Fructose and Pectin Detection in Fruit-Based Food Products by Surface-Enhanced Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanoparticle Preparation and Characterization
2.3. Sample Preparation
2.4. Micro-Raman Spectroscopy and SERS Measurements
2.5. Data Analysis
3. Results
3.1. Nanoparticle Characterization
3.2. Raman and SERS Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nie, S.M.; Emery, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Delfino, I.; Bizzarri, A.R.; Cannistraro, S. Single Molecule detection of Yeast Cytochrome c by Surface-Enhanced Raman Spectroscopy. Biophys. Chem. 2005, 113, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Delfino, I.; Bizzarri, A.R.; Cannistraro, S. Time-dependent study of single-molecule SERS signal from yeast cytochrome c. Chem. Phys. 2006, 326, 356–362. [Google Scholar] [CrossRef]
- Schlücker, S. Surface-Enhanced Raman Spectroscopy: Concepts and Chemical Applications. Angew. Chem. Int. Ed. 2014, 53, 4756–4795. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, A.; Cervo, S.; Sergo, V. Label-free surface-enhanced Raman spectroscopy of biofluids: Fundamental aspects and diagnostic application. Anal. Bioanal. Chem. 2015, 407, 8265–8277. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Guan, Z.Q.; Xu, H.X.; Moskovits, M. Surface-enhanced Raman spectroscopy and nanogeometry: The plasmonic origin of SERS. J. Phys. Chem. C 2007, 111, 17985–17988. [Google Scholar] [CrossRef]
- Kneipp, J.; Kneipp, H.; Kneipp, K. SERS—A single-molecule and nanoscale tool for bioanalytics. Chem. Soc. Rev. 2008, 37, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, E. Nanoparticle SERS Substrates, in Surface Enhanced Raman Spectroscopy: Analytical, Biophysical and Life Science Applications; Schlücker, S., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010. [Google Scholar]
- Delfino, I. Light Scattering Methods for Tracking Gold Nanoparticles Aggregation Induced by Biotin-Neutravidin Interaction. Biophys. Chem. 2013, 177, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chu, W.; Foroushani, A.D.; Wang, H.; Li, D.; Liu, J.; Barrow, C.J.; Wang, X.; Yang, W. New Gold Nanostructures for Sensor Applications: A Review. Materials 2014, 7, 5169–5201. [Google Scholar] [CrossRef]
- Polavarapu, L.; Mourdikoudis, S.; Pastoriza-Santos, I.; Pérez-Juste, J. Nanocrystal engineering of noble metals and metal chalcogenides: Controlling the morphology, composition and crystallinity. CrystEngComm 2015, 17, 3727–3262. [Google Scholar] [CrossRef]
- Polavarapu, L.; Liz-Marzán, L.M. Growth and galvanic replacement of silver nanocubes in organic media. Nanoscale 2013, 5, 4355–4361. [Google Scholar] [CrossRef] [PubMed]
- Kress-Rogers, E.; Brimelow, C.J. Instrumentations and Sensors for the Food Industry; Woodhead Publishing Limited: Abington Hall, Abington, Cambridge, UK, 2001. [Google Scholar]
- Chao, K.; Kim, M.S.; Lawrence, K.C. Optical methods for food inspection. Sens. Instrum. Food Qual. Saf. 2008, 2, 73–74. [Google Scholar] [CrossRef]
- Liu, B.; Han, G.; Zhang, Z.; Liu, R.; Jiang, C.; Wang, S.; Han, M.Y. Shell thickness-dependent Raman enhancement for rapid identification and detection of pesticide residues at fruit peels. Anal. Chem. 2012, 84, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.P.; Franca, A.S.; Irudayaraj, J. Surface-Enhanced Raman Spectroscopy Applied to Food Safety. Annu. Rev. Food Sci. Technol. 2013, 4, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Camerlingo, C.; Portaccio, M.; Tatè, R.; Lepore, M.; Delfino, I. Surface-Enhanced Raman Spectroscopy Study of Commercial Fruit Juices. In Proceedings of the 3rd International Electronic Conference on Sensors and Applications, Sciforum Electronic Conference Series, 15–30 November 2016; Available online: https://sciforum.net/conference/ecsa-3 (accessed on 11 April 2017).
