The Effects of Dithiothreitol on DNA
Abstract
:1. Introduction
2. Materials and Methods
2.1. DNA-Oligonucleotides
- Primer 1: 5′-ATTTTTCTAAGTCTTTTAGATCGAACGACTCAGAATGATGCATGTATACTAAACTCACAAATTAGAGC-3′
- Primer 2: 5′-TTTTTTTTTTTTTTTTTTTTTTTTTGCTTTCTCATAGCTCACGCTG-3′
- IN-fw: 5′-AACTGGCGCGCCATGGCTTCTGAC-3′
- IN-rv: 5′-TTAATCTTCGTCCTGACGAGAAGCAACG-3′
- 5′-Amine-Oligo A: 5′-Am-TTTAGTCAGTGTGGAAAACTCTAGCAGT-3′
- Oligo B: 5′-ACTGCTAGAGATTTTCCACACTGACTAAA-3′
- Acceptor-circle-fw: 5′-CCGCCCTGCAGCCTCAATGCACATGTTTGGCTCCC-3′
- Acceptor-circle-rv: 5′-TAATTCTGCAGACGATAGCGGTACATCTCGG-3′
- Detection probe: 5′-FAM-CCTCAATGCACATGTTTGGCTCC-3′
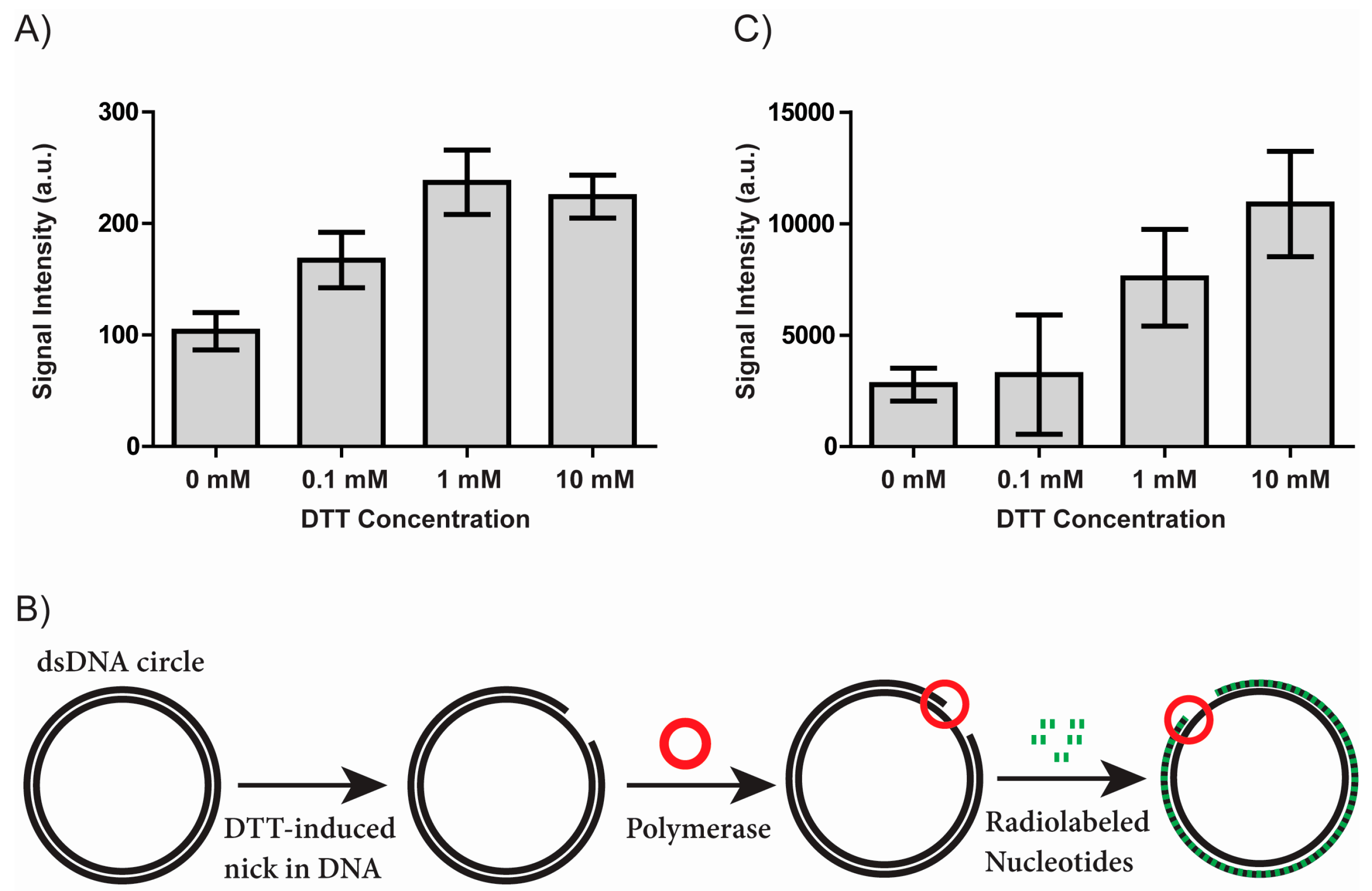
2.2. Nicking of Supercoiled Plasmid
2.3. Polymerase Enabled Nick Detection (Modified Nick Translation Assay)
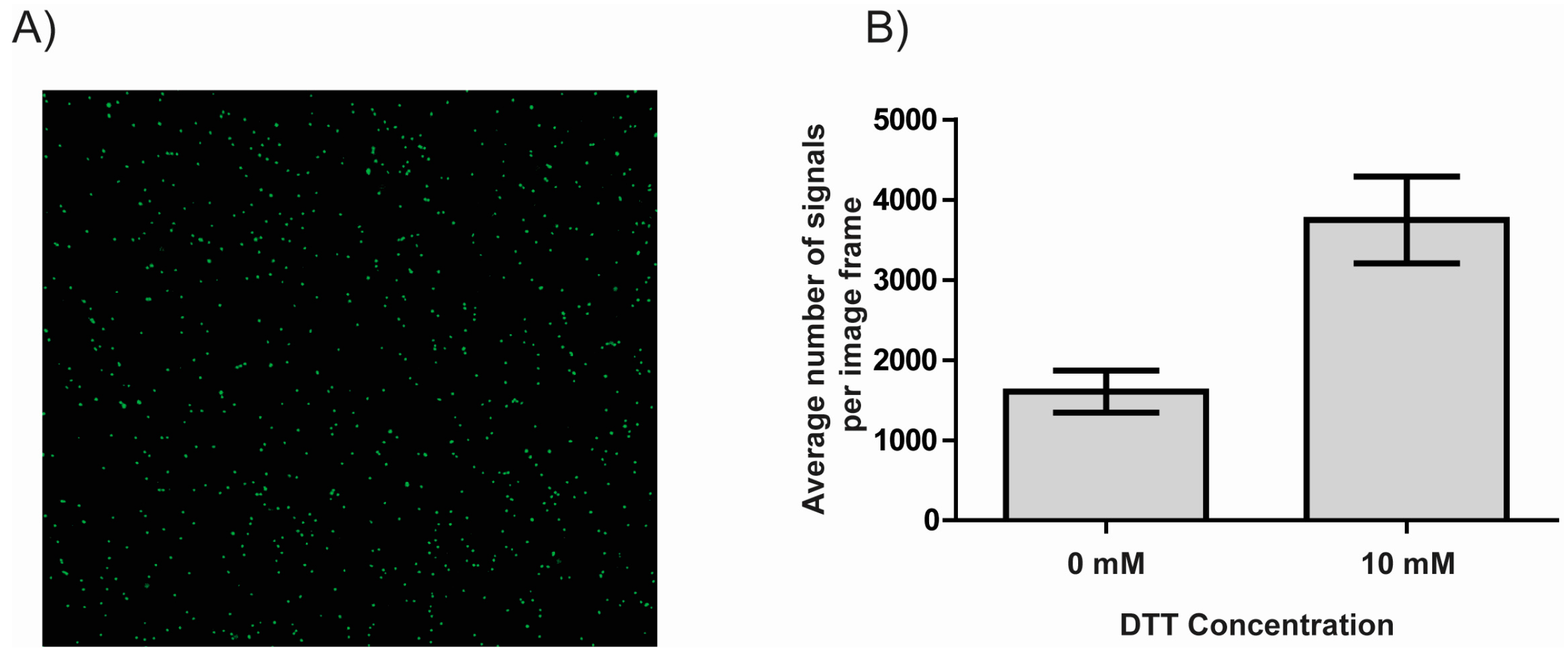
2.4. DNA Adhesion Assay
2.5. Purification of IN
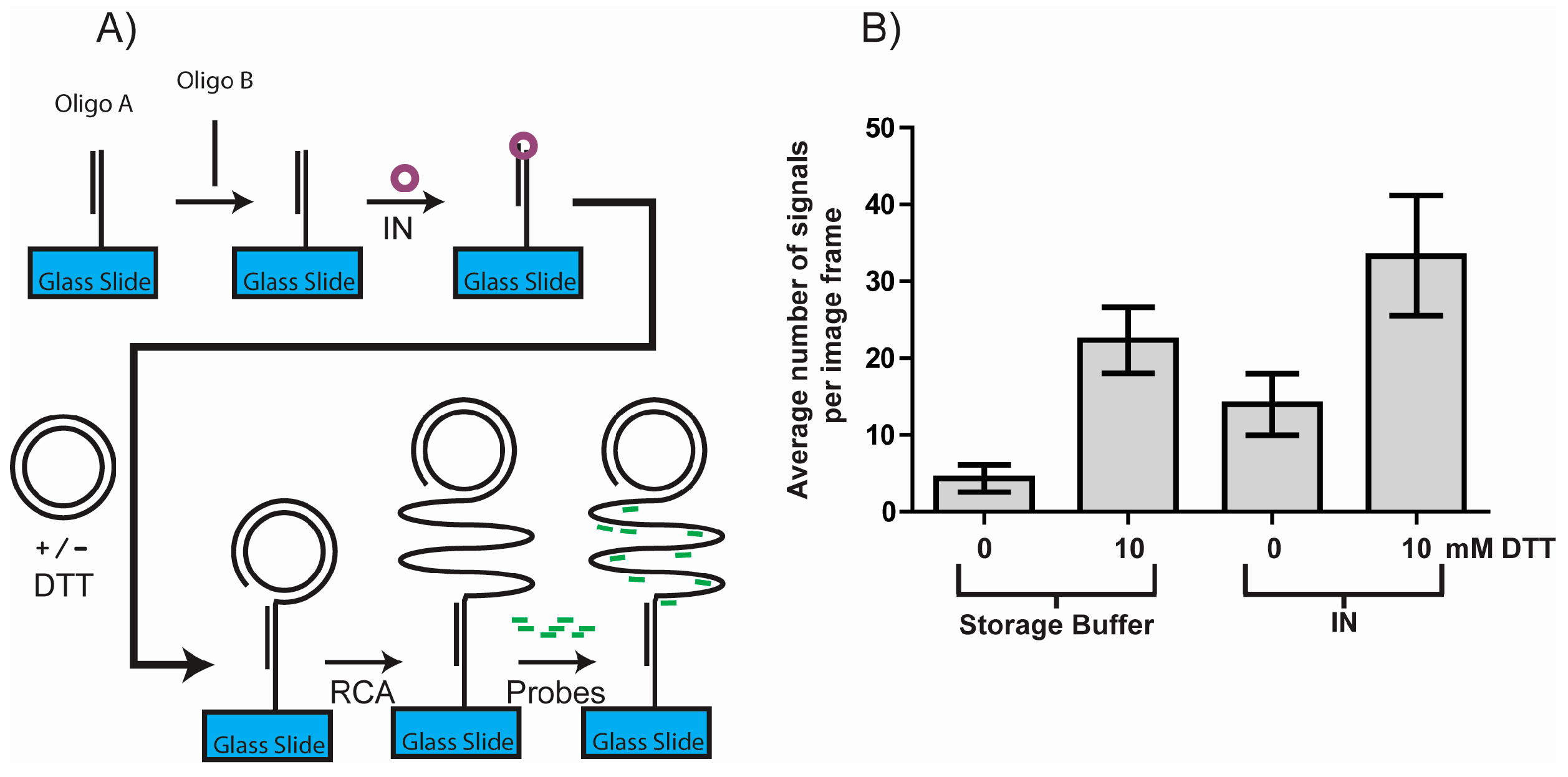
2.6. Generation of DNA Acceptor Circles
2.7. Rolling Circle Amplification-Based IN Detection
3. Results and Discussion
3.1. DTT Creates Single Stranded Nicks in Covalently Closed DNA Circles
3.2. DTT Facilitates the Immobilization of DNA to NHS-Ester Coated Microscopy Slides
3.3. DTT Influences the Outcome of a RCA DNA Sensor System
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| bp | Base pairs |
| ddH2O | Double distilled water |
| DTT | Dithiothreitol |
| EDTA | Ethylenediaminetetraacetic acid |
| IN | Retroviral integrase |
| IPTG | Isopropyl β-d-thiogalactoside |
| NHS | N-Hydroxysuccinimide |
| PCR | Polymerase Chain Reaction |
| PMSF | phenylmethylsulfonyl fluoride |
| RCA | Rolling circle amplification |
| REEAD | Rolling circle amplification enzyme activity detection |
| Tris | Tris(hydroxymethyl)aminomethane |
References
