Easy-to-Fabricate and High-Sensitivity LSPR Type Specific Protein Detection Sensor Using AAO Nano-Pore Size Control
Abstract
:1. Introduction
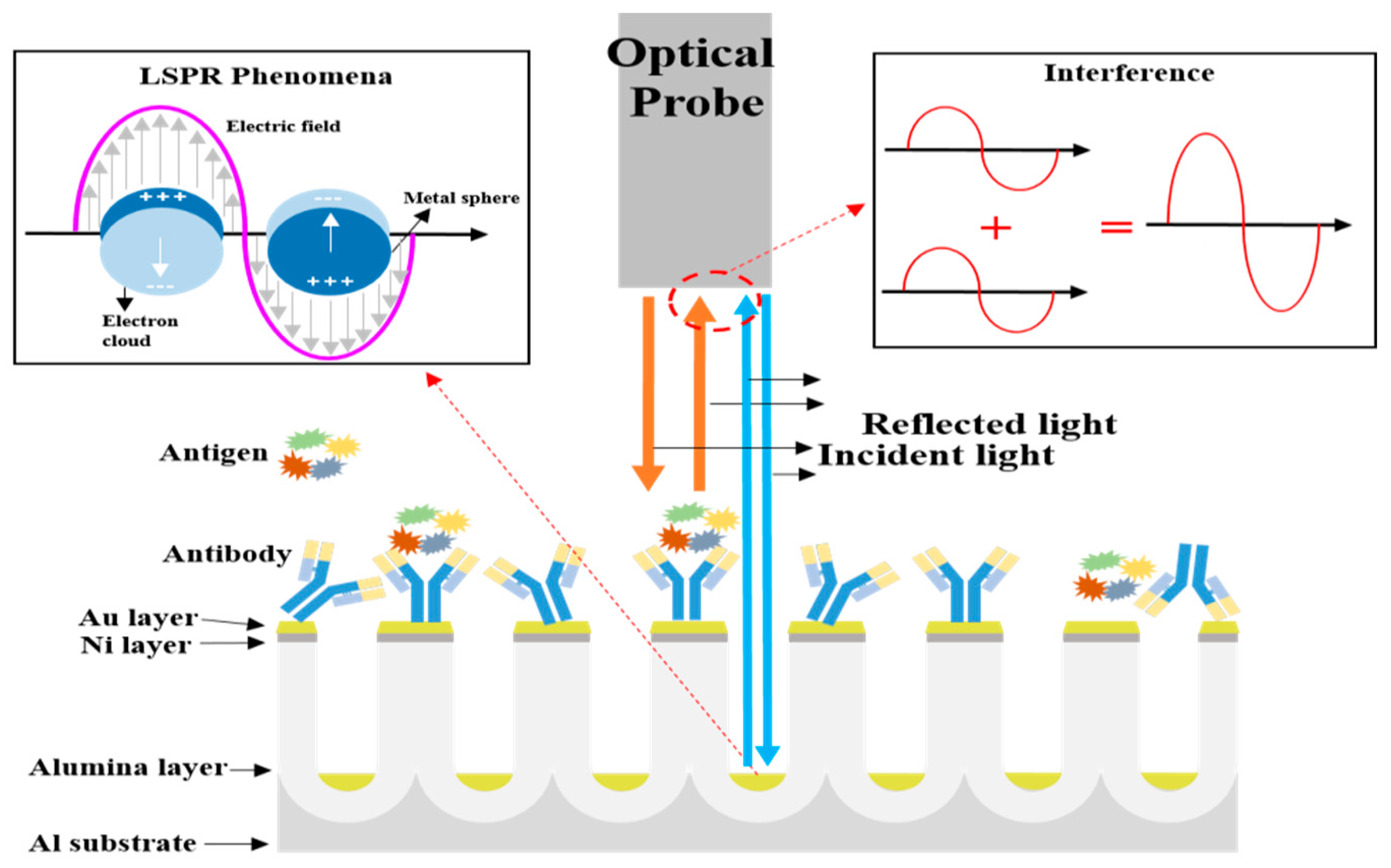
2. Principle of the Proposed Biosensor
3. Materials and Methods
3.1. Fabrication of the Surface-Area-Controlled AAO Nano-Structure
3.2. Sensing Membrane Preparation
3.3. Sensing System for the SAA1 Detection Setup
4. Results and Discussion
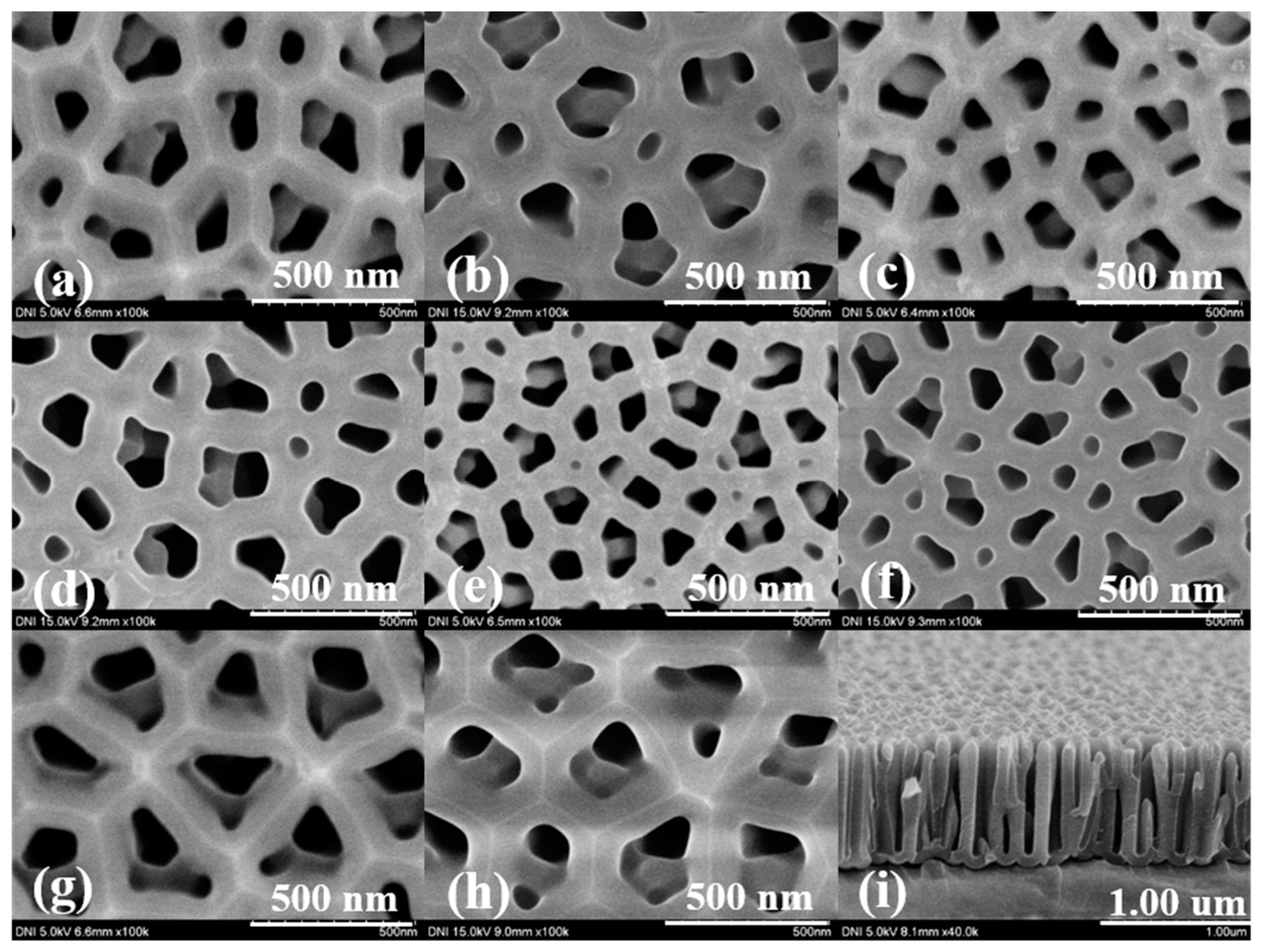
4.1. Surface Characteristics of the Fabricated AAO Chip
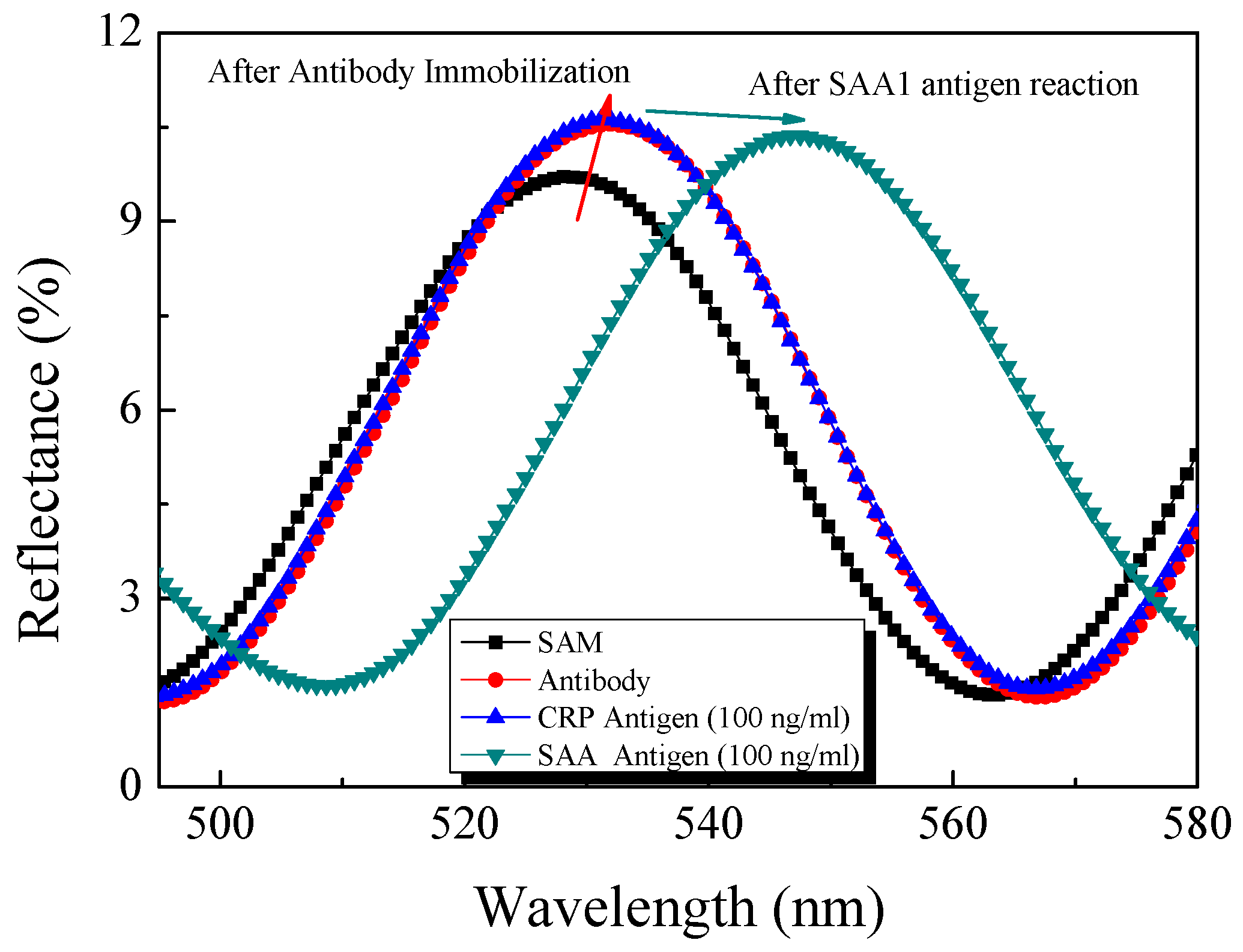
4.2. Optical Properties of the Fabricated AAO Chip
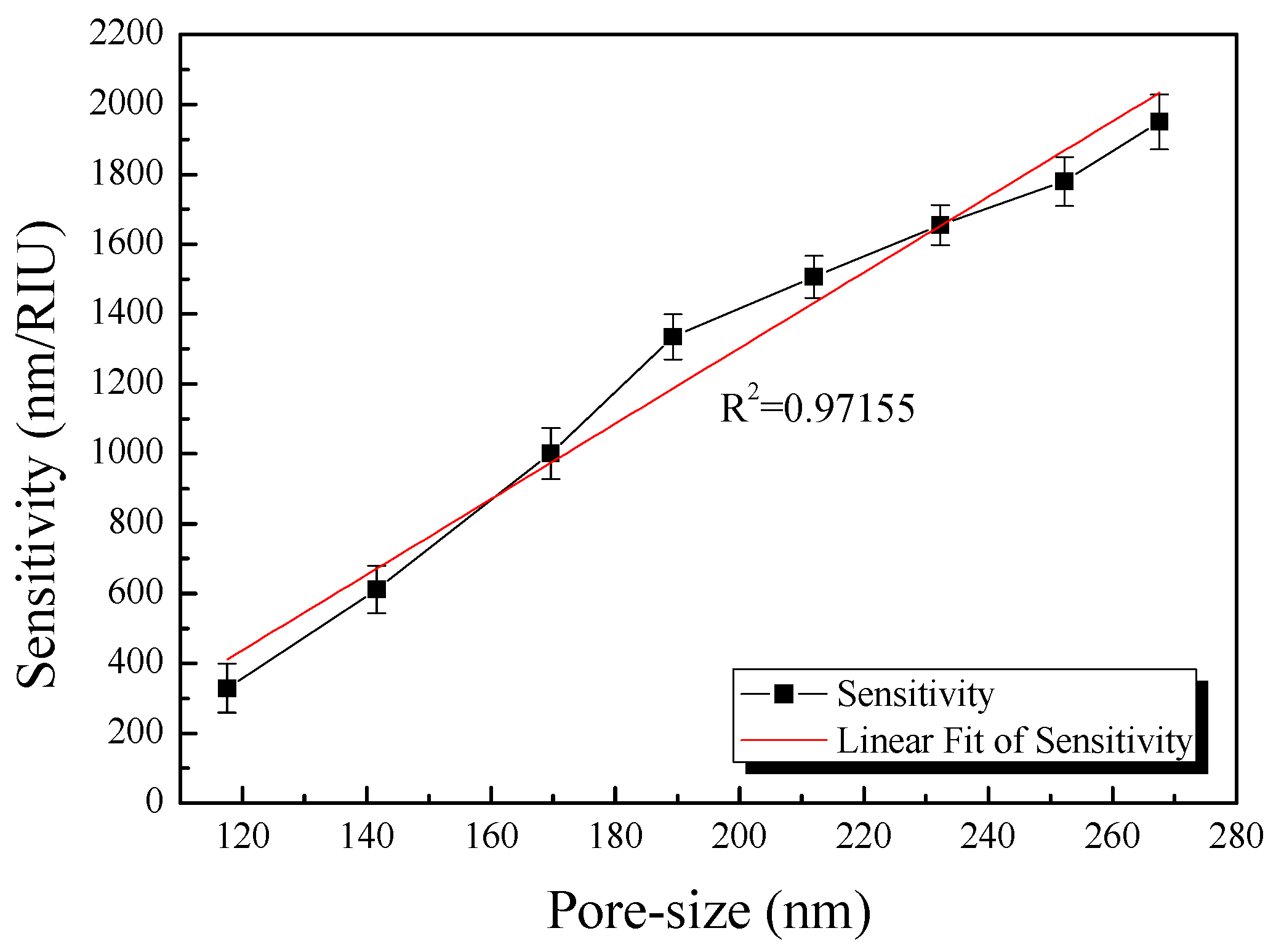
4.3. Optimization of the AAO Fabrication Conditions
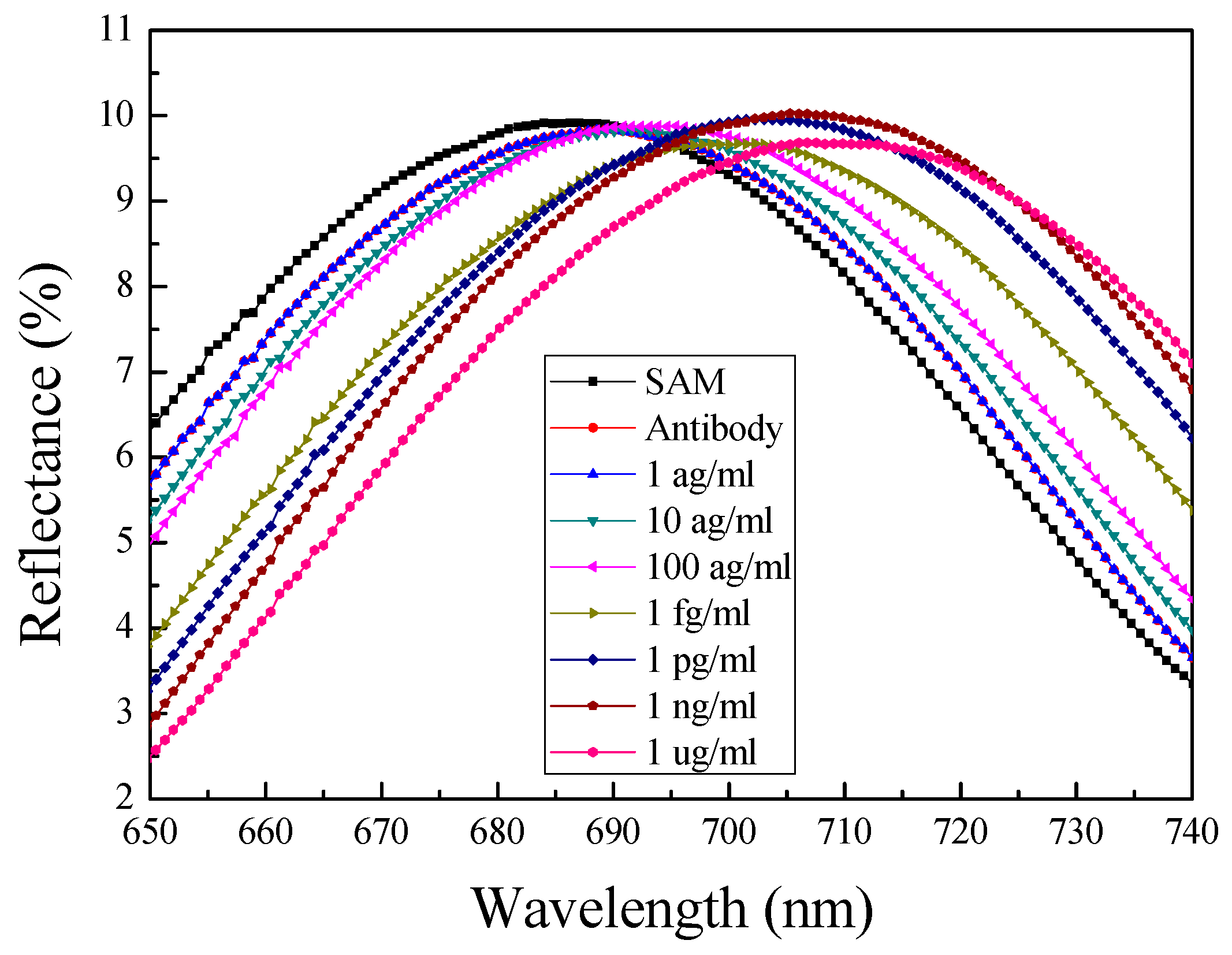
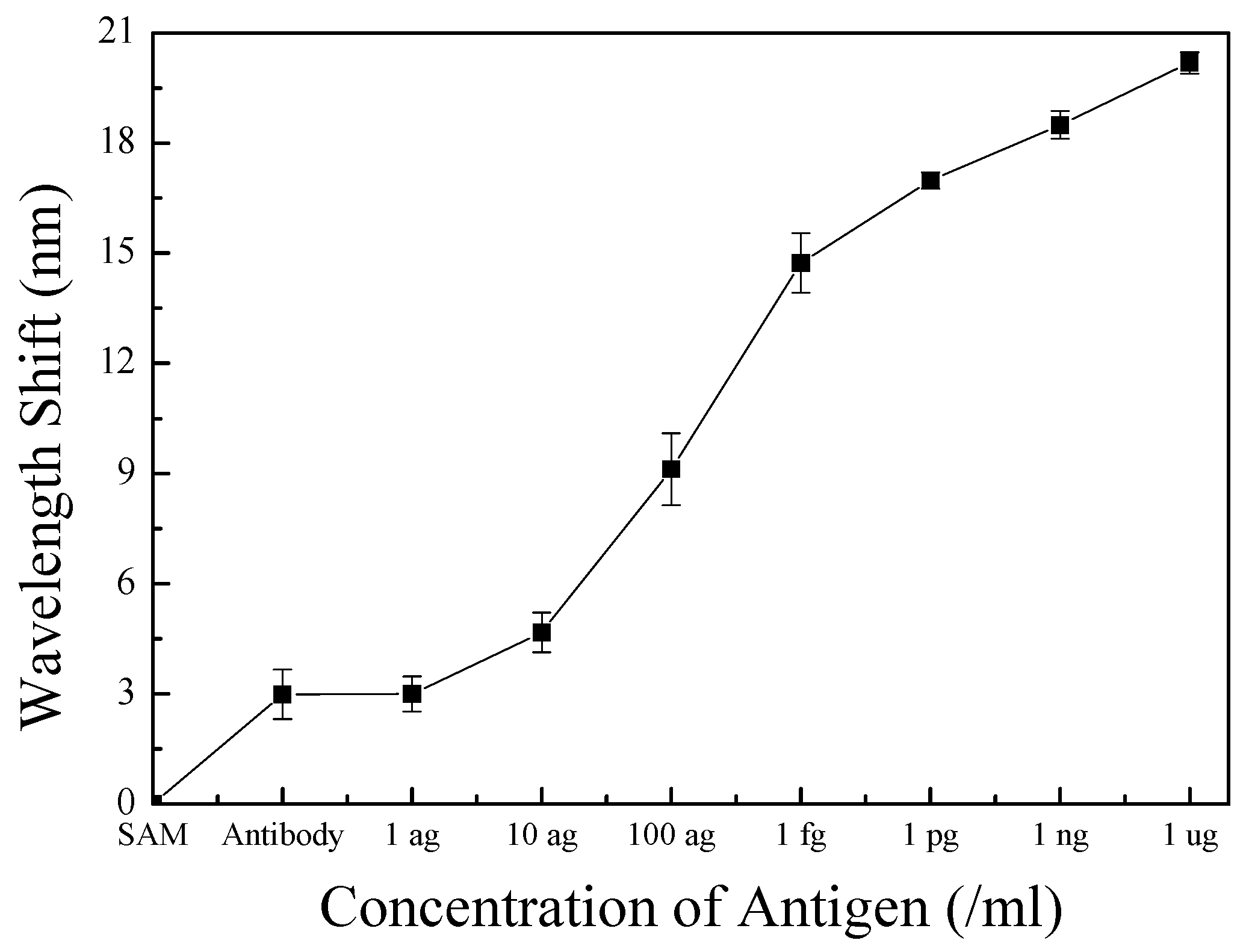
4.4. Response Properties of the Optical Biosensor
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hamadouche, M.; Boutin, P.; Daussange, J.; Bolander, M.E.; Sedel, L. Alumina-on-alumina total hip arthroplasty. J. Bone Jt. Surg. Am. 2002, 84, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.S.; Prinz, F.B.; Smith, R.L. Nanoscale Technology in Biological System; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Li, W.B.; Ren, K.N.; Zhou, J.H. Aluminum-based localized surface plasmon resonance for biosensing. TrAC Trends Anal. Chem. 2016, 80, 486–494. [Google Scholar] [CrossRef]
- Santos, A.; Kumeria, T.; Losic, D. Nanoporous anodic aluminum oxide for chemical sensing and biosensors. TrAC Trends Anal. Chem. 2013, 44, 25–38. [Google Scholar] [CrossRef]
- Chen, S.; Liu, L.; Zhou, J.; Jiang, J. Controlling antibody orientation on charged self-assembled monolayers. Langmuir 2003, 19, 2859–2864. [Google Scholar] [CrossRef]
- Brolo, A.G. Plasmonics for future biosensor. Nat. Photonics 2012, 6, 709–713. [Google Scholar] [CrossRef]
- Gao, T.; Lu, J.; Rothberg, L.J. Biomolecular sensing using near-null single wavelength arrayed imaging reflectometry. Anal. Chem. 2006, 78, 6622–6627. [Google Scholar] [CrossRef] [PubMed]
- Ligler, F.S.; Breimer, M.; Golden, J.P.; Nivens, D.A.; Dodson, J.P.; Green, T.M.; Haders, D.P.; Sadik, O.A. Integrating waveguide biosensor. Anal. Chem. 2002, 74, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Kwon, H.C.; Yeom, S.H.; Kwon, D.H.; Kang, S.W. MOSFET-BJT hybrid mode of the gated lateral bipolar junction transistor for C-reactive protein detection. Biosens. Bioelectron. 