- Khlebtsov, B.N.; Khanadeev, V.A.; Panfilova, E.V.; Bratashov, D.N.; Khlebtso, N.G. Gold nanoisland films as reproducible SERS substrates for highly sensitive detection of fungicides. ACS Appl. Mater. Interfaces 2015, 7, 6518–6529. [Google Scholar] [CrossRef] [PubMed]
- Mandrile, L.; Giovannozzi, A.; Durbiano, F.; Rossi, A.M. Surface-enhanced Raman spectroscopy: A metrological tool for fungicide detection. In Proceedings of the 2nd IMEKOFOOD Metrology Promoting Objective and Measurable Food Quality and Safety, Benevento, Italy, 2–5 October 2016; pp. 280–286. [Google Scholar]
- Liu, P.; Zhou, B.; Liu, X.; Sun, X.; Li, H.; Lin, M. Detection of pesticides in fruits by Surface-Enhanced Raman Spectroscopy coupled with gold nanostructures. Food. Bioprocess Technol. 2013, 6, 710–718. [Google Scholar] [CrossRef]
- Radu, A.I.; Kuellmer, M.; Giese, B.; Huebner, U.; Weber, K.; Cialla-May, D.; Popp, J. Surface-Enhanced Raman Spectroscopy (SERS) in food analytics: Detection of vitamins B2 and B12 in cereals. Talanta 2016, 160, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Camerlingo, C.; Zenone, F.; Delfino, I.; Diano, N.; Mita, D.G.; Lepore, M. Investigation on clarified fruit juice composition by using visible light micro-Raman spectroscopy. Sensors 2007, 7, 2049–2061. [Google Scholar] [CrossRef]
- Delfino, I.; Camerlingo, C.; Portaccio, M.; Della Ventura, B.; Mita, L.; Mita, D.G.; Lepore, M. Visible micro-Raman spectroscopy for determining glucose content in beverage industry. Food Chem. 2011, 127, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Delfino, I.; Camerlingo, C.; Portaccio, M.; Lepore, M. Univariate and multivariate analysis of Raman spectra for quantitative determination of sugars in beverage industry. In Lecture Notes in Electrical Engineering (LNEE); Springer: Heidelberg, Germany, 2014; Volume 162, pp. 375–379. [Google Scholar]
- Ellis, D.I.; Brewster, V.L.; Dunn, W.B.; Allwood, J.W.; Golovanov, A.P.; Goodacre, R. Fingerprinting food: Current technologies for the detection of food adulteration and contamination. Chem. Soc. Rev. 2012, 41, 5706–5727. [Google Scholar] [CrossRef] [PubMed]
- Ilaslan, K.; Boyaci, I.H.; Topcu, A. Rapid analysis of glucose, fructose and sucrose contents of commercial soft drinks using Raman spectroscopy. Food Control 2015, 48, 56–61. [Google Scholar] [CrossRef]
- Meng, J.; Qin, S.; Zhang, L.; Yang, L. Designing of a novel gold nanodumbbells SERS substrate for detection of prohibited colorants in drinks. Appl. Surf. Sci. 2016, 366, 181–186. [Google Scholar] [CrossRef]
- Camerlingo, C.; Portaccio, M.; Lepore, M. Olive oil characterization by visible micro-Raman spectroscopy. In Proceedings of the 2nd IMEKOFOODS—Metrology Promoting Objective and Measurable Food Quality and Safety, Benevento, Italy, 2–5 October 2016; pp. 287–290. [Google Scholar]
- Feng, S.; Hu, Y.; Ma, L.; Lu, X. Development of molecularly imprinted polymers-surface-enhanced Raman spectroscopy/colorimetric dual sensor for determination of chlorpyrifos in apple juice. Sens. Actuators B Chem. 2017, 241, 750–757. [Google Scholar] [CrossRef]
- Frens, G. Particle size and sol stability in metal colloids. Colloid Polym. Sci. 1972, 250, 736–741. [Google Scholar] [CrossRef]
- Delfino, I.; Lepore, M.; Tatè, R.; Portaccio, M. Preparation and Characterization of Au Nanoparticles for Theranostic Applications. In Proceedings of the First International Electronic. Conference on Sensors and Applications, 1–16 June 2014; Available online: https://sciforum.net/conference/ecsa-1 (accessed on 11 April 2017).