- Stougaard, M.; Lohmann, J.S.; Mancino, A.; Celik, S.; Andersen, F.F.; Koch, J.; Knudsen, B.R. Single-molecule detection of human topoisomerase I cleavage-ligation activity. ACS Nano 2009, 3, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Flusberg, B.A.; Webster, D.R.; Lee, J.H.; Travers, K.J.; Olivares, E.C.; Clark, T.A.; Korlach, J.; Turner, S.W. Direct detection of DNA methylation during single-molecule, real-time sequencing. Nat. Methods 2010, 7, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Getz, E.B.; Xiao, M.; Chakrabarty, T.; Cooke, R.; Selvin, P.R. A comparison between the sulfhydryl reductants tris(2-carboxyethyl)phosphine and dithiothreitol for use in protein biochemistry. Anal. Biochem. 1999, 273, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Netto, L.E.S.; Stadtman, E.R. The Iron-Catalyzed Oxidation of Dithiothreitol Is a Biphasic Process: Hydrogen Peroxide Is Involved in the Initiation of a Free Radical Chain of Reactions. Arch. Biochem. Biophys. 1996, 333, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Newton, G.L.; Gonick, G.; Fahey, R.C.; John, F.; Apr, N.; Wardt, J.F. Radioprotection of DNA by Thiols: Relationship between the Net Charge on a Thiol and Its Ability to Protect DNA. Radiat. Res. Soc. 2016, 114, 11–27. [Google Scholar] [CrossRef]
- Held, K.D.; Harrop, H.A.; Michael, B.D. Effects of oxygen and sulphydryl-containing compounds on irradiated transforming DNA. Part I. Actions of dithiothreitol. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1981, 40, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Solen, G.; Edgren, M.; Scott, O.C.; Revesz, L. Radioprotection by dithiothreitol (DTT) at varying oxygen concentrations: Predictions of a modified competition model and theory evaluation. Int. J. Radiat. Biol. 1991, 59, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, E.J.; Hyun, J.W.; Kim, S.H.; Mar, W.; Kim, J.K. Reduction of radiation-induced chromosome aberration and apoptosis by dithiothreitol. Arch. Pharm. Res. 1998, 21, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.J.; Douglas, K.T. Chemical cleavage of plasmid DNA by glutathione in the presence of Cu(II) ions. The Cu(II)-thiol system for DNA strand scission. Biochem. J. 1991, 275 Pt 3, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.J.; Douglas, K.T. Single-strand cleavage of DNA by Cu(II) and thiols: A powerful chemical DNA-cleaving system. Biochem. Biophys. Res. Commun. 