The Development of Indicator Cotton Swabs for the Detection of pH in Wounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Indicator Cotton Swabs
2.3. Measurements
2.4. Determination of Endotoxin
2.5. Cytotoxicity Screening of Eluates/Extracts of Cotton Swab Components
2.6. Cytotoxicity Screening in Direct Contact with Tips of Cotton Swabs
3. Results and Discussion
3.1. Choice of Dyes and Evaluation of the pH Indicator Cotton Swabs Using a Color Measurement Device
3.2. Sensitivities of ICS1 and ICS2, and the Effect of Sterilization
3.3. Temperature Effect on Sensitivity
3.4. Toxicity Testing of the Cotton Swabs According to ISO Guidelines
3.5. Testing of Wound Dressings and Horse Serum Using ICS1 and ICS2
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thawer, H.A.; Houghton, P.E.; Woodbury, M.G.; Keast, D.; Campbell, K. A comparison of computer-assisted and manual wound size measurement. Ostomy Wound Manag. 2002, 48, 46–53. [Google Scholar]
- Siddall, S.S. Wound healing. An assessment tool. Home Healthc. Nurse 1983, 1, 35–40. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Hoey, C. Topical antimicrobial therapy for treating chronic wounds. Clin. Pract. 2009, 49, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- De Laat, E.H.; Scholte op Reimer, W.J.; van Achterberg, T. Pressure ulcers: Diagnostics and interventions aimed at wound-related complaints: A review of the literature. J. Clin. Nurs. 2005, 14, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Fleck, C.A. Palliative dilemmas: Wound odour. Wound Care Can. 2006, 4, 10–14. [Google Scholar]
- Casalinuovo, I.A.; Di Pierro, D.; Coletta, M.; Di Francesco, P. Application of electronic noses for disease diagnosis and food spoilage detection. Sensors 2006, 6, 1428–1439. [Google Scholar] [CrossRef]
