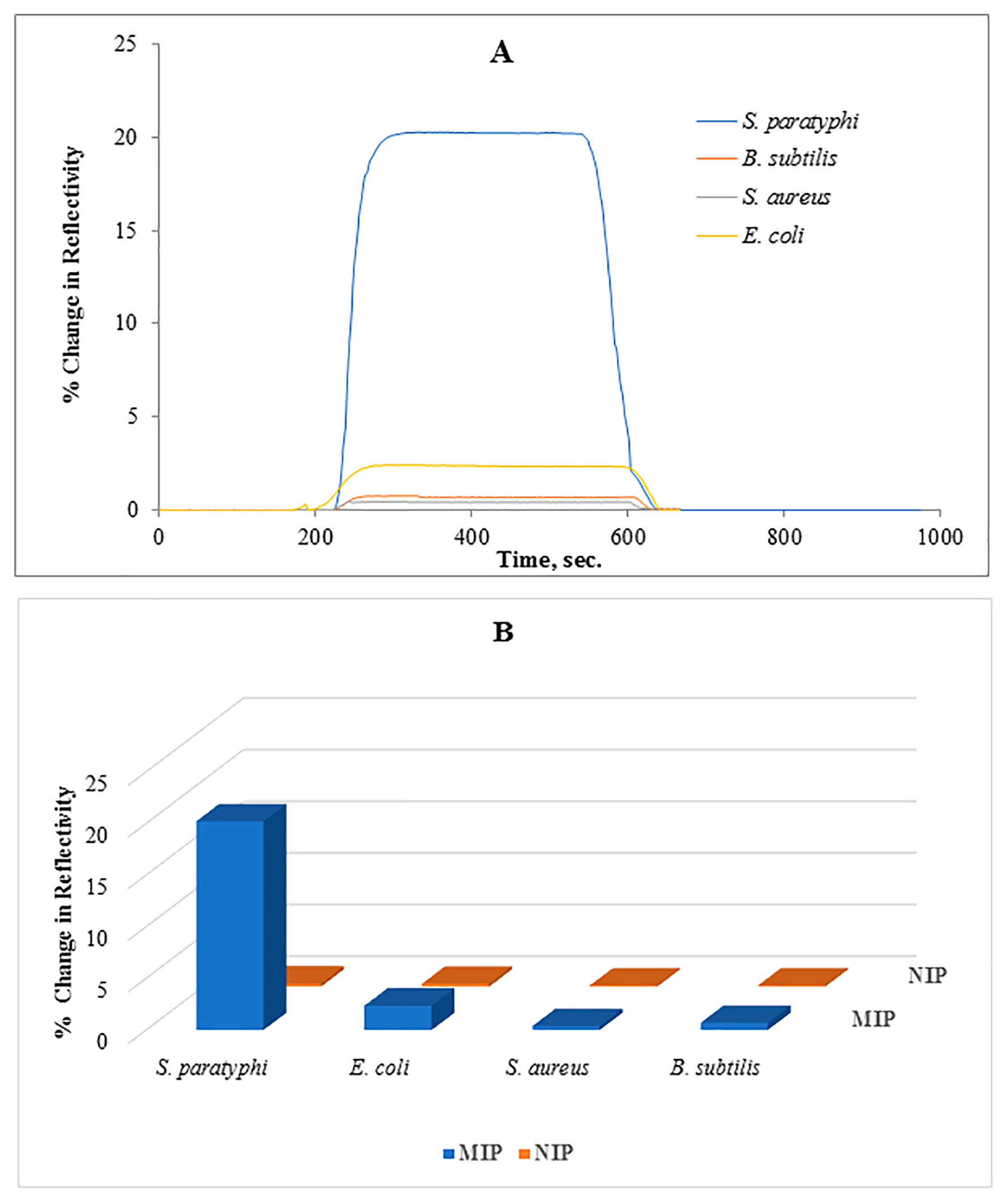
3.3. Selectivity of the Salmonella paratyphi Imprinted SPR Chip
3.4. Real Sample and Reusability Studies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, Y.; Wang, H.; Zhang, P.; Sun, C.; Wang, X.; Wang, X.; Yang, R.; Wang, C.; Zhou, L. Rapid multiplex detection of 10 foodborne pathogens with an up-converting phosphor technology-based 10-channel lateral flow assay. Sci. Rep. 2016, 6, 21342. [Google Scholar] [CrossRef] [PubMed]
- Ivnitski, D.; Abdel-Hamid, I.; Atanasov, P.; Wilkins, E. Biosensors for detection of pathogenic bacteria. Biosens. Bioelectron. 1999, 14, 599–624. [Google Scholar] [CrossRef]
- Wang, Y.; Salazar, J.K. Culture-independent rapid detection methods for bacterial pathogens and toxins in food matrices. Compr. Rev. Food Sci. Food Saf. 2016, 15, 183–205. [Google Scholar] [CrossRef]
- İdil, N.; Hedström, M.; Denizli, A.; Mattiasson, B. Whole cell based microcontact imprinted capacitive biosensor for the detection of Escherichia coli. Biosens. Bioelectron. 2017, 87, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.D.; Tran, T.B.; Nguyen, D.T.X.; Min, J. Magnetic silica nanotube-assisted impedimetric immunosensor for the separation and label-free detection of Salmonella typhimurium. Sens. Actuators B Chem. 2014, 197, 314–320. [Google Scholar] [CrossRef]
- Mandal, P.K.; Biswas, A.K.; Choi, K.; Pal, U.K. Methods for rapid detection of foodborne pathogens: An overview. Am. J. Food Technol. 2011, 6, 87–102. [Google Scholar] [CrossRef]
- Zhao, X.; Lin, C.W.; Wang, J.; Oh, D.H. Advances in rapid detection methods for foodborne pathogens. J. Microbiol. Biotechnol. 2014, 24, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Lin, Y.; Liang, T.; Chen, Z.; Li, J.; Yue, Z.; Lv, J.; Jiang, Q.; Yi, C. Ultrasensitive detection and rapid identification of multiple foodborne pathogens with naked eyes. Biosens. Bioelectron. 2015, 71, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.P.; Taitt, C.A.R. Biosensor-based detection of foodborne pathogens. In Foodborne Pathogens: Microbiology and Molecular Biology; Fratamico, P., Bhunia, A.K., Smith, J.L., Eds.; Caister Academic Press: Norfolk, UK, 2005. [Google Scholar]
- Rasooly, A.; Herold, K.E. Biosensors for the analysis of food- and waterborne pathogens and their toxins. J. AOAC Int. 2006, 89, 873–883. [Google Scholar] [PubMed]
