Twenty-Four-Hour Real-Time Continuous Monitoring of Cerebral Edema in Rabbits Based on a Noninvasive and Noncontact System of Magnetic Induction
Abstract
:1. Introduction
2. Materials and Methods
2.1. MIPS Detection Principle
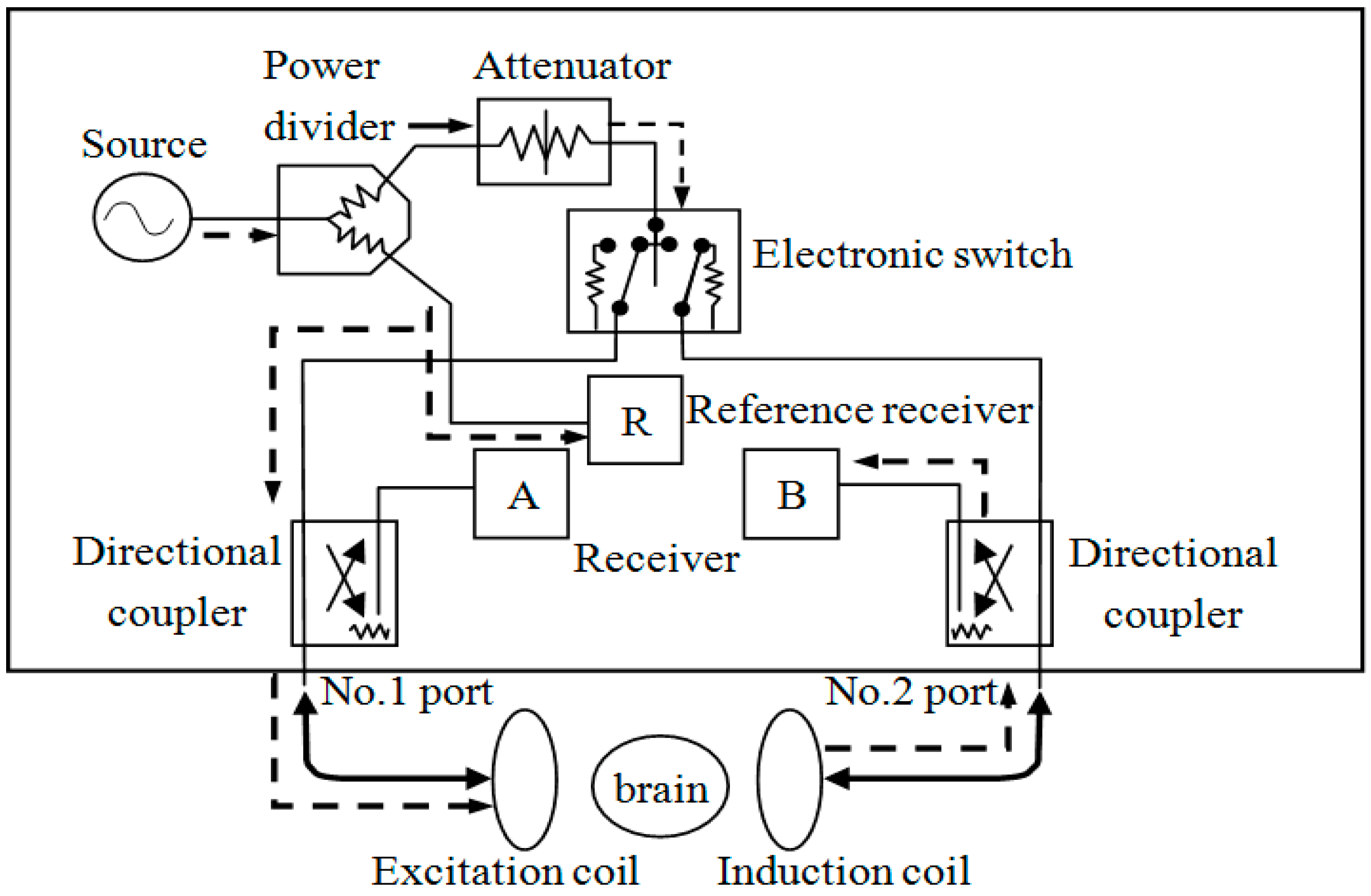
2.2. Construction of the System
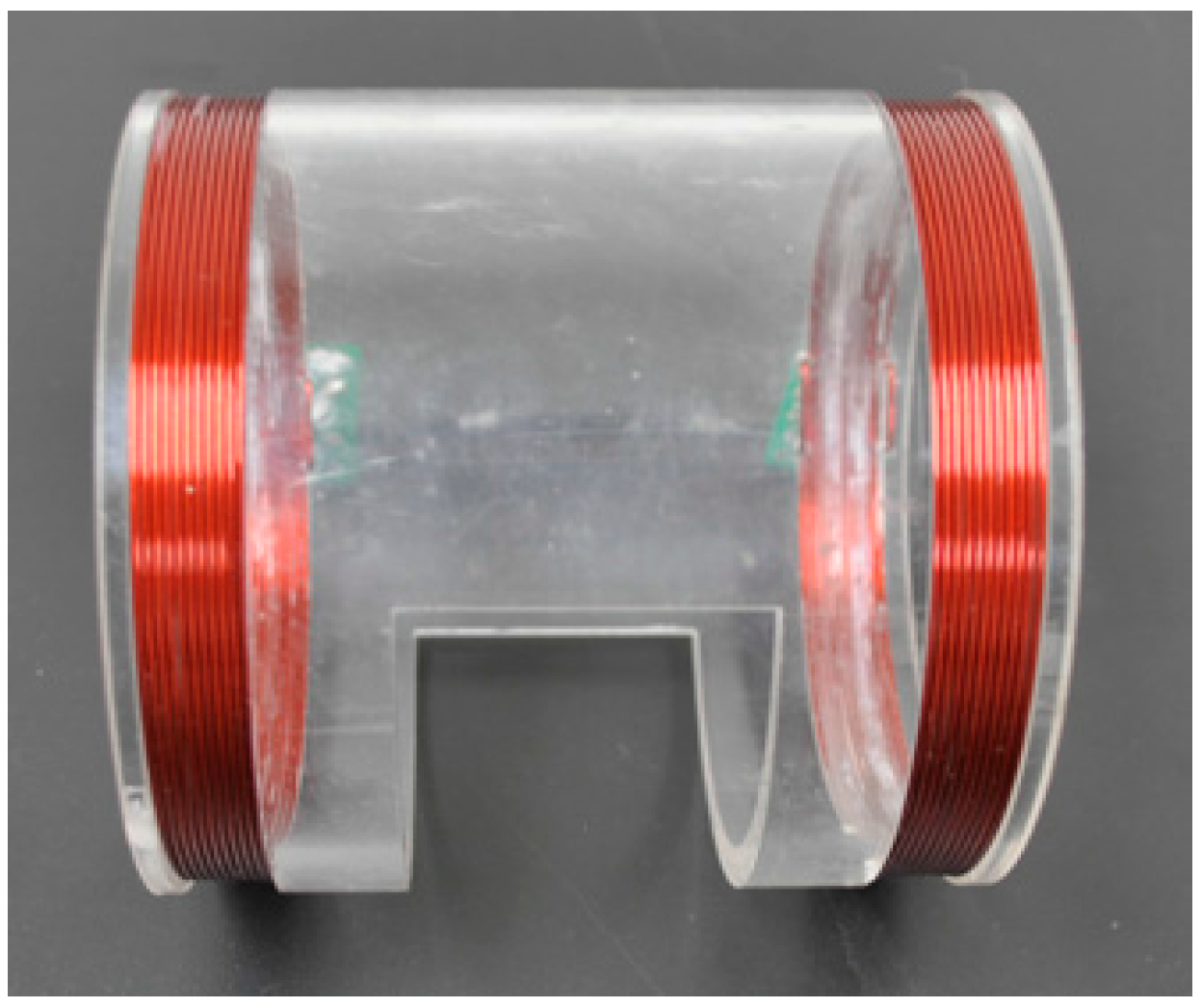
2.2.1. Two-Coil Sensor
2.2.2. Design of the Magnetic Induction Brain Monitor
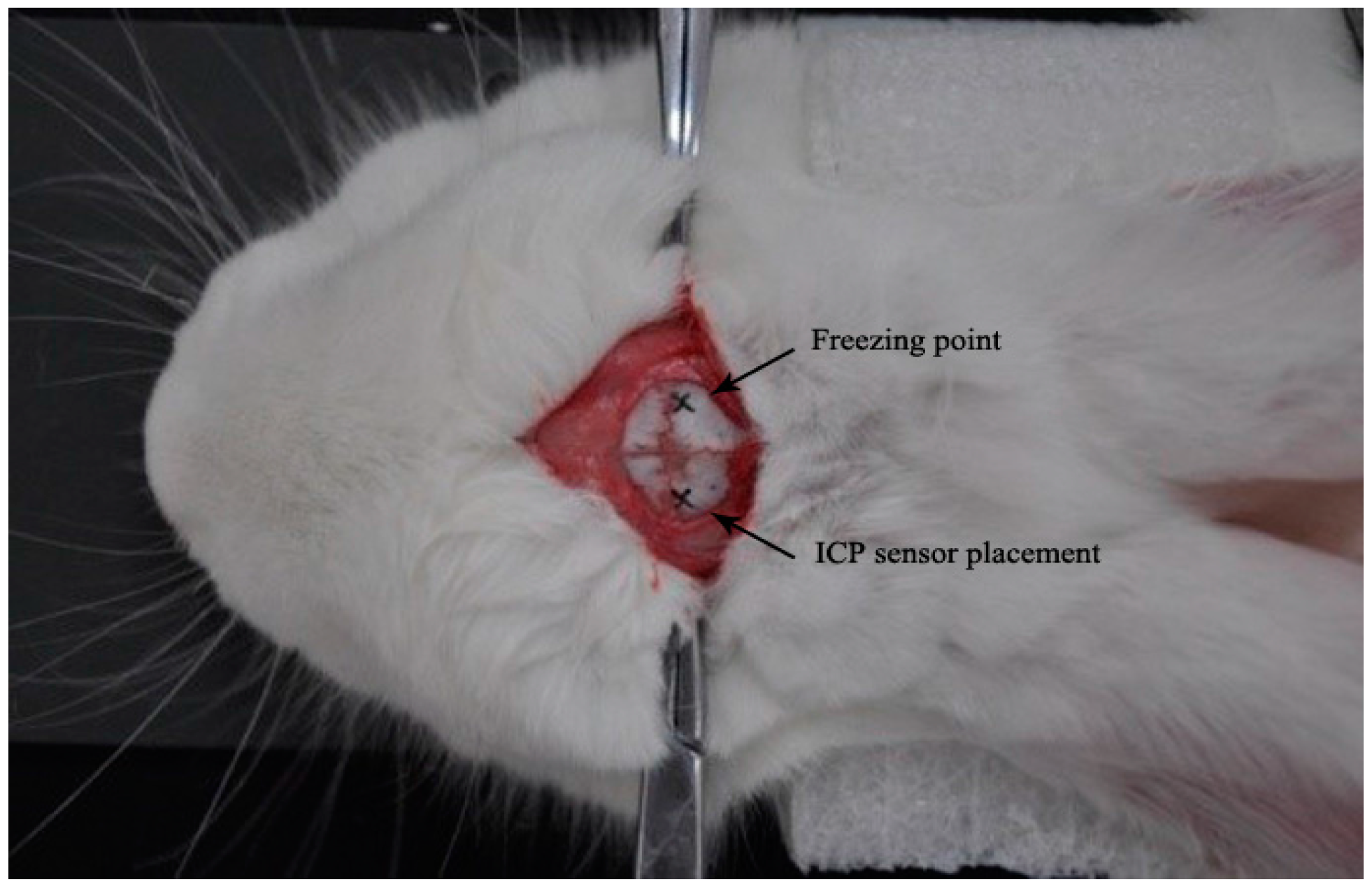
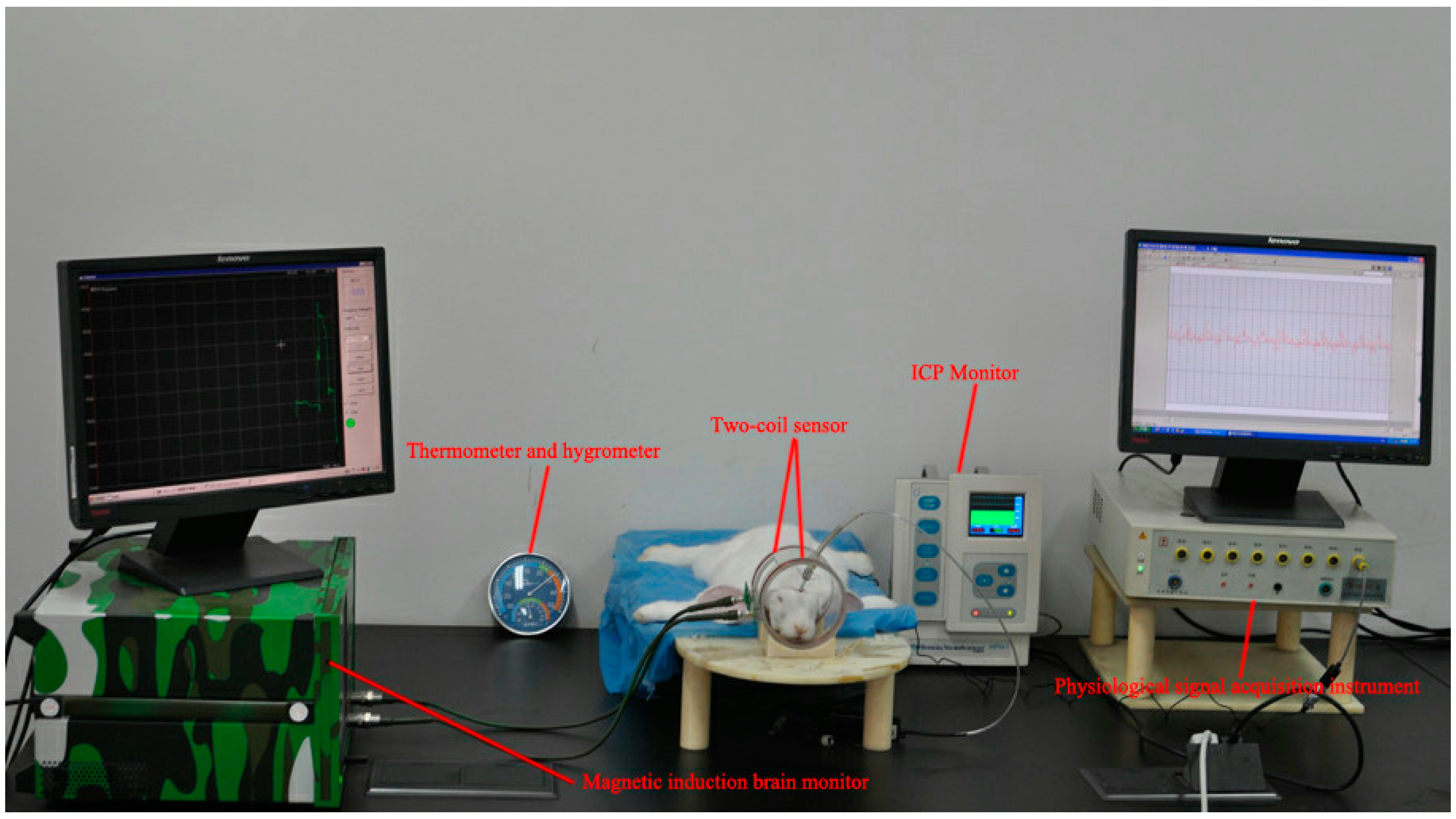
2.3. Animal Experimental Design
2.4. MIPS and ICP Data Analysis
2.5. Brain Water Content Analysis
2.6. Statistical Analysis
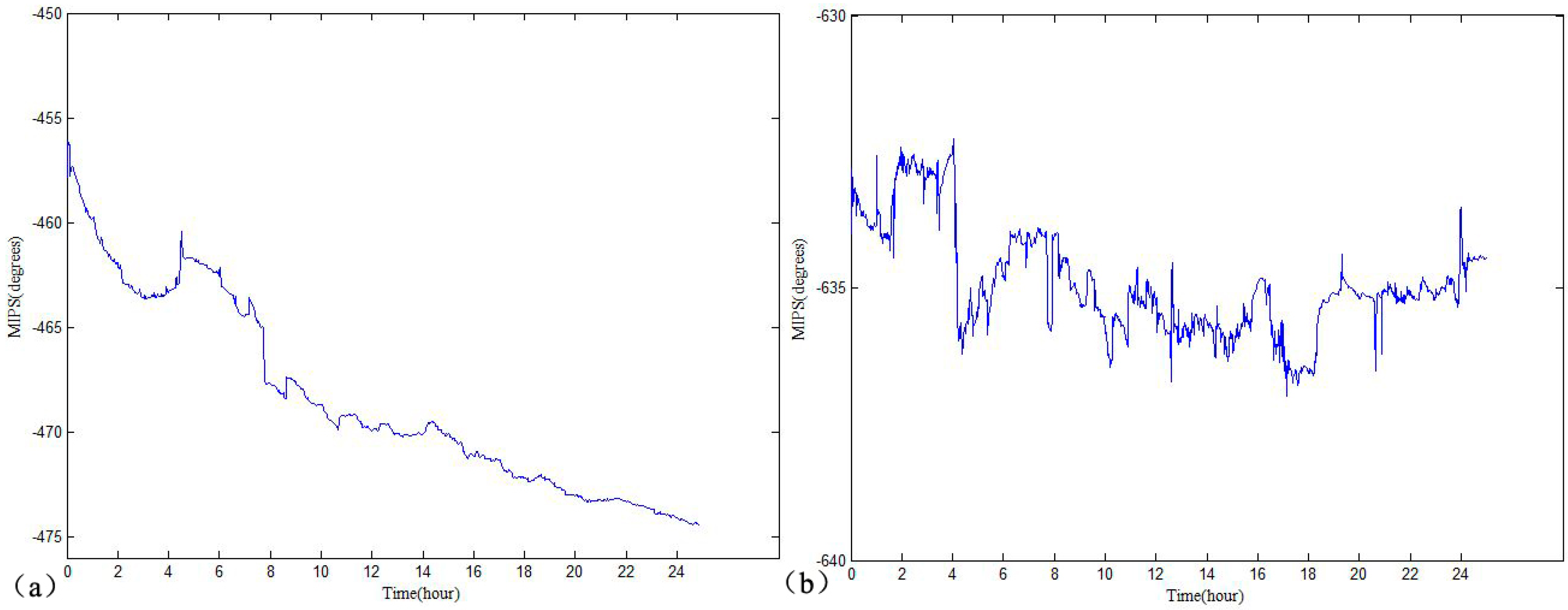
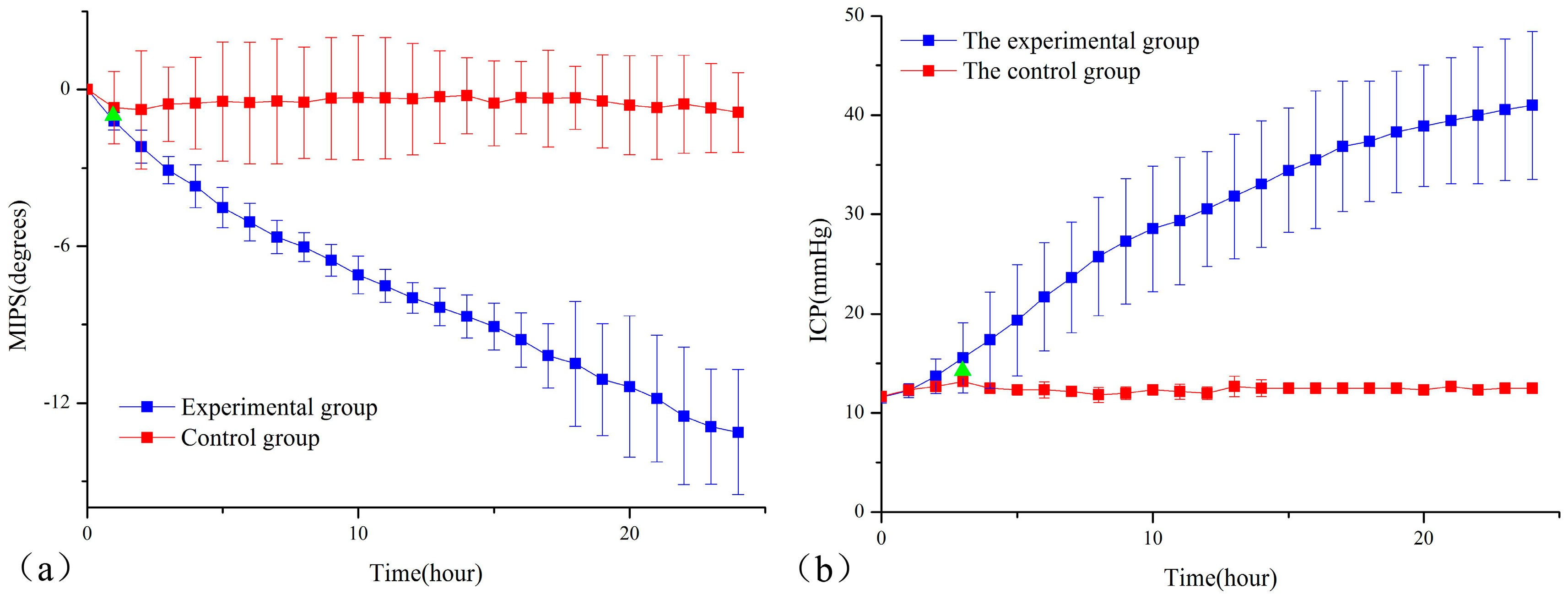
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mcbride, D.W.; Szu, J.I.; Hale, C. Reduction of cerebral edema after traumatic brain injury using an osmotic transport device. J. Neurotrauma 2014, 31, 1948–1954. [Google Scholar] [CrossRef] [PubMed]
- Blixt, J.; Svensson, M.; Gunnarson, E.; Wanecek, M. Aquaporins and blood-brain barrier permeability in early edema development after traumatic brain injury. Brain Res. 2015, 1611, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Wykes, V.; Vindlacheruvu, R. Intracranial pressure, cerebral blood flow and brain oedema. Surgery 2015, 33, 355–362. [Google Scholar] [CrossRef]