- Worsley, G.J.; Attree, S.L.; Noble, J.E.; Horgan, A.M. Rapid duplex immunoassay for wound biomarkers at the point-of-care. Biosens. Bioelectr. 2012, 34, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Pirnay, J.-P.; De Vos, D.; Duinslaeger, L.; Reper, P.; Vandenvelde, C.; Cornelis, P.; Vanderkelen, A. Quantitation of Pseudomonas aeruginosa in wound biopsy samples: From bacterial culture to rapid ‘real-time’ polymerase chain reaction. Crit. Care 2000, 4, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; McCarty, S.; Hunt, J.A.; Woods, E.J. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen. 2014, 22, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Ansari, U.; Ali, M.N. Real-time wound management through integrated pH sensors: A review. Sens. Rev. 2015, 35, 183–189. [Google Scholar] [CrossRef]
- Gethin, G. The significance of surface pH in chronic wounds. Wounds UK 2007, 3, 52–56. [Google Scholar]
- Parra, J.L.; Paye, M.; The EEMCO Group. EEMCO guidance for the in vivo assessment of skin surface pH. Skin Pharmacol. Appl. Skin Physiol. 2003, 16, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Cutting, K.F. Wound exudate: Composition and functions. Br. J. Commun. Nurs. 2003, 8, 4–9. [Google Scholar] [CrossRef]
- Wilson, I.A.; Henry, M.; Quill, R.D.; Byrne, P.J. The pH of varicose ulcer surfaces and its relationship to healing. J. Vasc. Dis. 1979, 8, 339–342. [Google Scholar]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: A new perspective for wound-therapy. Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Eberlein, T.; Abel, M.; Wild, T.; Riesinger, T. Ergebnisse zur In-time-, bed-side-Messung von pH-Wert und Wundtemperatur. In Proceedings of the 1. Wund-D.A.CH Dreiländerkongress, Friedrichshafen, Germany, 10–12 October 2013. Abstract No. 30. [Google Scholar]
- Van der Schueren, L.; De Clerck, K. Coloration and application of pH-sensitive dyes on textile materials. Color. Technol. 2012, 128, 82–90. [Google Scholar] [CrossRef]