2011, 28, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Kang, B.H.; Lee, J.S.; Jeong, H.M.; Yeom, S.H.; Kim, K.J.; Kwon, D.H.; Kang, S.W. Sensitivity alterable biosensor based on gated lateral BJT for CRP detection. J. Semicond. Technol. Sci. 2013, 13, 1–7. [Google Scholar] [CrossRef]
- Knaack, S.A.; Eddington, J.; Leonard, Q.; Cerrina, F.; Onellion, M. Dense arrays of nanopores as X-rays lithography masks. Appl. Phys. Lett. 2004, 84, 3388–3390. [Google Scholar] [CrossRef]
- Elnathan, R.; Kwiat, M.; Pevzner, A.; Engel, Y.; Burstein, L.; Khatchtourints, A.; Lichtenstein, A.; Kantaev, R.; Patolsky, F. Biorecognition layer engineering: Overcoming screening limitation of nanowire-based FET devices. Nano Lett. 2012, 12, 5245–5254. [Google Scholar] [CrossRef] [PubMed]
- Azahar, A.; Saurabh, S.; Pratima, R.S.; Venu, R.; Ved, V.A.; Kim, C.G.; Renu, J.; Ransi, D.M. Highly efficient bienzyne functionalized nanocomposite-based microfluidics biosensor platform for biomedical application. Sci. Rep. 2013, 3, 1–9. [Google Scholar]