- Lim, W.Q.; Gao, Z. Plasmonic nanoparticles in biomedicine. Nano Today 2016, 11, 168–188. [Google Scholar] [CrossRef]
- Alvarez, S.; Riera, F.A.; Alvarez, R.; Coca, J.; Cuperus, F.P.; Bouwer, S.T.; Boswinkel, C.; van Gemert, R.W.; Veldsink, J.W.; Giorno, L.; et al. A new integrated membrane process for producing clarified apple juice and apple juice aroma concentrate. J. Food Eng. 2000, 46, 109–125. [Google Scholar] [CrossRef]
- Cerreti, M.; Liburdi, K.; Benucci, I.; Esti, M. The effect of pectinase and protease treatment on turbidity and on haze active molecules in pomegranate juice. LWT—Food Sci. Tech. 2016, 73, 326–333. [Google Scholar] [CrossRef]
- Malik, V.S.; Hu, F.B. Fructose and Cardiometabolic Health: What the Evidence From Sugar-Sweetened Beverages Tells Us. J. Am. Coll. Cardiol. 2015, 66, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Bernfeld, P. Amylase α and β. Meth. Enzymol. 1955, 1, 149–150. [Google Scholar]
- Grassin, C.; Fauquembergue, P. Progress in Biotechnology 14: Pectins and Pectinases; Visser, J., Voragen, A.G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; pp. 453–463. [Google Scholar]
- Youn, K.S.; Hong, J.H.; Bae, D.H.; Kim, S.J.; Kim, S.D. Effective clarifying process of reconstituted apple juice using membrane filtration with filter-aid pretreatment. J. Membr. Sci. 2004, 228, 179–186. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Ferni, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV-Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Njoki, P.N.; Lim, I.S.; Mott, D.; Park, H.-Y.; Khan, B.; Mishra, S.; Sujakumar, R.; Luo, J.; Zhong, C.-J. Size Correlation of optical and spectroscopic properties for gold nanoparticles. J. Phys. Chem. C 2007, 111, 14664–14669. [Google Scholar] [CrossRef]
- Camerlingo, C.; Zenone, F.; Gaeta, G.M.; Riccio, R.; Lepore, M. Wavelet data processing of micro-Raman spectra of biological samples. Meas. Sci. Technol. 2006, 17, 298–303. [Google Scholar] [CrossRef]
- Delfino, I.; Perna, G.; Lasalvia, M.; Capozzi, V.; Manti, L.; Camerlingo, C.; Lepore, M. Visible micro-Raman spectroscopy of single human mammary epithelial cells exposed to X-ray radiation. J. Biomed. Opt. 2015, 20. [Google Scholar] [CrossRef] [PubMed]
- Camerlingo, C.; Zenone, F.; Perna, G.; Capozzi, V.; Cirillo, N.; Gaeta, G.M.; Lepore, M. An investigation on micro-Raman spectra and wavelet data treatment for Pemphigus Vulgaris follow-up monitoring. Sensors 2008, 8, 3656–3664. [Google Scholar] [CrossRef] [PubMed]
- Mathlouthi, M.; Luu, D.V. Laser-Raman spectra of D-fructose in aqueous solution. Carbohydr. Res. 1980, 78, 225–233. [Google Scholar] [CrossRef]
- Söderholm, S.; Roos, Y.H.; Meinander, N.; Hotokka, M. Raman spectra of fructose and glucose in the amorphous and crystalline states. J. Raman Spectrosc. 1999, 30, 1009–1018. [Google Scholar] [CrossRef]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 2007, 38, 1133–1147. [Google Scholar] [CrossRef]
- Engelsen, S.B.; Noorgard, L. Comparative vibrational spectroscopy for determination of quality parameters in amidated pectins as evaluated by chemometrics. Carbohydr. Polym. 1996, 30, 9–24. [Google Scholar] [CrossRef]
- Raman Spectra of Carbohydrates. Available online: www.models.life.ku.dk/specarb (accessed on 10 October 2016).