- Kumar, P.; Honnegowda, T.M. Effect of limited access dressing on surface pH of chronic wounds. Plast. Aesthet. Res. 2015, 2, 257–260. [Google Scholar] [CrossRef]
- Steyaert, I.; Vancoillie, G.; Hoogenboom, R.; De Clerck, K. Dye immobilization in halochromic nanofibers through blend electrospinning of a dye-containing copolymer and polyamide-6. Polym. Chem. 2015, 6, 2685–2694. [Google Scholar] [CrossRef]
- Kassal, P.; Zubak, M.; Scheipl, G.; Mohr, G.J.; Steinberg, M.D.; Murković Steinberg, I. Smart bandage with wireless connectivity for optical monitoring of pH. Sens. Actuators B Chem. 2017, 246, 455–460. [Google Scholar] [CrossRef]
- Meier, R.J.; Schreml, S.; Wang, X.D.; Landthaler, M.; Babilas, P.; Wolfbeis, O.S. Simultaneous photographing of oxygen and pH In vivo using sensor films. Angew. Chem. Int. Ed. 2011, 50, 10893–10896. [Google Scholar] [CrossRef] [PubMed]
- Mohr, G.J.; Müller, H. Tailoring colour changes of optical sensor materials by combining indicator and inert dyes and their use in sensor layers, textiles and non-wovens. Sens. Actuators B Chem. 2015, 206, 788–793. [Google Scholar] [CrossRef]
- Schaude, C.; Meindl, C.; Fröhlich, E.; Attard, J.; Mohr, G.J. Developing a sensor layer for the optical detection of amines during food spoilage. Talanta 2017, 170, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Capeletti, L.B.; Dos Santos, J.H.Z.; Moncada, E. Dual-target sensors: The effect of the encapsulation route on pH measurements and ammonia monitoring. J. Sol-Gel Sci. Technol. 2012, 64, 209–218. [Google Scholar] [CrossRef]