- Yeom, S.H.; Kim, O.G.; Kang, B.H.; Kim, K.J.; Yuan, H.; Kwon, D.H.; Kim, H.R.; Kang, S.W. Highly sensitive nano-porous lattice biosensor based on localized surface plasmon resonance and interference. Opt. Express 2011, 19, 22882–22891. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Kerman, K.; Saito, M.; Sathuluri, R.R.; Endo, T.; Yamamura, S.; Kwon, Y.S.; Tamiya, E. Label-free DNA biosensor based on localized surface plasmon resonance coupled with interferometry. Anal. Chem. 2007, 79, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- LEE, W.; Ji, R.; Gosele, U.; Nielsch, K. Fast fabrication of long-range ordered porous alumina membranes by hard anodization. Nat. Mater. 2006, 5, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T. Serum amyloid A (SAA): A concise review of biology, assay methods and clinical usefulness. Clin. Chem. Lab. Med. 1999, 37, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Howard, B.A.; Wang, M.Z.; Campa, M.J.; Corro, C.; Fitzgerald, M.C.; Patz, E.F. Identification and validation of a potential cancer serum biomarker detected by matrix-assisted laser desorption/ionization-time of flight spectra analysis. Proteomics 2003, 3, 1720–1724. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C.; Chen, C.J.; Chu, H.C.; Chang, W.K.; Yu, J.C.; Chen, Y.J.; Wen, L.L.; Huang, S.C.; Ku, C.H.; Liu, Y.C.; et al. Evaluation of serum amyloid A as ab for gastric cancer. Ann. Surg. Oncol. 