1989, 162, 1111–1117. [Google Scholar] [CrossRef]
- Held, K.D.; Biaglow, J.E. Role of copper in the oxygen radical-mediated toxicity of the thiol-containing radioprotector dithiothreitol in mammalian cells. Radiat. Res. 1993, 134, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Halliwell, B.; Gajewski, E.; Dizdaroglu, M. Copper-Ion-Dependent Damage to the Bases in Dna in the Presence of Hydrogen-Peroxide. Biochem. J. 1991, 273, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Mira, A.; Gimenez, E.M.; Bolzán, A.D.; Bianchi, M.S.; López-Larraza, D.M. Effect of Thiol Compounds on Bleomycin-Induced DNA and Chromosome Damage in Human Cells. Arch. Environ. Occup. Health 2013, 68, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Tartier, L.; McCarey, Y.L.; Biaglow, J.E.; Kochevar, I.E.; Held, K.D. Apoptosis induced by dithiothreitol in HL-60 cells shows early activation of caspase 3 and is independent of mitochondria. Cell Death Differ. 2000, 7, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Hanna, P.M.; Mason, R.P. Direct Evidence for Inhibition of Free-Radical Formation from Cu(i) and Hydrogen-Peroxide by Glutathione and Other Potential Ligands using the Epr Spin-Trapping Technique. Arch. Biochem. Biophys. 1992, 295, 205–213. [Google Scholar] [CrossRef]
- Misra, H.P. Generation of Superoxide during the Autoxidation. J. Biol. Chem. 1974, 246, 6886–6890. [Google Scholar]
- Ménová, P.; Raindlová, V.; Hocek, M. Scope and limitations of the nicking enzyme amplification reaction for the synthesis of base-modified oligonucleotides and primers for PCR. Bioconjug. Chem. 2013, 24, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Niemz, A.; Ferguson, T.M.; Boyle, D.S. Point-of-care nucleic acid testing for infectious diseases. Trends Biotechnol. 2011, 29, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Hede, M.; Fjelstrup, S.; Knudsen, B. DNA Sensors for Malaria Diagnosis. Nano Life 2015, 5, 1541003. [Google Scholar] [CrossRef]