- International Organization for Standardization. 10993-1. Biological Evaluation of Medical Devices Part 1: Evaluation and Testing in the Risk Management Process; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- International Organization for Standardization. 10993-12. Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials; International Organization for Standardization: Geneva, Switzerland, 2007. [Google Scholar]
- Jacobs, J.P.; Jones, C.M.; Baille, J.P. Characteristics of a human diploid cell designated MRC-5. Nature 1970, 227, 168–170. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. 10993-5. Biological Evaluation of Medical Devices—Part 5: Test for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Van Tienhoven, E.A.E.; Korbee, D.; Schipper, L.; Verharen, H.W.; De Jong, W.H. In vitro and in vivo (cyto) toxicity assays using PVC and LDPE as model materials. J. Biomed. Mater. Res. Part A 2006, 78, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Mohr, G.J.; Müller, H.; Bussemer, B.; Stark, A.; Carofiglio, T.; Trupp, S.; Heuermann, R.; Henkel, T.; Escudero, D.; Gonzalez, L. Design of acidochromic dyes for facile preparation of pH sensor layers. Anal. Bioanal. Chem. 2008, 392, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Zajko, S.; Klimant, I. The effects of different sterilization procedures on the optical polymer oxygen sensors. Sens. Actuators B Chem. 2013, 177, 86–93. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. Fiber Optic Chemical Sensors and Biosensors; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Schaude, C.; Mohr, G.J. Indicator washcloth for detecting alkaline washing solutions to prevent dermatitis patients and babies from skin irritation. Fash. Text. 2017, 4, 7. [Google Scholar] [CrossRef]
- Sendroy, J.; Rodkey, F.L. Apparent dissociation constant of phenol red as determined by spectrophotometry and by visual colorimetry. Clin. Chem. 1961, 7, 646–654. [Google Scholar] [PubMed]