2006, 14, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Willets, K.A.; Van Duyne, R.P. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B Condens. Phase 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Miller, M.M.; Lazarides, A.A. Sensitivity of metal nanoparticle surface plasmon resonance to the dielectric environment. J. Phys. Chem. B 2005, 109, 21556–21565. [Google Scholar] [CrossRef] [PubMed]
- Rossi, B.B. Optics; Addison Wesley: Boston, MA, USA, 1957. [Google Scholar]
- Huang, K.; Li, Y.; Wu, Z.; Li, C.; Lai, H.; Kang, J.Y. Asymmetric light reflectance effect in AAO on glass. Opt. Express 2011, 19, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Whitney, A.V.; Elam, J.W.; Zou, S.; Zinovev, A.V.; Stair, P.C.; Schatz, G.C.; Van Duyne, R.P. Localized surface plasmon resonance nanosensor: A high-resolution distance-dependence study using atomic layer deposition. J. Phys. Chem. B 2005, 109, 20522–20528. [Google Scholar] [CrossRef] [PubMed]
- Parkhutik, V.P.; Shershulsky, V.I. Theoretical modeling of porous oxide growth on aluminium. J. Phys. D Appl. Phys. 1992, 25, 1258–1263. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structure of anodic alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, B.; Leong, E.S.P.; Yang, P.; Zong, Y.; Si, G.; Teng, J.; Maier, S.A. Enhanced surface plasmon resonance on a smooth silver film with a seed growth layer. ACS Nano 2010, 4, 3139–3146. [Google Scholar] [CrossRef] [PubMed]
- Chaki, N.K.; Aslam, M.; Sharma, J.; Vijayamohanan, K. Applications of self-assembled monolayers in materials chemistry. J. Chem. Sci. 2001, 113, 659–670. [Google Scholar] [CrossRef]
- Onuma, K.; Oyane, A.; Kokubo, T.; Treboux, G.; Kanzaki, N.; Ito, A. Nucleation of calcium phosphate on 11- mercaptoundecanoic acid self-assembled monolayer in a pseudophysiological solution. J. Phys. Chem. B 2000, 104, 11950–11956. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.J. Porous Aanodic aluminum oxide: Anodization and templated Ssynthesis of functional nanostructure. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef] [PubMed]
- Glantz, S.A.; Slinker, B.K. Primer of Applied Regression and Analysis of Variance; McGraw-Hill: New York, NY, USA, 1990. [Google Scholar]
