A Versatile Electronic Tongue Based on Surface Plasmon Resonance Imaging and Cross-Reactive Sensor Arrays—A Mini-Review
Abstract
:1. Introduction
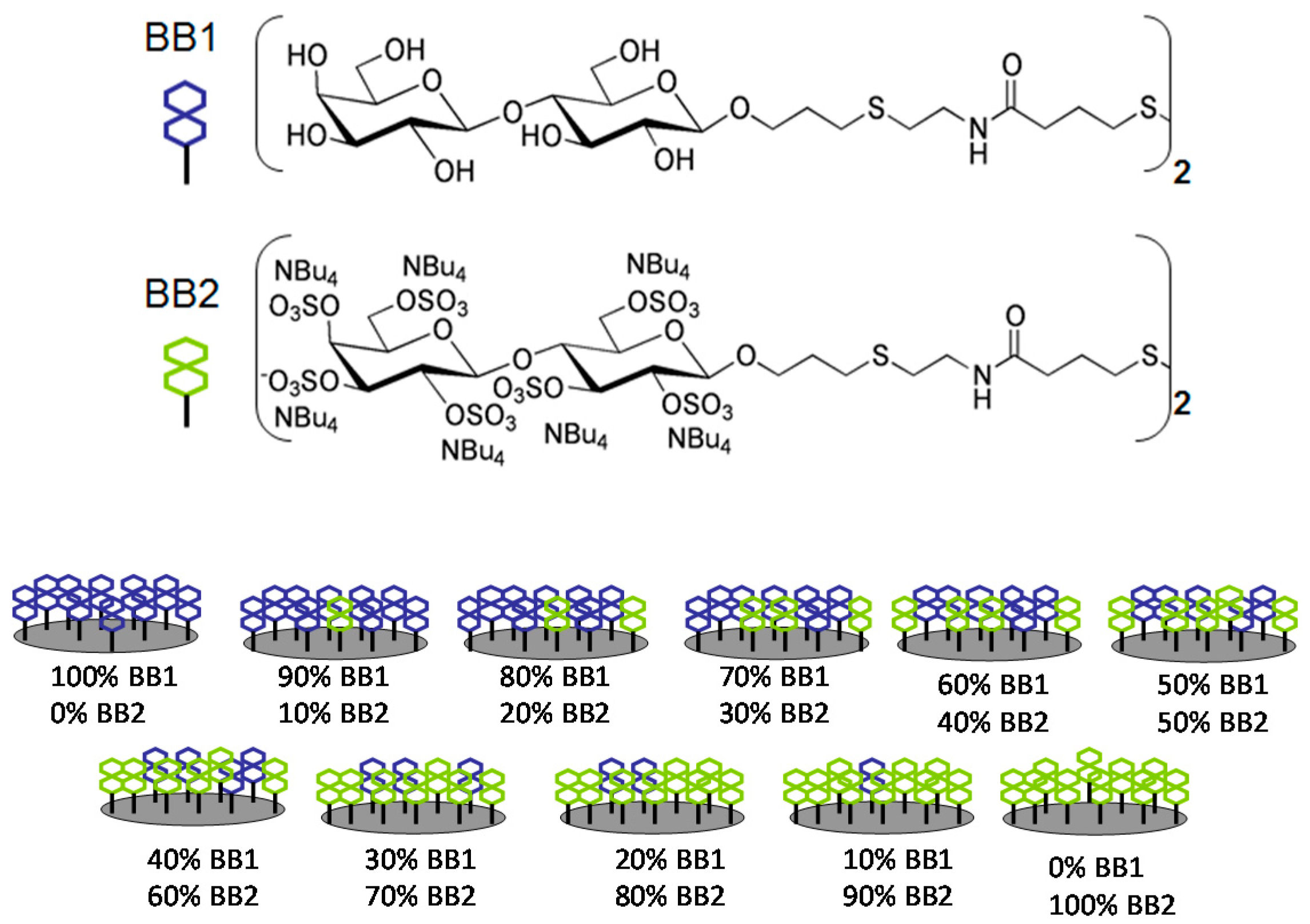
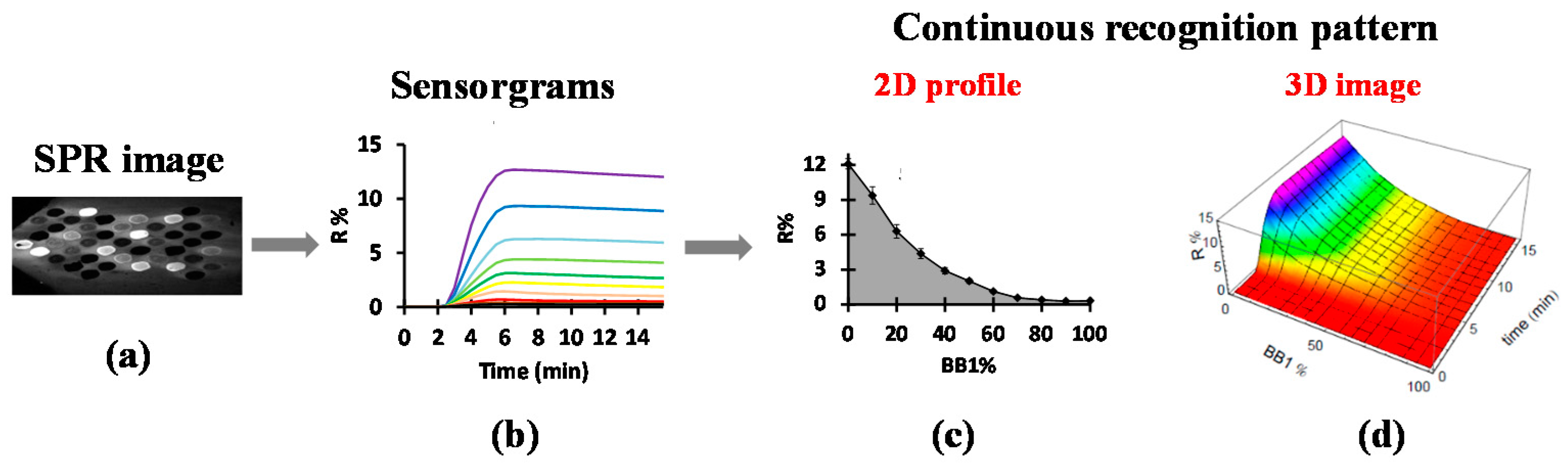
2. Electronic Tongue Based on SPRi and Cross-Reactive Sensor Array
3. Diverse Applications of the eTs
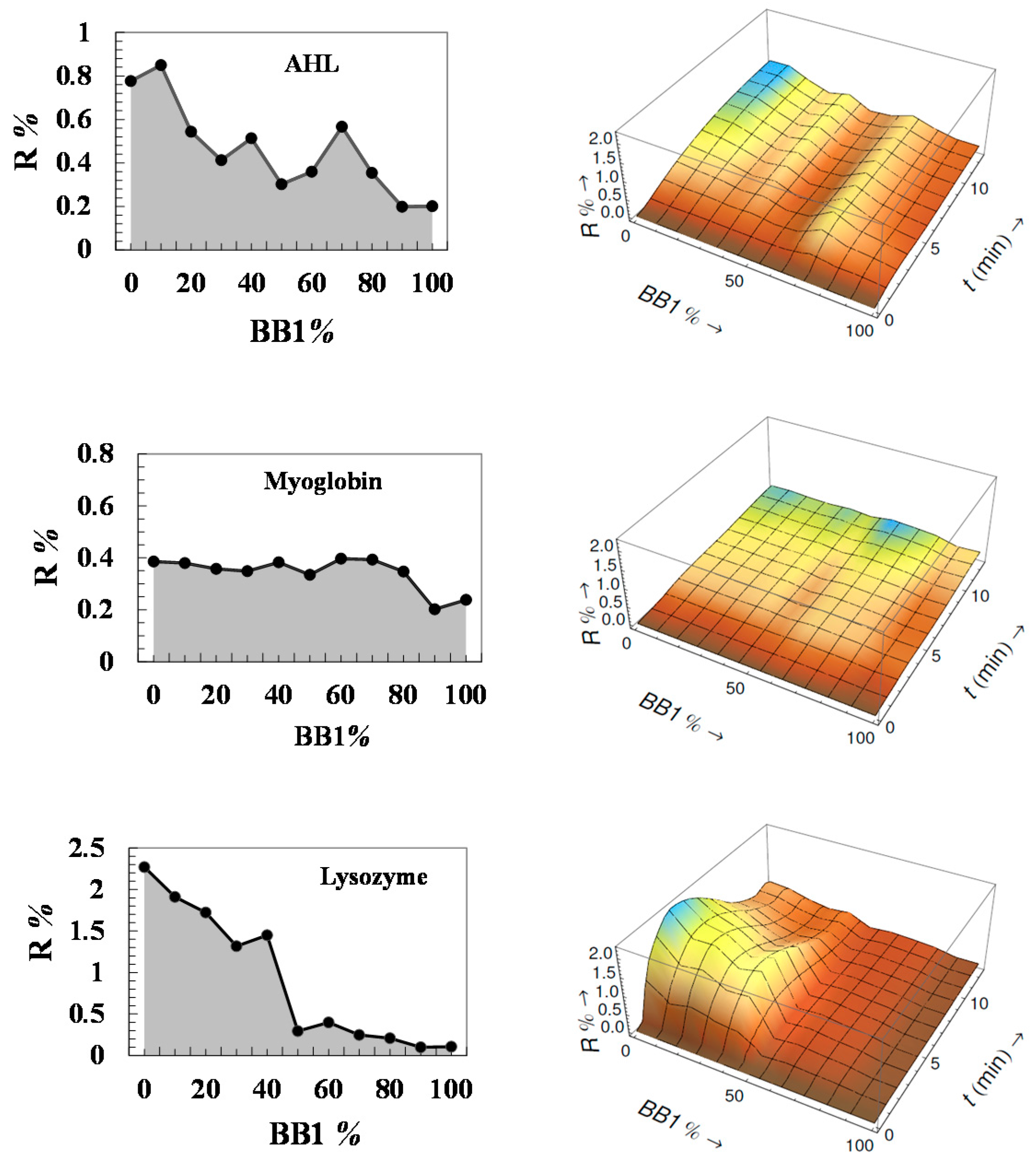
3.1. Analysis of Pure Proteins
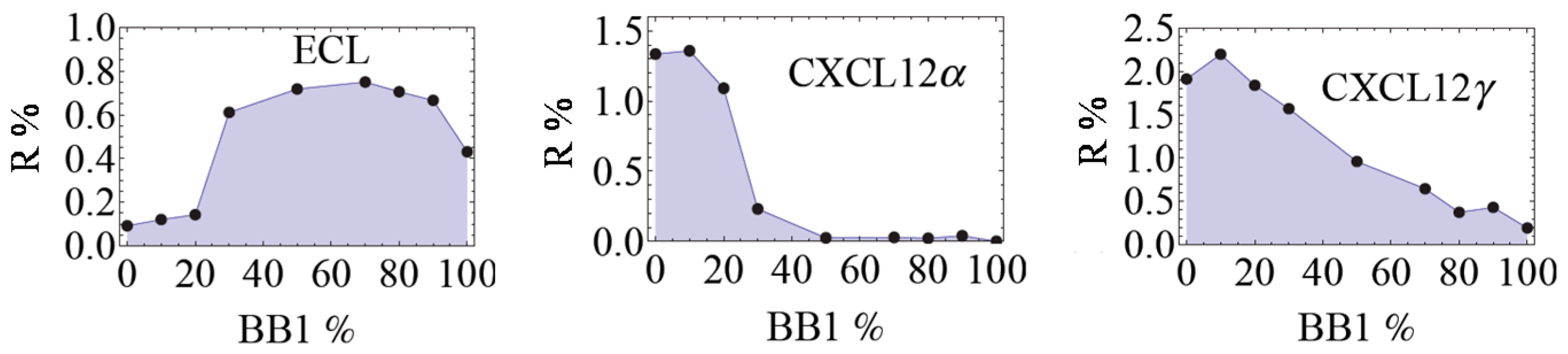
3.2. Analysis of Protein Mixtures
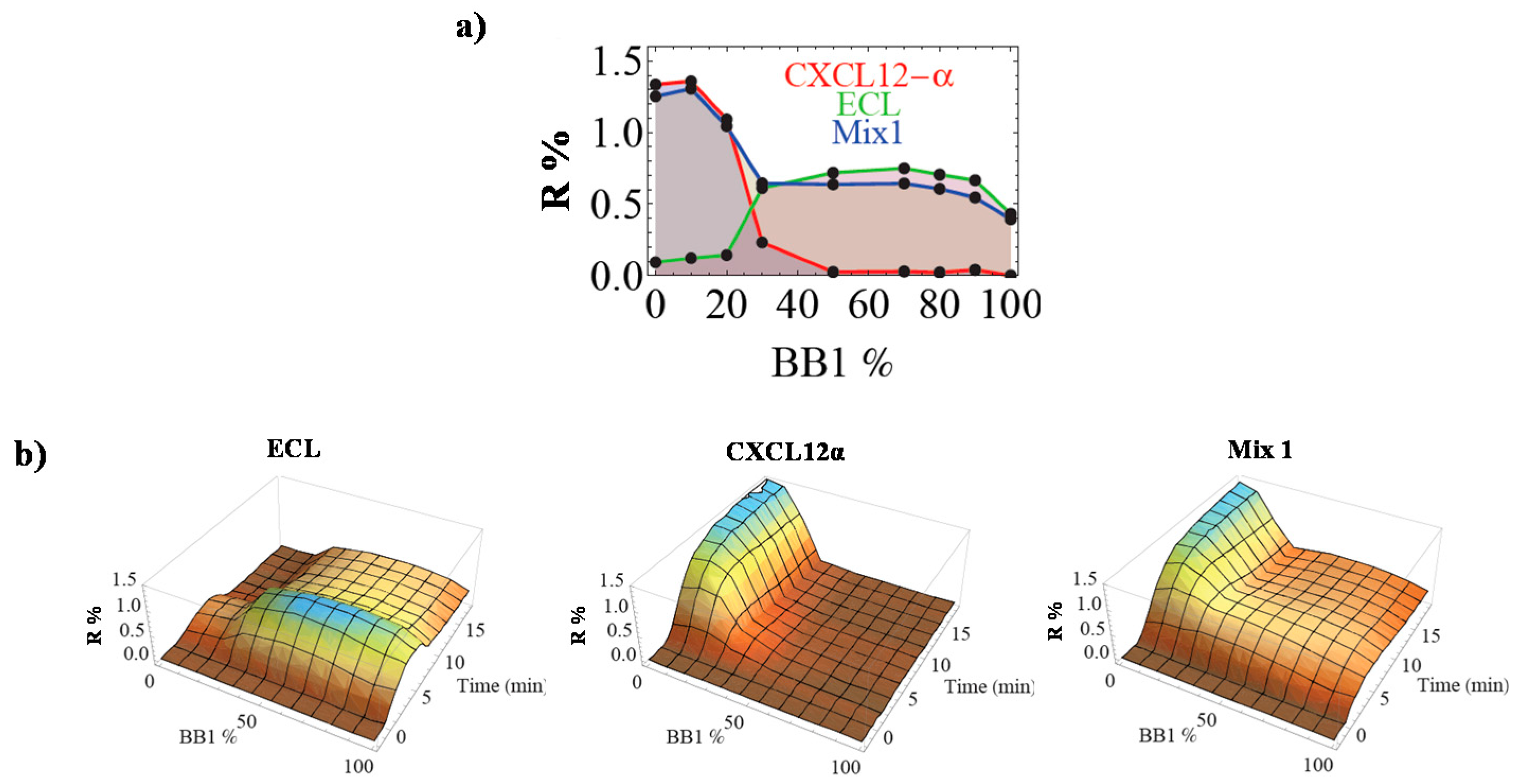
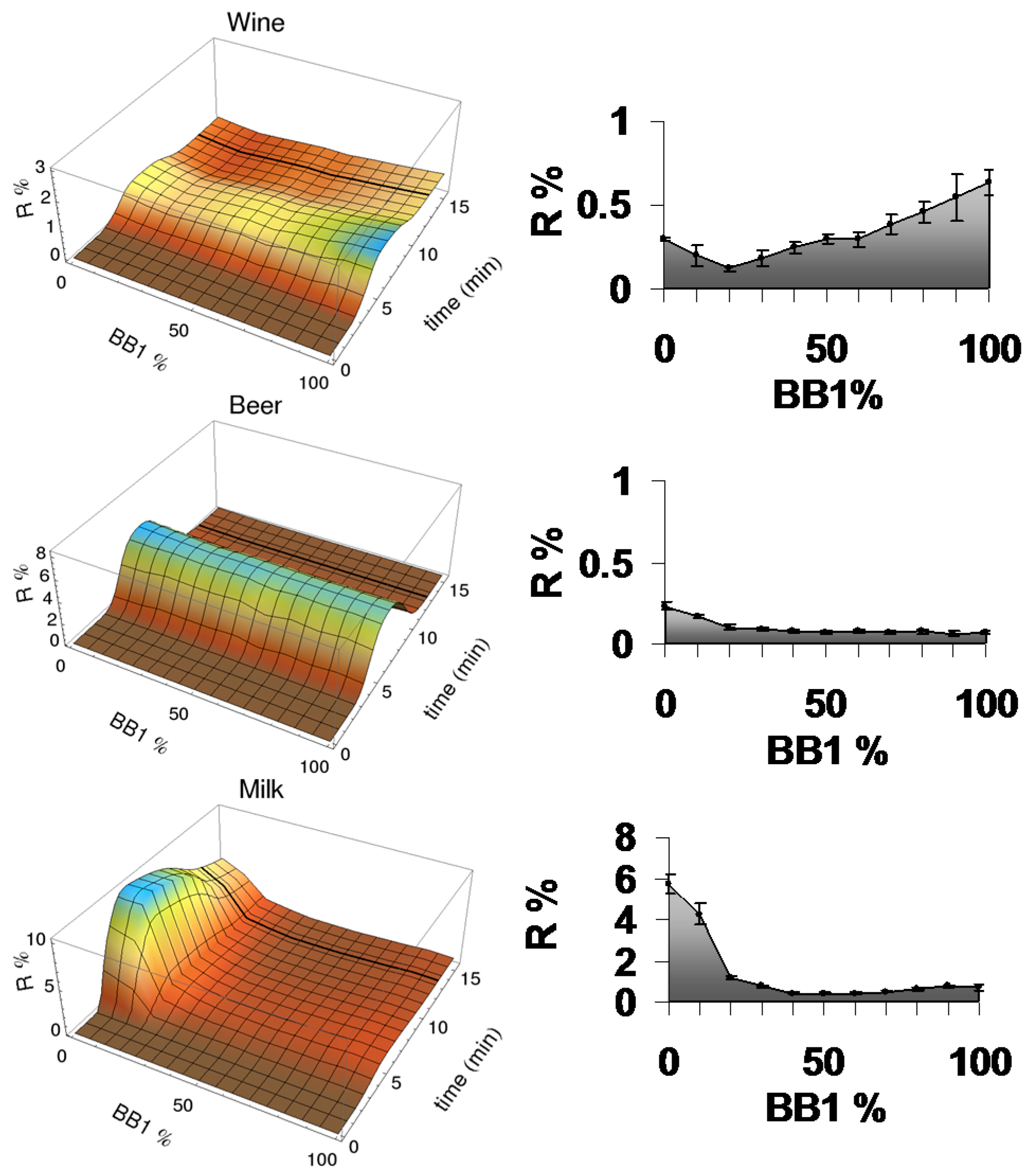
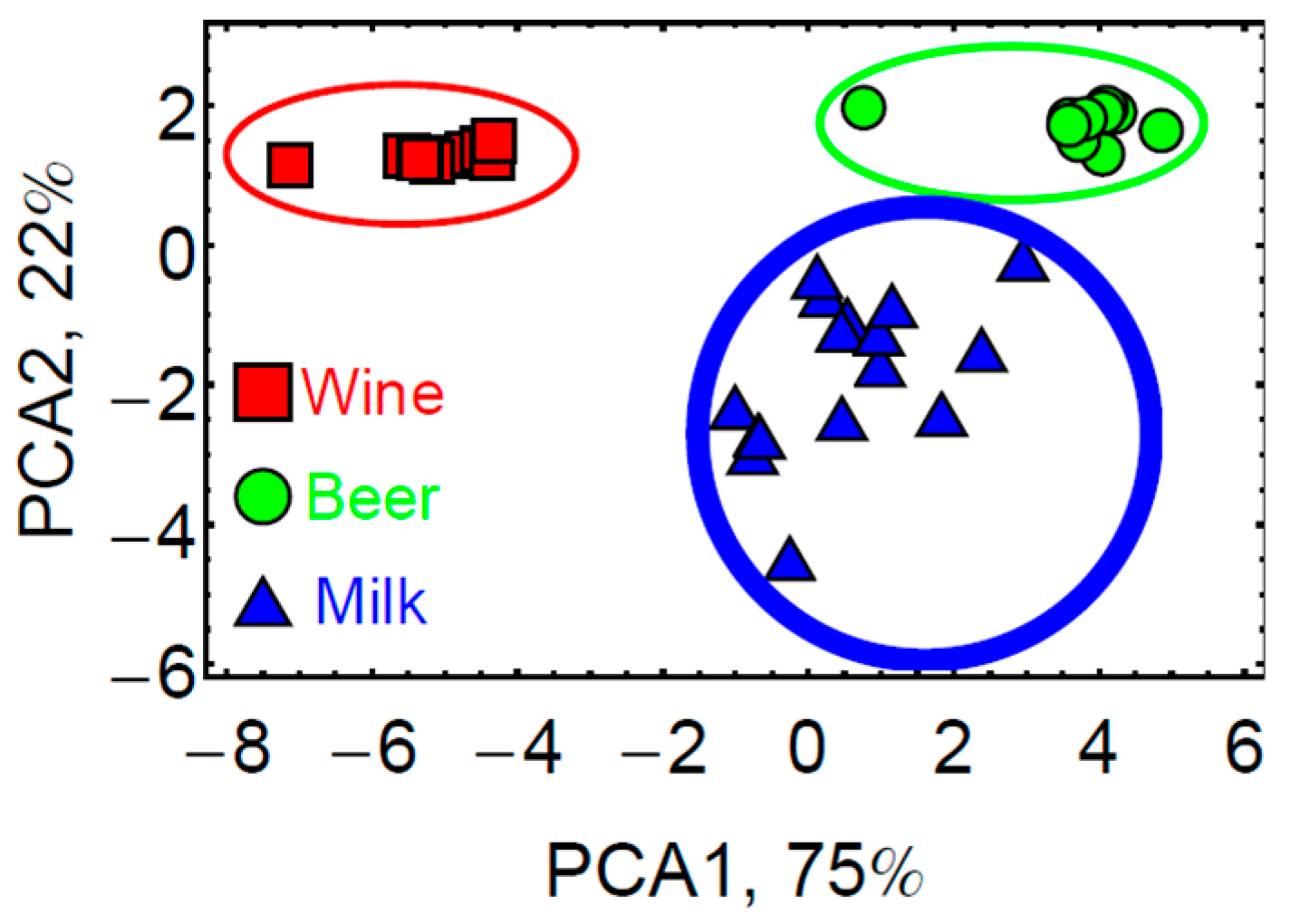
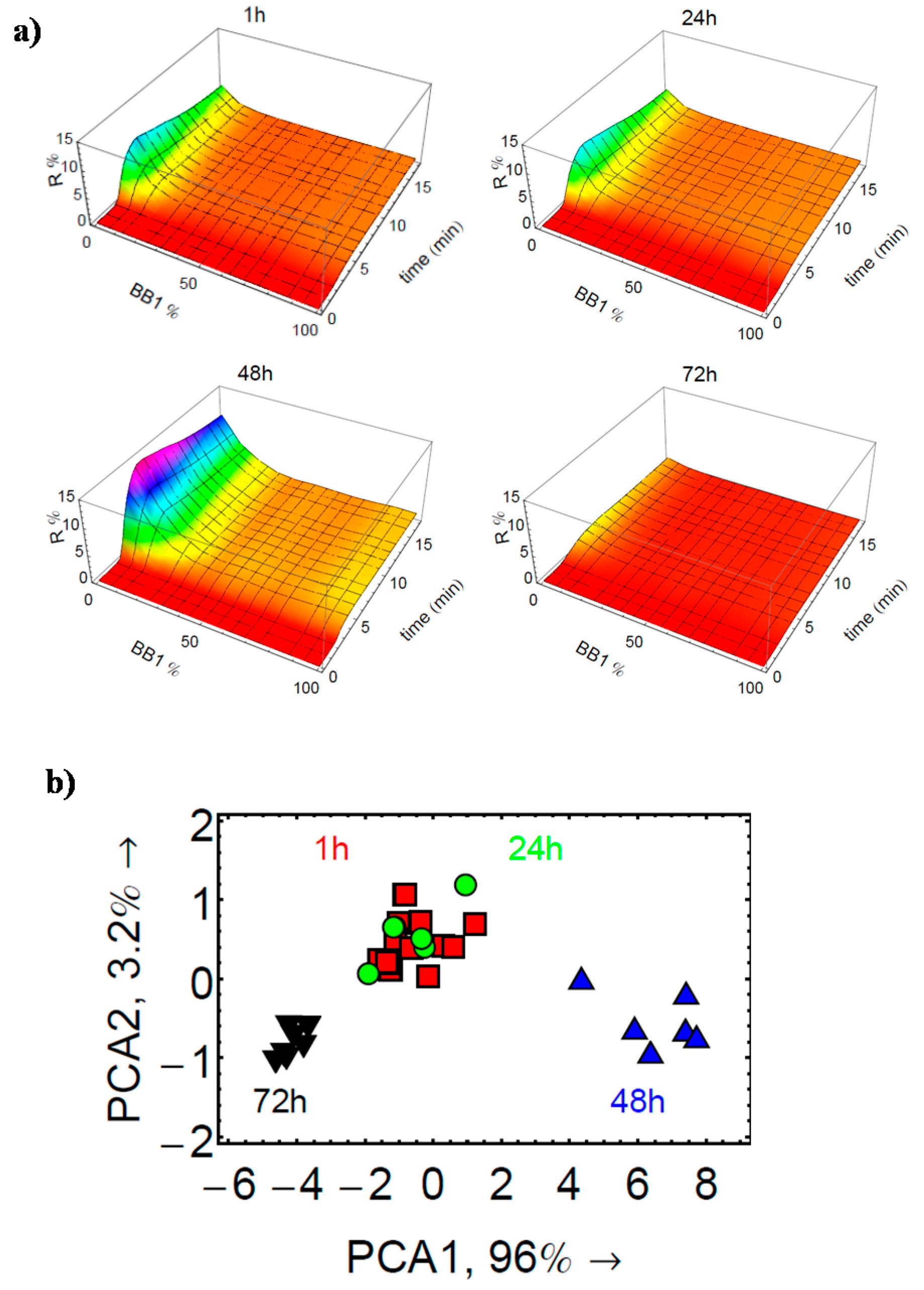
3.3. Analysis of Complex Mixtures
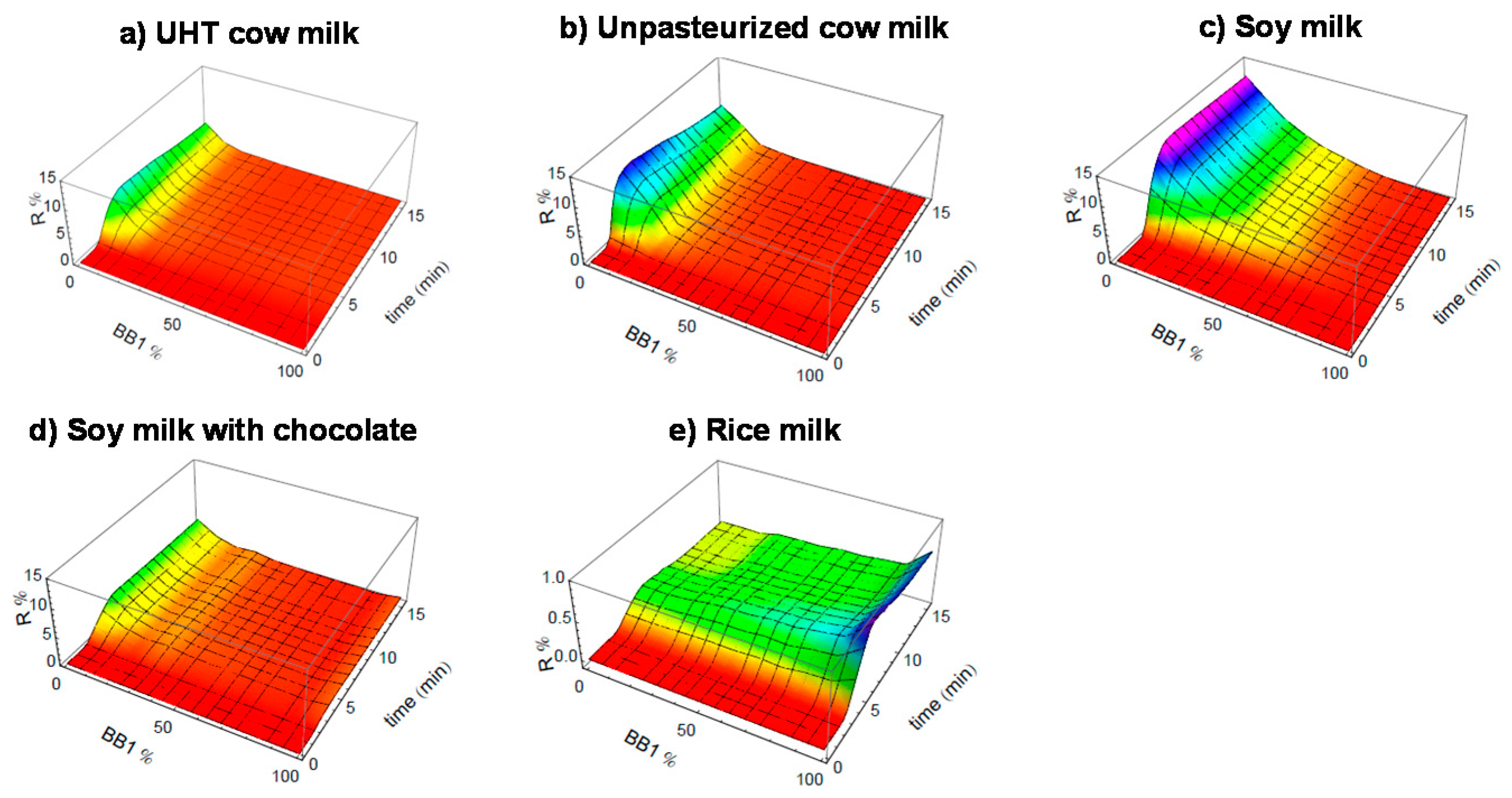
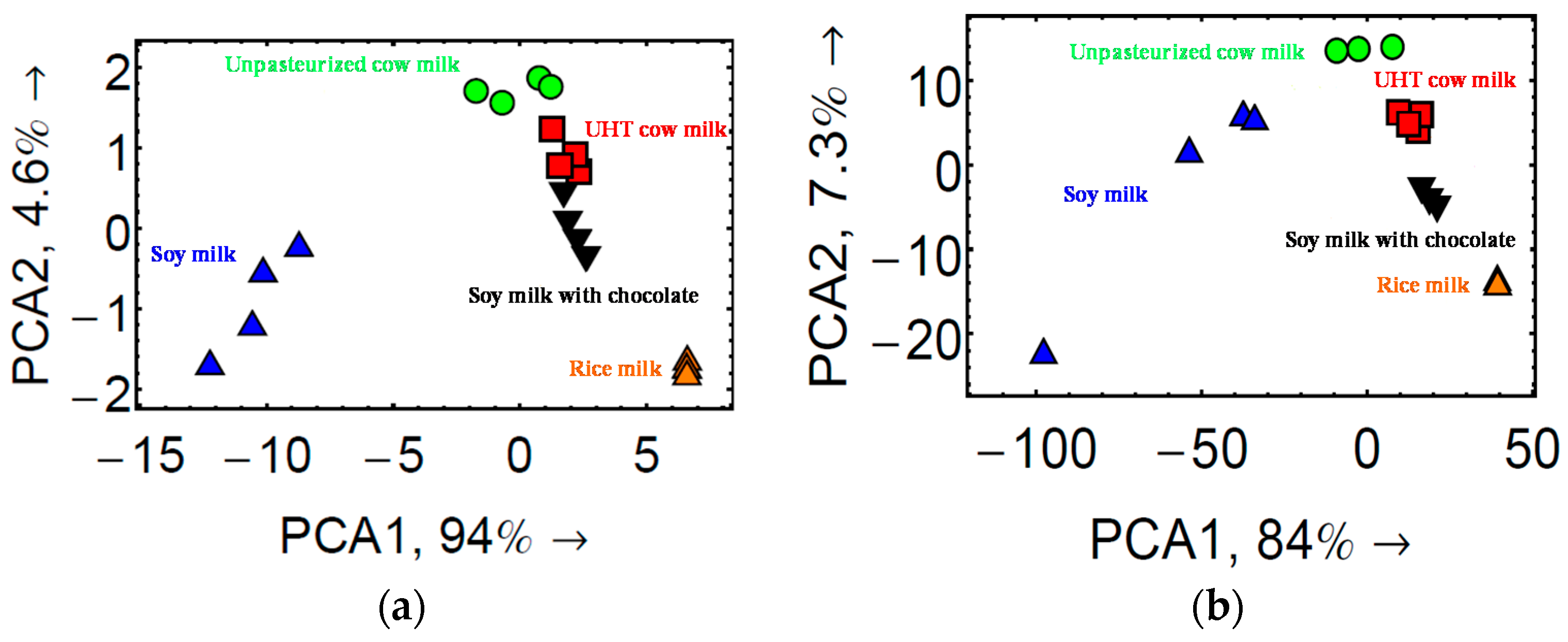
3.4. Monitoring Spoilage of Milk
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lvova, L.; D’Amico, A.; Pede, A.; Di Natale, C.; Paolesse, R. Metallic Sensors in Multisensor Analysis. In Multisensor Systems for Chemical Analysis: Materials and Sensors; Lvova, L., Kirsanov, D., Di Natale, C., Legin, A., Eds.; Pan Stanford Publishing Pte. Ltd.: Singapore, 2014; pp. 69–138. [Google Scholar]