- Gamsjaeger, A.; Baranska, M.; Schulz, H.; Heiselmayer, P. Discrimination of carotenoid and flavonoid content in petals of pansy cultivars (Viola x wittrockiana) by FT-Raman spectroscopy. J. Raman Spectr. 2011, 42, 1240–1247. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Solovchenko, A.E.; Smagin, A.I.; Gitelson, A.A. Apple flavonols during fruit adaptation to solar radiation: Spectral features and technique for non-destructive assessment. J. Plant Physiol. 2005, 162, 151–160. [Google Scholar] [CrossRef] [PubMed]
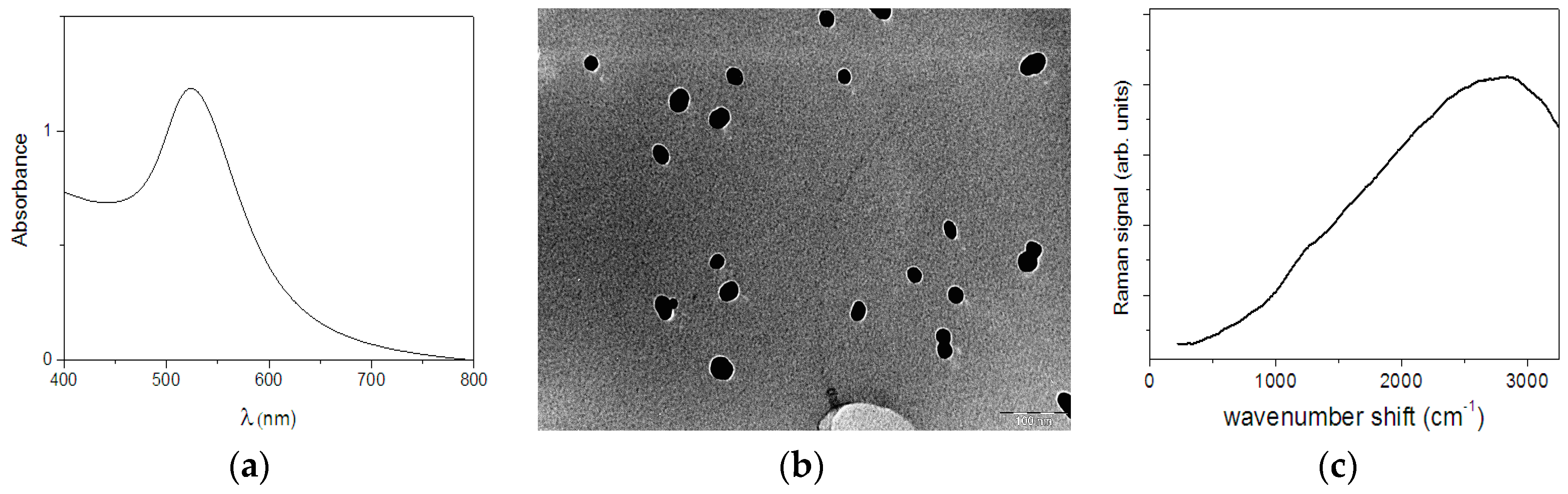

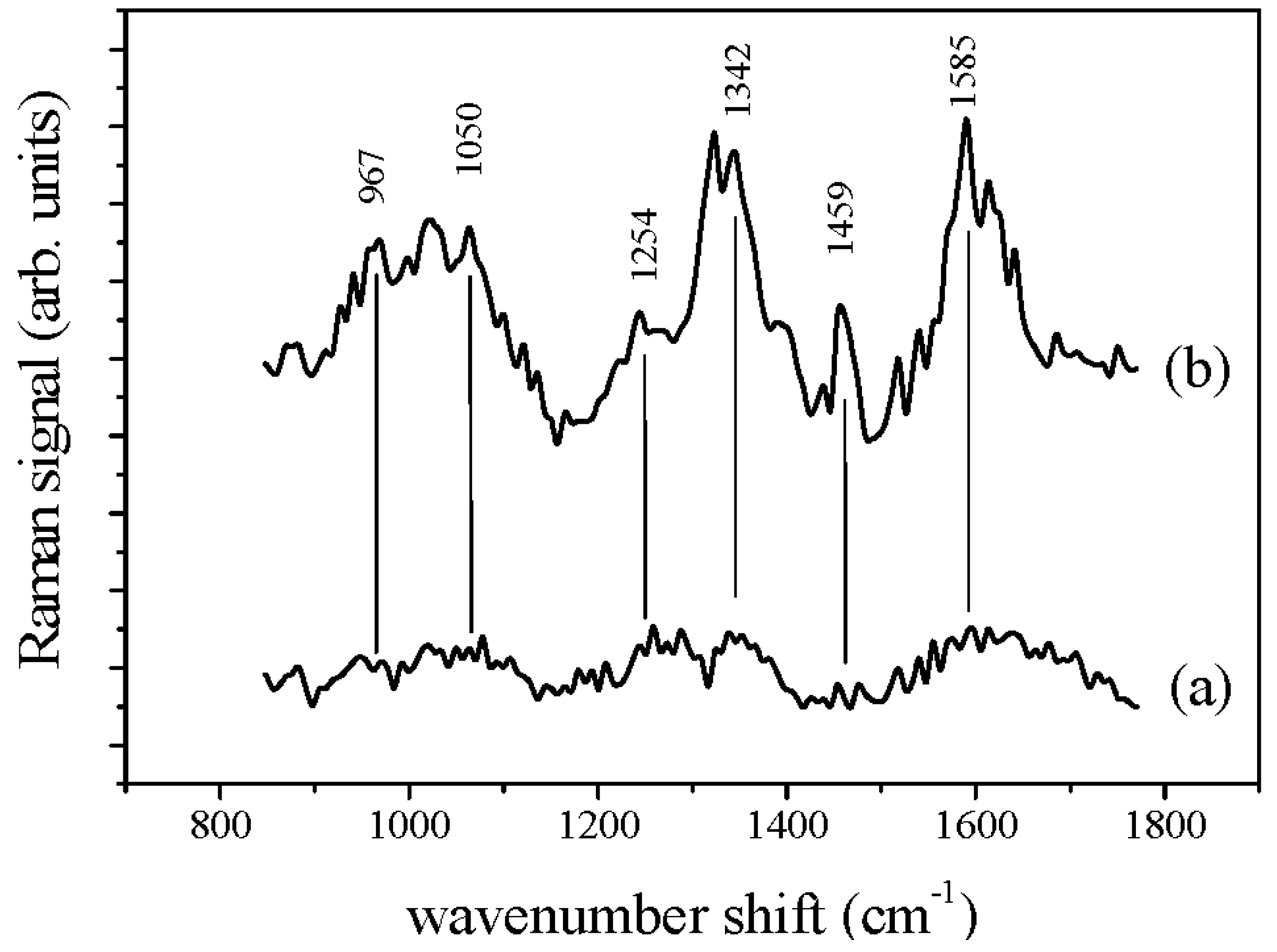
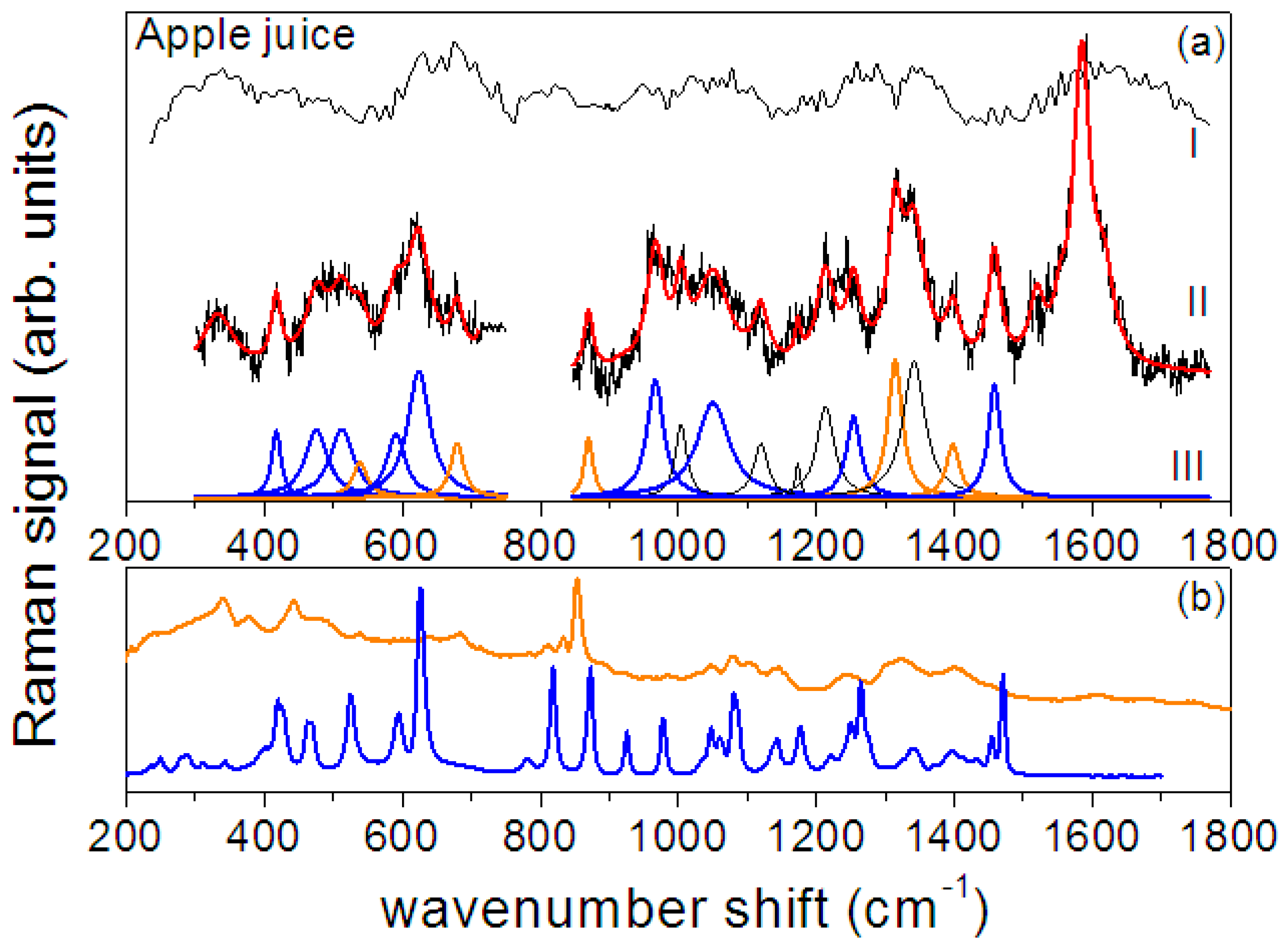
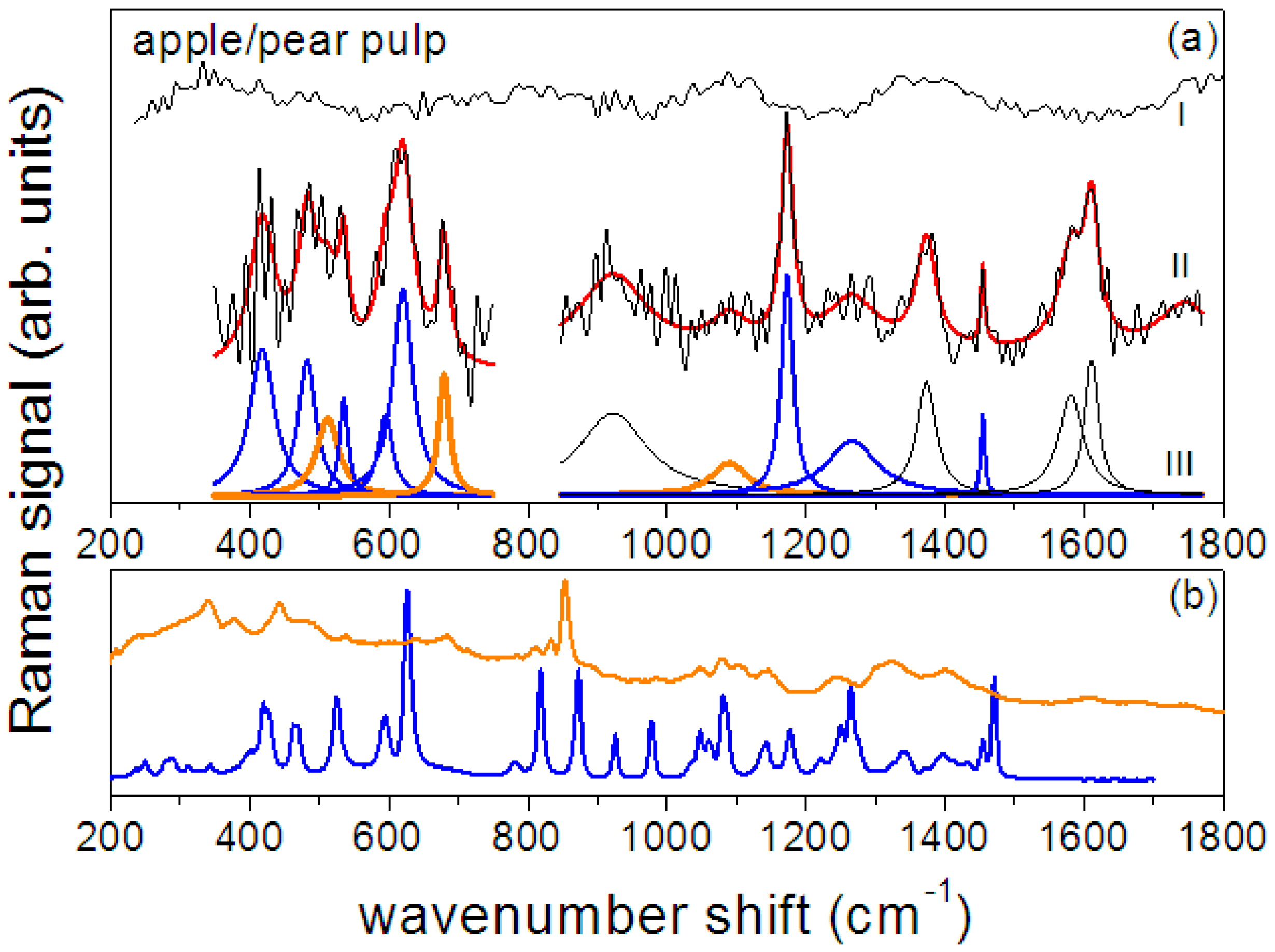