- Draper, N.R. Applied Regression Analysis; Wiley-Interscience: Hoboken, NJ, USA, 1998. [Google Scholar]
- Bence, N.F.; Sampat, R.M.; Kopito, R.R. Impairment of the ubiquitin-proteasome system by protein aggregation. Science 2001, 292, 1552–1555. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Cabrera-Abreu, J.C.; Davies, P.; Matek, Z.; Murphy, M.S. Performance of blood tests in diagnosis of inflammatory bowel disease in a specialist clinic. Arch. Dis. Child. 2004, 89, 69–71. [Google Scholar] [PubMed]


| Applied Voltage (V) | 2nd Anodizing Time (min) |
|---|---|
| 60 | 25 |
| 70 | 20 |
| 80 | 18 |
| 90 | 15 |
| 100 | 11 |
| 110 | 8.5 |
| 120 | 6 |
| 130 | 4 |
| Applied Voltage (V) | Pore Size (nm) | Percentage of Pore Area (%) | Percentage of Non-Pore Area (%) |
|---|---|---|---|
| 60 | 117 ± 2.967 | 30.98 | 69.02 ± 0.545 |
| 70 | 141 ± 2.521 | 27.78 | 72.22 ± 0.671 |
| 80 | 169 ± 2.942 | 25.62 | 74.38 ± 0.367 |
| 90 | 189 ± 3.247 | 21.60 | 78.40 ± 0.841 |
| 100 | 212 ± 3.156 | 19.61 | 80.39 ± 0.541 |
| 110 | 232 ± 2.782 | 17.16 | 82.84 ± 0.412 |
| 120 | 252 ± 2.897 | 13.67 | 86.33 ± 0.352 |