- Del Valle, M. Electronic tongues employing electrochemical sensors. Electroanal 2010, 22, 1539–1555. [Google Scholar] [CrossRef]
- Ciosek, P.; Wroblewski, W. Potentiometric electronic tongues for foodstuff and biosample recognition—An overview. Sensors 2011, 11, 4688–4701. [Google Scholar] [CrossRef] [PubMed]
- Escuder-Gilabert, L.; Peris, M. Review: Highlights in recent applications of electronic tongues in food analysis. Anal. Chim. Acta 2010, 665, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Winquist, F. Voltammetric electronic tongues—Basic principles and applications. Microchim. Acta 2008, 163, 3–10. [Google Scholar] [CrossRef]
- Scampicchio, M.; Ballabio, D.; Arecchi, A.; Cosio, S.M.; Mannino, S. Amperometric electronic tongue for food analysis. Microchim. Acta 2008, 163, 11–21. [Google Scholar] [CrossRef]
- Pioggia, G.; Di Francesco, F.; Marchetti, A.; Ferro, A.; Leardi, R.; Ahluwalia, A. A composite sensor array impedentiometric electronic tongue Part II. Discrimination of basic tastes. Biosens. Bioelectron. 2007, 22, 2624–2628. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.X.; Jaffrezic-Renault, N.; Martelet, C.; Zhang, A.D.; Minic-Vidic, J.; Gorojankina, T.; Persuy, M.A.; Pajot-Augy, E.; Salesse, R.; Akimov, V.; et al. A novel detection strategy for odorant molecules based on controlled bioengineering of rat olfactory receptor 17. Biosens. Bioelectron. 2007, 22, 1550–1555. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.X.; Jaffrezic-Renault, N.; Martelet, C.; Tlili, C.; Zhang, A.; Pernollet, J.C.; Briand, L.; Gomila, G.; Errachid, A.; Samitier, J.; et al. Study of Langmuir and Langmuir-Blodgett films of odorant-binding protein/amphiphile for odorant biosensors. Langmuir 2005, 21, 4058–4065. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Méndez, M.L.; Preedy, V.R. Electronic Noses and Tongues in Food Science; Academic Press: London, UK, 2016; pp. 1–309. [Google Scholar]
- Baldwin, E.A.; Bai, J.H.; Plotto, A.; Dea, S. Electronic noses and tongues: Applications for the food and pharmaceutical industries. Sensors 2011, 11, 4744–4766. [Google Scholar] [CrossRef] [PubMed]