| Crystalline Fructose | Fructose in Aqueous Solution | Assignment | ||
|---|---|---|---|---|
| Position (cm−1) | Relative Intensity (%) | Position (cm−1) | Relative Intensity (%) | |
| 290 | 10 | - | - | - |
| 344 | 8 | 333 | 81 | C-O-C bending |
| 423 | 39 | 424 | 81 | C-C-C bending (pyranose) |
| 467 | 39 | 492 | 36 | C-C-C bending (furanose) |
| 527 | 37 | 517 | 42 | C-C-O bending (pyranose) |
| 592 | 32 | - | - | - |
| 627 | 100 a | 622 | 100 a | C-C-O bending |
| - | - | 678 | 20 | C-C-O bending (furanose) |
| 778 | 9 | - | - | - |
| 817 | 68 | 815 | 81 | C-C stretching (pyranose) |
| 870 | 63 | 872 | 66 | C-C stretching (furanose) |
| 923 | 19 | 940 | 19 | C-C-H bending (furanose) |
| 977 | 24 | - | - | C-C-H bending (pyranose) |
| 1049 | 46 | 1058 | 20 | C-O stretching |
| 1082 | 51 | 1095 | 128 | C-O-H bending |
| 1139 | 31 | - | - | C-O stretching (pyranose) |
| 1176 | 33 | - | - | C-O stretching (furanose) |
| 1241 | 25 | - | - | - |
| 1264 | 44 | 1272 | 43 | CH2 twisting |
| 1338 | 18 | 1340 | 52 | - |
| - | - | 1370 | 97 | CH2 wagging |
| 1404 | 18 | 1412 | 31 | - |
| 1466 | 38 | 1460 | 100 | CH2 bending |
| Apple Juice—Peak Position (cm−1) | Apple/Pear Pulp—Peak Position (cm−1) | Fructose | Pectin |
|---|---|---|---|
| 417 | 417 | X | |
| 475 | 482 | X | |
| 512 | 512 | X | |
| 538 | 535 | X | |
| 591 | 595 | X | |
| 624 | 620 | X | |