- Geng, T.; Bhunia, A.K. Optical biosensors in foodborne pathogen detection. In Smart Biosensor Technology; Knopf, G.K., Bassi, A.S., Eds.; Taylor and Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Dey, D.; Goswami, T. Optical biosensors: A revolution towards quantum nanoscale electronics device fabrication. Biomed. Res. Int. 2011. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yang, X.; Gong, D.; Liu, F.; Hu, W.; Cai, W.; Huang, J.; Yang, M. Investigation for terminal reflection optical fiber SPR glucose sensor and glucose sensitive membrane with immobilized GODs. Opt. Express 2017, 25, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, H.; Şener, G.; Memon, N.; Bhanger, M.I.; Nizamani, S.M.; Üzek, R.; Denizli, A. Molecularly imprinted surface resonance (SPR) based sensing of bisphenol A for its selective detection in aqueous systems. Anal. Methods 2015, 7, 4661–4670. [Google Scholar] [CrossRef]
- Ertürk, G.; Özen, H.; Tümer, M.A.; Mattiasson, B.; Denizli, A. Microcontact imprinting based surface resonance (SPR) biosensor for real time and ultrasensitive detection of prostate specific antigen (PSA) from clinical samples. Sens. Actuators B Chem. 2016, 224, 823–832. [Google Scholar] [CrossRef]
- Bokken, G.; Corbee, R.J.; van Knapen, F.; Bergwerff, A.A. Immunochemical detection of Salmonella group B, D and E using an optical surface plasmon resonance biosensor. FEMS Microbiol. Lett. 2003, 222, 75–82. [Google Scholar] [CrossRef]
- Oh, B.K.; Kim, Y.K.; Park, K.W.; Lee, W.H.; Choi, J.W. Surface plasmon resonance immunosensor for the detection of Salmonella typhimurium. Biosens. Bioelectron. 2004, 19, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.K.; Lee, W.; Kim, Y.K.; Lee, W.H.; Choi, J.W. Surface plasmon resonance immunosensor using self-assembled protein G for the detection of Salmonella paratyphi. J. Biotechnol. 2004, 111, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Koubova, V.; Brynda, E.; Karasova, L.; Skvor, J.; Homola, J.; Dostalek, J.; Tobiska, P.; Rosicky, J. Detection of foodborne pathogens using surface plasmon resonance biosensors. Sens. Actuators B 2001, 74, 100–105. [Google Scholar] [CrossRef]
- Barlen, B.; Mazumdar, S.D.; Lezrich, O.; Kampfer, P.; Keusgen, M. Detection of Salmonella by surface plasmon resonance. Sensors 2007, 7, 1427–1446. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, Y.; Li, Q.; Yu, T.; Cheng, W.; Wang, L.; Ju, H.; Ding, S. Label-free and high-sensitive detection of Salmonella using a surface plasmon resonance DNA-based biosensor. J. Biotechnol. 2012, 160, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Yi, S.Y.; Woubit, A.; Kim, M. A Portable surface plasmon resonance biosensor for rapid detection of Salmonella typhimurium. Appl. Sci. Converg. Technol. 2016, 25, 61–65. [Google Scholar] [CrossRef]