- Smyth, H.; Cozzoino, D. Instrumental methods (spectroscopy, electronic nose, and tongue) as tools to predict taste and aroma in beverages: advantages and limitations. Chem. Rev. 2013, 113, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Wadehra, A.; Patil, P.S. Application of electronic tongues in food processing. Anal. Methods UK 2016, 8, 474–480. [Google Scholar] [CrossRef]
- Tuantranont, A.; Wisitsora-at, A.; Sritongkham, P.; Jaruwongrungsee, K. A review of monolithic multichannel quartz crystal microbalance: A review. Anal. Chim. Acta 2011, 687, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.X.; Huang, Y.; Mason, A.; Zeng, X.Q. Multichannel monolithic quartz crystal microbalance gas sensor array. Anal. Chem. 2009, 81, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Leonte, I.I.; Sehra, G.; Cole, M.; Hesketh, P.; Gardner, J.W. Taste sensors utilizing high-frequency SH-SAW devices. Sens. Actuator B Chem. 2006, 118, 349–355. [Google Scholar] [CrossRef]
- Sehra, G.; Cole, M.; Gardner, J.W. Miniature taste sensing system based on dual SH-SAW sensor device: An electronic tongue. Sens. Actuator B Chem. 2004, 103, 233–239. [Google Scholar] [CrossRef]