- Committee for Medicinal Products for Human Use. EMEA/CHMP/BWP/452081/2007. Guideline on the Replacement of Rabbit Pyrogen Testing by an Alternative Test for Plasma Derived Medicinal Products; European Medicine Agency: London, UK, 2009. [Google Scholar]
- United States Pharmacopeia. 34-NF29, U. <87>, Biological Reactivity Test, In Vitro—Direct Contact Test; The United States Pharmacopeia: Rockville, MD, USA, 2011. [Google Scholar]
- United States Pharmacopeia. United States Pharmacopeial Convention USP 23; The United States Pharmacopeia: Rockville, MD, USA, 1995. [Google Scholar]
- Csako, G.; Tsai, C.M.; Hochstein, H.D.; Elin, R.J. The concentration, physical state, and purity of bacterial endotoxin affect its detoxification by ionizing radiation. Radiat. Res. 1986, 108, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Ekwall, B. Overview of the Final MEIC Results: II. The in vitro-In vivo evaluation, including the selection of a practical battery of cell tests for prediction of acute lethal blood concentrations in humans. Toxicol. In Vitro 1999, 13, 665–673. [Google Scholar] [CrossRef]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar] [PubMed]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [PubMed]
- Uzun, M.; Anand, S.C.; Shah, T. The effect of wound dressings on the pH stability of fluids. J. Wound Care 2012, 21, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Escudero, D.; Trupp, S.; Bussemer, B.; Mohr, G.J.; Gonzalez, L. Spectroscopic properties of azobenzene-based pH indicator dyes: A quantum chemical and experimental study. J. Chem. Theory Comput. 2011, 7, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
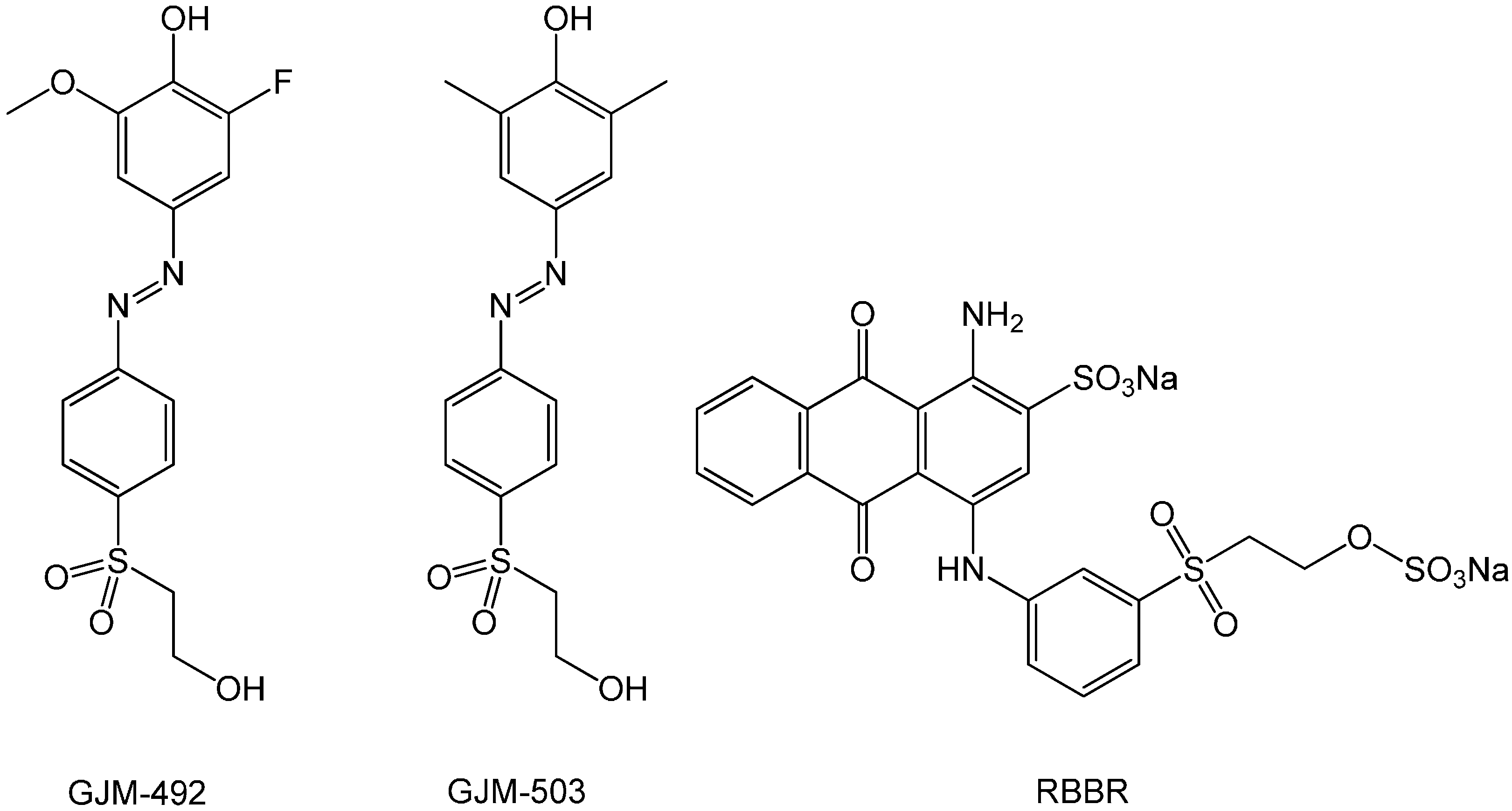

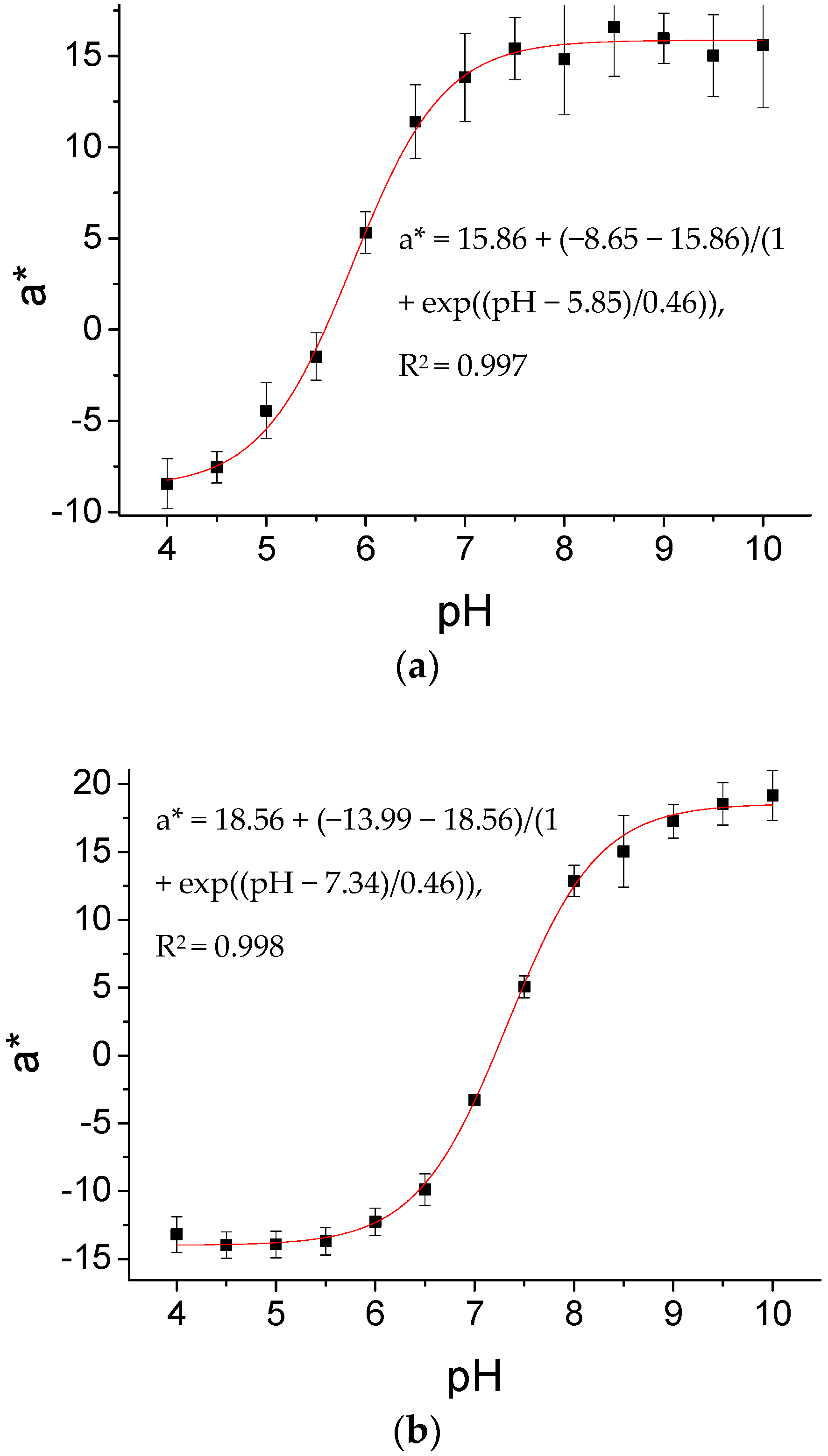
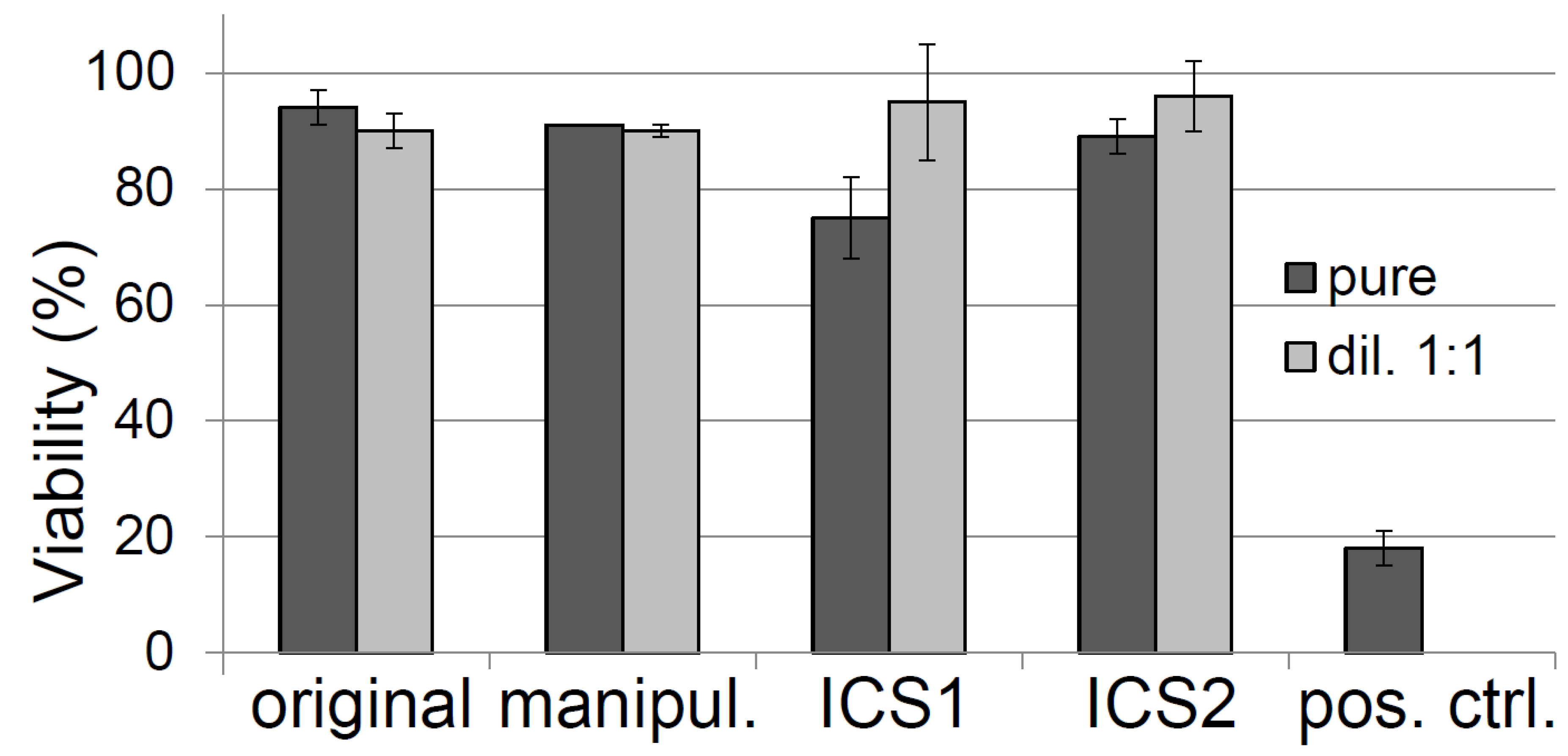
| ICS1 (Not Sterilized) | ICS1 (Sterilized) | ICS2 (Not Sterilized) | ICS2 (Sterilized) | |
|---|---|---|---|---|
| Five swabs measured once | 5.89 (0.07) | 5.85 (0.06) | 7.38 (0.11) | 7.34 (0.05) |