- Maciel, C.B.; Sheth, K.N. Malignant MCA Stroke: An Update on Surgical Decompression and Future Directions. Curr. Atheroscler. Rep. 2015, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wijdicks, E.F.; Sheth, K.N.; Carter, B.S. Recommendations for the management of cerebral and cerebellar infarction with swelling: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 1222–1238. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, S.; Koziol, J.A.; Martinez, F. Sonographic monitoring of mass effect in stroke patients treated with hypothermia. Correlation with intracranial pressure and matrix metalloproteinase 2 and 9 expression. J. Neurol. Sci. 2009, 276, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Dong, W.; Ji, X. A new method of noninvasive brain-edema monitoring in stroke: Cerebral electrical impedance measurement. Neurol. Res. 2013, 28, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, H. Magnetic induction tomography. Meas. Sci. Technol. 2001, 12, 1126–1131. [Google Scholar] [CrossRef]
- Scharfetter, H.; Casañas, R.; Rosell, J. Biological tissue characterization by magnetic induction spectroscopy (MIS): Requirements and limitations. IEEE Trans. Biomed. Eng. 2003, 50, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, H.; Stewart, W.R.; Gough, W. Magnetic Induction Tomography: A Measuring System for Biological Tissues. Ann. NY Acad. Sci. 1999, 873, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jin, G.; Qin, M.; Wan, Z.; Wang, J.; Wang, C.; Guo, W.; Xu, L.; Ning, X.; Xu, J.; et al. The detection of chronic cerebral hemorrhage in rabbits with magnetic induction. J. Phys. Conf. Ser. 2012, 407, 12014–12023. [Google Scholar] [CrossRef]