- Cole, M.; Sehra, G.; Gardner, J.W.; Varadan, V.K. Development of smart tongue devices for measurement of liquid properties. IEEE Sens. J. 2004, 4, 543–550. [Google Scholar] [CrossRef]
- Zhang, C.; Suslick, K.S. Colorimetric sensor array for soft drink analysis. J. Agric. Food Chem. 2007, 55, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Bailey, D.P.; Suslick, K.S. Colorimetric sensor arrays for the analysis of beers: A feasibility study. J. Agric. Food Chem. 2006, 54, 4925–4931. [Google Scholar] [CrossRef] [PubMed]
- Suslick, B.A.; Feng, L.; Suslick, K.S. Discrimination of Complex Mixtures by a Colorimetric Sensor Array: Coffee Aromas. Anal. Chem. 2010, 82, 2067–2073. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.T.; Griffin, M.J.; Zhong, Z.; McCleskey, S.C.; Anslyn, E.V.; McDevitt, J.T. Differential receptors create patterns that distinguish various proteins. Angew. Chem. Int. Ed. 2005, 44, 6375–6378. [Google Scholar] [CrossRef] [PubMed]
- Baldini, L.; Wilson, A.J.; Hong, J.; Hamilton, A.D. Pattern-based detection of different proteins using an array of fluorescent protein surface receptors. J. Am. Chem. Soc. 2004, 126, 5656–5657. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.C.; Baldini, L.; Hong, J.; Wilson, A.J.; Hamilton, A.D. Pattern recognition of proteins based on an array of functionalized porphyrins. J. Am. Chem. Soc. 2006, 128, 2421–2425. [Google Scholar] [CrossRef] [PubMed]
- De, M.; Rana, S.; Akpinar, H.; Miranda, O.R.; Arvizo, R.R.; Bunz, U.H.F.; Rotello, V.M. Sensing of proteins in human serum using conjugates of nanoparticles and green fluorescent protein. Nat. Chem. 2009, 1, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.X.; Genua, M.; Batista, D.T.; Calemczuk, R.; Buhot, A.; Fornarelli, P.; Koubachi, J.; Bonnaffe, D.; Saesen, E.; Laguri, C.; et al. Continuous Evolution Profiles for Electronic-Tongue-Based Analysis. Angew. Chem. Int. Edit 2012, 51, 10394–10398. [Google Scholar] [CrossRef] [PubMed]
- Scarano, S.; Mascini, M.; Turner, A.P.F.; Minunni, M. Surface plasmon resonance imaging for affinity-based biosensors. Biosens. Bioelectron. 2010, 25, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.T.; Kim, G. SPR microscopy and its applications to high-throughput analyses of biomolecular binding events and their kinetics. Biomaterials 2007, 28, 2380–2392. [Google Scholar] [CrossRef] [PubMed]