- Bereli, N.; Andaç, M.; Baydemir, G.; Say, R.; Galaev, I.Y.; Denizli, A. Protein recognition via ion-coordinated molecularly imprinted supermacroporous cryogels. J. Chromatogr. A 2008, 1190, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Osman, B.; Uzun, L.; Beşirli, N.; Denizli, A. Microcontact imprinted surface resonance sensor for myoglobin detection. Mater. Sci. Eng. C 2013, 33, 3609–3614. [Google Scholar] [CrossRef] [PubMed]
- Ertürk, G.; Hedström, M.; Tümer, A.; Denizli, A.; Mattiasson, B. Real time PSA detection with PSA imprinted (PSA-MIP) capacitive biosensors. Anal. Chim. Acta 2015, 891, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Bakhshpour, M.; Özgür, E.; Bereli, N.; Denizli, A. Microcontact imprinted quartz crystal microbalance nanosensor for protein C recognition. Colloids Surf. B 2017, 151, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, E.; Majidi, D.; Özgür, E.; Denizli, A. Whole cell imprinting based Escherichia coli sensors: A study for SPR and QCM. Sens. Actuators B Chem. 2015, 209, 714–721. [Google Scholar] [CrossRef]
- Bole, A.L.; Manesiotis, P. Advanced materials for the recognition and capture of whole cells and microorganisms. Adv. Mater. 2016, 28, 5349–5366. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, E.; Özgür, E.; Bereli, N.; Türkmen, D.; Denizli, A. Plastic antibody based surface plasmon resonance nanosensors for selective atrazine detection. Mater. Sci. Eng. C 2017, 73, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Saylan, Y.; Akgönüllü, S.; Çimen, D.; Derazshamshir, A.; Bereli, N.; Yılmaz, F.; Denizli, A. Surface plasmon resonance based nanosensors for detection of triazine pesticides. Sens. Actuators B Chem. 2017, 241, 446–454. [Google Scholar] [CrossRef]
- Atay, S.; Pişkin, K.; Yılmaz, F.; Çakır, C.; Yavuz, H.; Denizli, A. Quartz crystal microbalance based biosensors for detecting highly metastatic breast cancer cells. Anal. Methods 2016, 8, 153–161. [Google Scholar] [CrossRef]
- Taylor, A.D.; Yu, Q.; Chen, S.; Homola, J.; Jiang, S. Comparison of E. coli O157:H7 preparation methods used for detection with surface plasmon resonance sensor. Sens. Actuators B Chem. 2005, 107, 202–208. [Google Scholar] [CrossRef]