- Sun, J.; Jin, G.; Qin, M.; Wan, Z.; Wang, J.; Wang, C.; Guo, W.; Xu, L.; Ning, X.; Xu, J.; et al. Detection of acute cerebral hemorrhage in rabbits by magnetic induction. Braz. J. Med. Biol. Res. 2014, 47, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Yan, Q.; Qin, M.; Jin, G.; Sun, J.; Ning, X.; Zhuang, W.; Peng, B.; Li, G. Detection of cerebral hemorrhage in rabbits by time-difference magnetic inductive phase shift spectroscopy. PLoS ONE 2015, 10, e0128127. [Google Scholar] [CrossRef] [PubMed]
- Zolgharni, M.; Ledger, P.D.; Armitage, D.W.; Holder, D.S.; Griffiths, H. Imaging cerebral haemorrhage with magnetic induction tomography: Numerical modelling. Physiol. Meas. 2009, 30, S187–S200. [Google Scholar] [CrossRef] [PubMed]
- Barai, A.; Watson, S.; Griffiths, H.; Patz, R. Magnetic induction spectroscopy: Noncontact measurement of the electrical conductivity spectra of biological samples. Meas. Sci. Technol. 2012, 23, 755–766. [Google Scholar] [CrossRef]
- O’Toole, M.D.; Marsh, L.A.; Davidson, J.L. Noncontact multi-frequency magnetic induction spectroscopy system for industrial-scale bio-impedance measurement. Meas. Sci. Technol. 2015, 26, 035102. [Google Scholar] [CrossRef]