- Genua, M.; Garcon, L.A.; Mounier, V.; Wehry, H.; Buhot, A.; Billon, M.; Calemczuk, R.; Bonnaffe, D.; Hou, Y.X.; Livache, T. SPR imaging based electronic tongue via landscape images for complex mixture analysis. Talanta 2014, 130, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.X.; Genua, M.; Garcon, L.A.; Buhot, A.; Calemczuk, R.; Bonnaffe, D.; Lortat-Jacob, H.; Livache, T. Electronic Tongue Generating Continuous Recognition Patterns for Protein Analysis. JoVE J. Vis. Exp. 2014, 91, 51901. [Google Scholar] [CrossRef] [PubMed]
- Garçon, L.A.; Hou, Y.J.; Genua, M.; Buhot, A.; Calemczuk, R.; Bonnaffé, D.; Hou, Y.; Livache, T. Landscapes of taste by a novel electronic tongue for the analysis of complex mixtures. Sens. Lett. 2014, 12, 1059–1064. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garçon, L.-A.; Genua, M.; Hou, Y.; Buhot, A.; Calemczuk, R.; Livache, T.; Billon, M.; Le Narvor, C.; Bonnaffé, D.; Lortat-Jacob, H.; et al. A Versatile Electronic Tongue Based on Surface Plasmon Resonance Imaging and Cross-Reactive Sensor Arrays—A Mini-Review. Sensors 2017, 17, 1046. https://doi.org/10.3390/s17051046