- Taylor, A.D.; Ladd, J.; Chen, S.; Homola, J.; Jiang, S. Quantitative and simultaneous detection of four foodborne bacterial pathogens with a multi-channel SPR sensor. Biosens. Bioelectron. 2006, 22, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, S.D.; Hartmann, M.; Kampfer, P.; Keusgen, M. Rapid method for detection of Salmonella in milk by surface plasmon resonance (SPR). Biosens. Bioelectron. 2007, 22, 2040–2046. [Google Scholar] [CrossRef] [PubMed]
- Tokonami, S.; Nakado, Y.; Takahashi, M.; İkemizu, M.; Kadoma, T.; Saimatsu, K.; Dung, L.Q.; Shilgi, H.; Nagaoka, T. Label-free and selective bacteria detection using a film with transferred bacterial configuration. Anal. Chem. 2013, 85, 4925–4929. [Google Scholar] [CrossRef] [PubMed]
- Son, J.R.; Kim, G.; Kothapalli, A.; Morgan, M.T.; Ess, D. Detection of Salmonella enteridis using a miniature optical surface plasmon resonance biosensor. J. Phys. 2007, 61, 1086–1090. [Google Scholar]
- Poller, A.M.; Spieker, E.; Lieberzeit, P.A.; Preininger, C. Surface imprints: Advantageous application of ready2use materials for bacterial quartz-crystal microbalance sensors. Appl. Mater. Interf. 2017, 9, 1129–1135. [Google Scholar] [CrossRef] [PubMed]

| Bacterial Strains | SPR Response, ΔR | SPR Response, ΔR | Selectivity Coefficient, k | Selectivity Coefficient, k | Relative Selectivity Coefficient, k’ |
|---|---|---|---|---|---|
| Imprinted | Non-imprinted | Imprinted | Non-imprinted | ||
| S. paratyphi | 20.25 | 0.25 | - | - | - |
| S. aureus | 0.4 | 0.28 | 50.63 | 0.89 | 56.70 |
| E. coli | 0.7 | 0.35 | 28.93 | 0.71 | 40.50 |
| B. subtilis | 2.3 | 0.35 | 8.80 | 0.71 | 12.33 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perçin, I.; Idil, N.; Bakhshpour, M.; Yılmaz, E.; Mattiasson, B.; Denizli, A. Microcontact Imprinted Plasmonic Nanosensors: Powerful Tools in the Detection of Salmonella paratyphi. Sensors 2017, 17, 1375. https://doi.org/10.3390/s17061375
Perçin I, Idil N, Bakhshpour M, Yılmaz E, Mattiasson B, Denizli A. Microcontact Imprinted Plasmonic Nanosensors: Powerful Tools in the Detection of Salmonella paratyphi. Sensors. 2017; 17(6):1375. https://doi.org/10.3390/s17061375
Chicago/Turabian StylePerçin, Işık, Neslihan Idil, Monireh Bakhshpour, Erkut Yılmaz, Bo Mattiasson, and Adil Denizli. 2017. "Microcontact Imprinted Plasmonic Nanosensors: Powerful Tools in the Detection of Salmonella paratyphi" Sensors 17, no. 6: 1375. https://doi.org/10.3390/s17061375
APA StylePerçin, I., Idil, N., Bakhshpour, M., Yılmaz, E., Mattiasson, B., & Denizli, A. (2017). Microcontact Imprinted Plasmonic Nanosensors: Powerful Tools in the Detection of Salmonella paratyphi. Sensors, 17(6), 1375. https://doi.org/10.3390/s17061375