- Jin, G.; Sun, J.; Qin, M.; Wang, C.; Guo, W.; Yan, Q.; Peng, B.; Pan, W. A special phase detector for magnetic inductive measurement of cerebral hemorrhage. PLoS ONE 2014, 9, e97179. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Sun, J.; Qin, M.; Tang, Q.; Xu, L.; Ning, X.; Xu, J.; Pu, X.; Chen, M. A new method for detecting cerebral hemorrhage in rabbits by magnetic inductive phase shift. Biosens. Bioelectron. 2014, 52, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Jin, G.; Qin, M.; Zhao, J.; Wang, J.; Sun, J. Experimental study on the detection of rabbit intracranial hemorrhage using four coil structures based on magnetic induction phase shift. Biomed Eng./Biomed. Technik 2016, 62, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.A.; Villanueva, C.; Vera, C.; Flores, O.; Reyes, R.D.; Rubinsky, B. The detection of brain ischaemia in rats by inductive phase shift spectroscopy. Physiol. Meas. 2009, 30, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.A.; Valencia, J.A.; Mora, A. Volumetric Electromagnetic Phase-Shift Spectroscopy of Brain Edema and Hematoma. PLoS ONE 2013, 8, e63223. [Google Scholar] [CrossRef] [PubMed]
- Kao, H.P.; Cardoso, R.; Shwedyk, E. Correlation of permittivity and water content during cerebral edema. IEEE Trans. Biomed. Eng. 1999, 46, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Klatzo, I. Neuropathological Aspects of Braix Edema. J. Neuropathol. Exp. Neurol. 