| 679 | 679 | X | |
| 730 | X | ||
| 870 | X | X | |
| 918 | 922 | X | |
| 967 | X | ||
| 1004 | |||
| 1050 | X | ||
| 1090 | X | ||
| 1120 | |||
| 1173 | 1173 | X | |
| 1213 | |||
| 1254 | 1266 | X | |
| 1315 | |||
| 1342 | X | ||
| 1373 | X | ||
| 1398 | |||
| 1458 | 1455 | X | |
| 1520 | |||
| 1585 | 1581 | ||
| 1554 | |||
| 1617 | 1611 | ||
| 1746 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camerlingo, C.; Portaccio, M.; Tatè, R.; Lepore, M.; Delfino, I. Fructose and Pectin Detection in Fruit-Based Food Products by Surface-Enhanced Raman Spectroscopy. Sensors 2017, 17, 839. https://doi.org/10.3390/s17040839
Camerlingo C, Portaccio M, Tatè R, Lepore M, Delfino I. Fructose and Pectin Detection in Fruit-Based Food Products by Surface-Enhanced Raman Spectroscopy. Sensors. 2017; 17(4):839. https://doi.org/10.3390/s17040839
Chicago/Turabian StyleCamerlingo, Carlo, Marianna Portaccio, Rosarita Tatè, Maria Lepore, and Ines Delfino. 2017. "Fructose and Pectin Detection in Fruit-Based Food Products by Surface-Enhanced Raman Spectroscopy" Sensors 17, no. 4: 839. https://doi.org/10.3390/s17040839
APA StyleCamerlingo, C., Portaccio, M., Tatè, R., Lepore, M., & Delfino, I. (2017). Fructose and Pectin Detection in Fruit-Based Food Products by Surface-Enhanced Raman Spectroscopy. Sensors, 17(4), 839. https://doi.org/10.3390/s17040839