| One swab measured five times | 5.75 (0.11) | 5.87 (0.07) | 7.38 (0.09) | 7.37 (0.03) |
| ICS1 (n = 10) | ICS2 (n = 10) | |
|---|---|---|
| pKa at 20 °C | 5.86 (0.22) | 7.31 (0.26) |
| pKa at 30 °C | 5.92 (0.14) | 7.39 (0.31) |
| pKa at 40 °C | 5.90 (0.08) | 7.43 (0.13) |
| pH of Solutions Measured by a pH Electrode | Mepilex | AQUACEL Extra | Suprasorb A |
|---|---|---|---|
| Phosphate buffer (pH 6.0) | 6.6 | 6.5 | 6.3 |
| Phosphate buffer (pH 7.0) | 7.3 | 7.5 | 6.6 |
| Phosphate buffer (pH 8.0) | 8.2 | 7.9 | 6.5 |
| Ringer solution (pH 6.0) | 7.1 | 5.1 | 5.2 |
| Ringer solution (pH 7.0) | 7.5 | 5.0 | 5.4 |
| Ringer solution (pH 8.0) | 7.7 | 5.1 | 5.6 |
| pH of Horse Serum Measured by a pH Electrode | ICS1 * | ICS2 * |
|---|---|---|
| 6.01 | 6.00 (0.06) | 6.39 (0.04) |
| 6.62 | 6.62 (0.18) | 6.81 (0.02) |
| 8.41 | 8.29 (0.36) | 8.54 (0.30) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaude, C.; Fröhlich, E.; Meindl, C.; Attard, J.; Binder, B.; Mohr, G.J. The Development of Indicator Cotton Swabs for the Detection of pH in Wounds. Sensors 2017, 17, 1365. https://doi.org/10.3390/s17061365
Schaude C, Fröhlich E, Meindl C, Attard J, Binder B, Mohr GJ. The Development of Indicator Cotton Swabs for the Detection of pH in Wounds. Sensors. 2017; 17(6):1365. https://doi.org/10.3390/s17061365
Chicago/Turabian StyleSchaude, Cindy, Eleonore Fröhlich, Claudia Meindl, Jennifer Attard, Barbara Binder, and Gerhard J. Mohr. 2017. "The Development of Indicator Cotton Swabs for the Detection of pH in Wounds" Sensors 17, no. 6: 1365. https://doi.org/10.3390/s17061365
APA StyleSchaude, C., Fröhlich, E., Meindl, C., Attard, J., Binder, B., & Mohr, G. J. (2017). The Development of Indicator Cotton Swabs for the Detection of pH in Wounds. Sensors, 17(6), 1365. https://doi.org/10.3390/s17061365