1967, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kawai, N.; Kawanishi, M.; Nagao, S. Treatment of cold injury-induced brain edema with a nonspecific matrix metalloproteinase inhibitor MMI270 in rats. J. Neurotrauma 2003, 20, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Rolland, W.B.; Lekic, T.; Krafft, P.R.; Hasegawa, Y.; Altay, O.; Hartman, R.; Ostrowski, R.; Manaenko, A.; Tang, J.; Zhang, J. Fingolimod reduces cerebral lymphocyte infiltration in experimental models of rodent intracerebral hemorrhage. Exp. Neurol. 2013, 241, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Bigdeli, M.R.; Hajizadeh, S.; Froozandeh, M.; Rasulian, B.; Heidarianpour, A.; Khoshbaten, A. Prolonged and intermittent normobaric hyperoxia induce different degrees of ischemic tolerance in rat brain tissue. Brain Res. 2007, 1152, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Laufer, S.; Solomon, S.B.; Rubinsky, B. Tissue characterization using electrical impedance spectroscopy data: A linear algebra approach. Physiol. Meas. 2015, 33, 997–1013. [Google Scholar] [CrossRef] [PubMed]
- Melhem, S.; Shutter, L.; Kaynar, A.M. A trial of intracranial pressure monitoring in traumatic brain injury. N. Engl. J. Med. 2012, 367, 2471–2481. [Google Scholar] [CrossRef]
- Thal, S.C.; Sporer, S.; Klopotowski, M.; Thal, S.E.; Woitzik, J.; Schmid-Elsaesser, R.; Plesnila, N.; Zausinger, S. Brain edema formation and neurological impairment after subarachnoid hemorrhage in rats. Am. Assoc. Neurol. Surg. 2009, 111, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Salci, K.; Enblad, P.; Piper, I.; Contant, C.; Nilsson, P. A model for studies of intracranial volume pressure dynamics in traumatic brain injury. J. Neurotrauma 2004, 21, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, X.; Hu, X. Establishment and evaluation of cryogenic brain injury model of rats. Chin. J. Neurosurg. Dis. Res. 2008, 7, 725–728. [Google Scholar]
- Stocchetti, N.; Picetti, E.; Berardino, M.; Buki, A.; Chesnut, R.M.; Fountas, K.N.; Horn, P.; Hutchinson, P.J.; Iaccarino, C.; Kolias, A.G.; et al. Clinical applications of intracranial pressure monitoring in traumatic brain injury. Acta Neurochir. 2014, 156, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tzeng, W.S.; Wang, C.; Cheng, B.; Chang, Y.; Chen, H.; Lin, P.; Huang, T.; Chuang, T.; Lin, J.; et al. Neuroprotective effect of agmatine in rats with transient cerebral ischemia using MR imaging and histopathologic evaluation. Magn. Reson. Imaging 2013, 31, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Park, K.J.; Kang, S.H.; Chae, Y.S.; Yu, M.O.; Cho, T.H.; Suh, J.K.; Lee, H.K.; Chung, Y.G. Influence of interleukin-6 on the development of peritumoral brain edema in meningiomas. J. Neurosurg. 2010, 112, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Thrane, A.S.; Thrane, V.R.; Plog, B.A.; Nedergaard, M. Filtering the muddied waters of brain edema. Trends Neurosci. 