- Juul, S.; Nielsen, C.J.; Labouriau, R.; Roy, A.; Tesauro, C.; Jensen, P.W.; Harmsen, C.; Kristoffersen, E.L.; Chiu, Y.L.; Frohlich, R.; et al. Droplet microfluidics platform for highly sensitive and quantitative detection of malaria-causing Plasmodium parasites based on enzyme activity measurement. ACS Nano 2012, 6, 10676–10683. [Google Scholar] [CrossRef] [PubMed]
- Juul, S.; Ho, Y.P.; Koch, J.; Andersen, F.F.; Stougaard, M.; Leong, K.W.; Knudsen, B.R. Detection of single enzymatic events in rare or single cells using microfluidics. ACS Nano 2011, 5, 8305–8310. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, J.; Thomsen, J.; Selnihhin, D.; Hede, M.S.; Kirsebom, F.C.M.; Franch, O.; Fjelstrup, S.; Stougaard, M.; Ho, Y.-P.; et al. Novel DNA sensor system for highly sensitive and quantitative retrovirus detection using virus encoded integrase as a biomarker. Nanoscale 2017, 9, 440–448. [Google Scholar] [CrossRef] [PubMed]
- John, D.C.; Douglas, K.T. Sequence-dependent reactivity of linear DNA to chemical cleavage by Cu(II): Thiol combinations including cysteine or glutathione. Biochem. J. 1993, 289 Pt 2, 463–468. [Google Scholar] [CrossRef] [PubMed]
- John, D.C.; Douglas, K.T. A common chemical mechanism used for DNA cleavage by copper(II) activated by thiols and ascorbate is distinct from that for copper(II): Hydrogen peroxide cleavage. Transit. Met. Chem. 1996, 21, 460–463. [Google Scholar] [CrossRef]
- Speisky, H.; Gomez, M.; Carrasco-Pozo, C.; Pastene, E.; Lopez-Alarcon, C.; Olea-Azar, C. Cu(I)-glutathione complex: A potential source of superoxide radicals generation. Bioorg. Med. Chem. 2008, 16, 6568–6574. [Google Scholar] [CrossRef] [PubMed]