Garçon L-A, Genua M, Hou Y, Buhot A, Calemczuk R, Livache T, Billon M, Le Narvor C, Bonnaffé D, Lortat-Jacob H, et al. A Versatile Electronic Tongue Based on Surface Plasmon Resonance Imaging and Cross-Reactive Sensor Arrays—A Mini-Review. Sensors. 2017; 17(5):1046. https://doi.org/10.3390/s17051046
Chicago/Turabian StyleGarçon, Laurie-Amandine, Maria Genua, Yanjie Hou, Arnaud Buhot, Roberto Calemczuk, Thierry Livache, Martial Billon, Christine Le Narvor, David Bonnaffé, Hugues Lortat-Jacob, and et al. 2017. "A Versatile Electronic Tongue Based on Surface Plasmon Resonance Imaging and Cross-Reactive Sensor Arrays—A Mini-Review" Sensors 17, no. 5: 1046. https://doi.org/10.3390/s17051046
APA StyleGarçon, L.-A., Genua, M., Hou, Y., Buhot, A., Calemczuk, R., Livache, T., Billon, M., Le Narvor, C., Bonnaffé, D., Lortat-Jacob, H., & Hou, Y. (2017). A Versatile Electronic Tongue Based on Surface Plasmon Resonance Imaging and Cross-Reactive Sensor Arrays—A Mini-Review. Sensors, 17(5), 1046. https://doi.org/10.3390/s17051046