2015, 38, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Heiss, W.D. Malignant MCA Infarction: Pathophysiology and Imaging for Early Diagnosis and Management Decisions. Cerebrovasc. Dis. 2015, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
| Sample | BWC (%) | |
|---|---|---|
| Mean | Standard Deviation | |
| experimental group | 81.59 | 0.88 |
| control group | 78.03 | 0.51 |
| Time | Sample | Variances | F | Significance | t | Significance (Two-Tailed) | 95% Confidence Interval of the Difference | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| 0 h | ICP1 | Equal variances assumed | 1.333 | 0.265 | −0.641 | 0.531 | −0.718 | 0.385 |
| Equal variances not assumed | −0.643 | 0.534 | −0.743 | 0.403 | ||||
| 1 h | MIPS1 | Equal variances assumed | 3.087 | 0.098 | 8.720 | 1.78 × 10−7 | 1.325 | 2.176 |
| Equal variances not assumed | 7.490 | 1.29 × 10−4 | 1.199 | 2.302 | ||||
| 1 h | ICP2 | Equal variances assumed | 0.029 | 0.868 | −0.596 | 0.559 | −0.759 | 0.426 |
| Equal variances not assumed | −0.620 | 0.548 | −0.757 | 0.423 | ||||
| 2 h | ICP3 | Equal variances assumed | 0.005 | 0.945 | 0.800 | 0.435 | −0.412 | 0.912 |
| Equal variances not assumed | 0.875 | 0.398 | −0.368 | 0.868 | ||||
| 3 h | ICP4 | Equal variances assumed | 0.754 | 0.398 | 2.502 | 0.024 | 0.115 | 1.385 |
| Equal variances not assumed | 2.941 | 0.01 | 0.207 | 1.293 | ||||
| Sample | Correlation Coefficient | p Value |
|---|---|---|
| No. 1 rabbit | −0.8742 | 0.01 |