- Hede, M.; Okorie, P.; Fruekilde, S.; Fjelstrup, S.; Thomsen, J.; Franch, O.; Tesauro, C.; Bugge, M.; Christiansen, M. Refined method for droplet microfluidics-enabled detection of Plasmodium falciparum encoded topoisomerase i in blood from malaria patients. Micromachines 2015, 6, 1505–1513. [Google Scholar] [CrossRef]
- Weng, C.-H.; Huang, C.-J.; Lee, G.-B. Screening of Aptamers on Microfluidic Systems for Clinical Applications. Sensors 2012, 12, 9514–9529. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Li, L.-J. Label-free electrical detection of DNA hybridization using carbon nanotubes and graphene. Nano Rev. 2010, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Millipore Milli-Q® Element System Water Quality Assessment. Available online: http://www.johnmorris.com.au/files/files/Merck_Millipore/MilliQ_Element_System_Water_Quality_Assessment.pdf (accessed on 19 September 2016).
- Oehme, M.; Lund, W. The purification of water for inorganic ultratrace analysis. Talanta 1980, 27, 223–225. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fjelstrup, S.; Andersen, M.B.; Thomsen, J.; Wang, J.; Stougaard, M.; Pedersen, F.S.; Ho, Y.-P.; Hede, M.S.; Knudsen, B.R. The Effects of Dithiothreitol on DNA. Sensors 2017, 17, 1201. https://doi.org/10.3390/s17061201
Fjelstrup S, Andersen MB, Thomsen J, Wang J, Stougaard M, Pedersen FS, Ho Y-P, Hede MS, Knudsen BR. The Effects of Dithiothreitol on DNA. Sensors. 2017; 17(6):1201. https://doi.org/10.3390/s17061201
Chicago/Turabian StyleFjelstrup, Søren, Marie Bech Andersen, Jonas Thomsen, Jing Wang, Magnus Stougaard, Finn Skou Pedersen, Yi-Ping Ho, Marianne Smedegaard Hede, and Birgitta Ruth Knudsen. 2017. "The Effects of Dithiothreitol on DNA" Sensors 17, no. 6: 1201. https://doi.org/10.3390/s17061201
APA StyleFjelstrup, S., Andersen, M. B., Thomsen, J., Wang, J., Stougaard, M., Pedersen, F. S., Ho, Y.-P., Hede, M. S., & Knudsen, B. R. (2017). The Effects of Dithiothreitol on DNA. Sensors, 17(6), 1201. https://doi.org/10.3390/s17061201