| 130 | 267 ± 2.564 | 11.44 | 88.56 ± 0.415 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-W.; Lee, J.-S.; Lee, S.-W.; Kang, B.-H.; Kwon, J.-B.; Kim, O.-S.; Kim, J.-S.; Kim, E.-S.; Kwon, D.-H.; Kang, S.-W. Easy-to-Fabricate and High-Sensitivity LSPR Type Specific Protein Detection Sensor Using AAO Nano-Pore Size Control. Sensors 2017, 17, 856. https://doi.org/10.3390/s17040856
Kim S-W, Lee J-S, Lee S-W, Kang B-H, Kwon J-B, Kim O-S, Kim J-S, Kim E-S, Kwon D-H, Kang S-W. Easy-to-Fabricate and High-Sensitivity LSPR Type Specific Protein Detection Sensor Using AAO Nano-Pore Size Control. Sensors. 2017; 17(4):856. https://doi.org/10.3390/s17040856
Chicago/Turabian StyleKim, Sae-Wan, Jae-Sung Lee, Sang-Won Lee, Byoung-Ho Kang, Jin-Beom Kwon, Ok-Sik Kim, Ju-Seong Kim, Eung-Soo Kim, Dae-Hyuk Kwon, and Shin-Won Kang. 2017. "Easy-to-Fabricate and High-Sensitivity LSPR Type Specific Protein Detection Sensor Using AAO Nano-Pore Size Control" Sensors 17, no. 4: 856. https://doi.org/10.3390/s17040856
APA StyleKim, S.-W., Lee, J.-S., Lee, S.-W., Kang, B.-H., Kwon, J.-B., Kim, O.-S., Kim, J.-S., Kim, E.-S., Kwon, D.-H., & Kang, S.-W. (2017). Easy-to-Fabricate and High-Sensitivity LSPR Type Specific Protein Detection Sensor Using AAO Nano-Pore Size Control. Sensors, 17(4), 856. https://doi.org/10.3390/s17040856