| No. 2 rabbit | −0.8208 | 0.01 |
| No. 3 rabbit | −0.7645 | 0.01 |
| No. 4 rabbit | −0.7865 | 0.01 |
| No. 5 rabbit | −0.8674 | 0.01 |
| No. 6 rabbit | −0.8857 | 0.01 |
| No. 7 rabbit | −0.7804 | 0.01 |
| No. 8 rabbit | −0.9153 | 0.01 |
| No. 9 rabbit | −0.9092 | 0.01 |
| No. 10 rabbit | −0.8027 | 0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Ma, K.; Sun, J.; Jin, G.; Qin, M.; Feng, H. Twenty-Four-Hour Real-Time Continuous Monitoring of Cerebral Edema in Rabbits Based on a Noninvasive and Noncontact System of Magnetic Induction. Sensors 2017, 17, 537. https://doi.org/10.3390/s17030537
Li G, Ma K, Sun J, Jin G, Qin M, Feng H. Twenty-Four-Hour Real-Time Continuous Monitoring of Cerebral Edema in Rabbits Based on a Noninvasive and Noncontact System of Magnetic Induction. Sensors. 2017; 17(3):537. https://doi.org/10.3390/s17030537
Chicago/Turabian StyleLi, Gen, Ke Ma, Jian Sun, Gui Jin, Mingxin Qin, and Hua Feng. 2017. "Twenty-Four-Hour Real-Time Continuous Monitoring of Cerebral Edema in Rabbits Based on a Noninvasive and Noncontact System of Magnetic Induction" Sensors 17, no. 3: 537. https://doi.org/10.3390/s17030537
APA StyleLi, G., Ma, K., Sun, J., Jin, G., Qin, M., & Feng, H. (2017). Twenty-Four-Hour Real-Time Continuous Monitoring of Cerebral Edema in Rabbits Based on a Noninvasive and Noncontact System of Magnetic Induction. Sensors, 17(3), 537. https://doi.org/10.3390/s17030537





