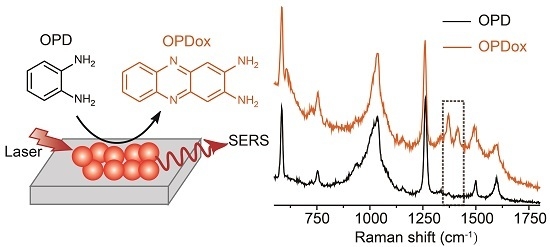
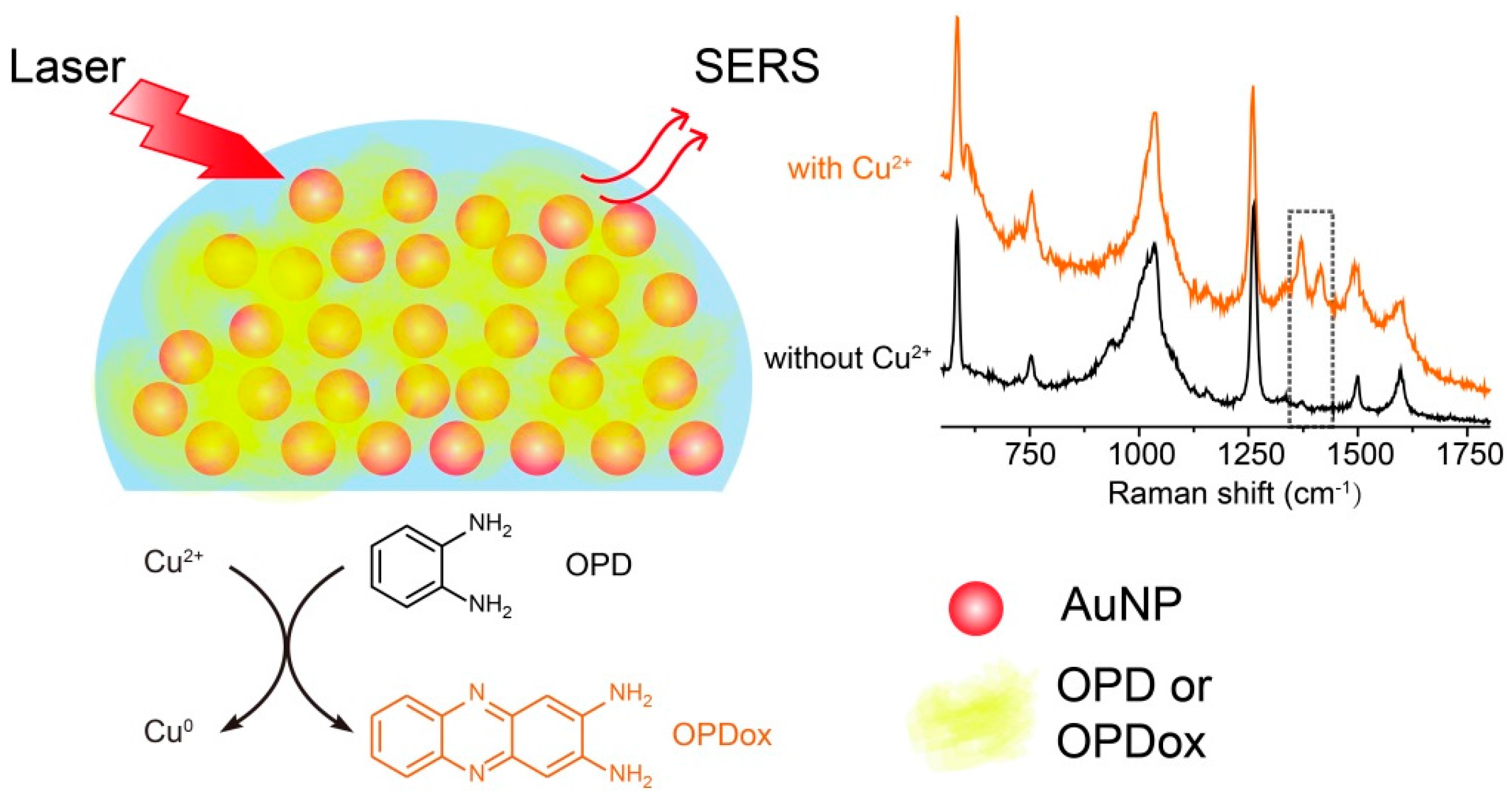
Real-Time Sensing of O-Phenylenediamine Oxidation on Gold Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Synthesis of AuNPs
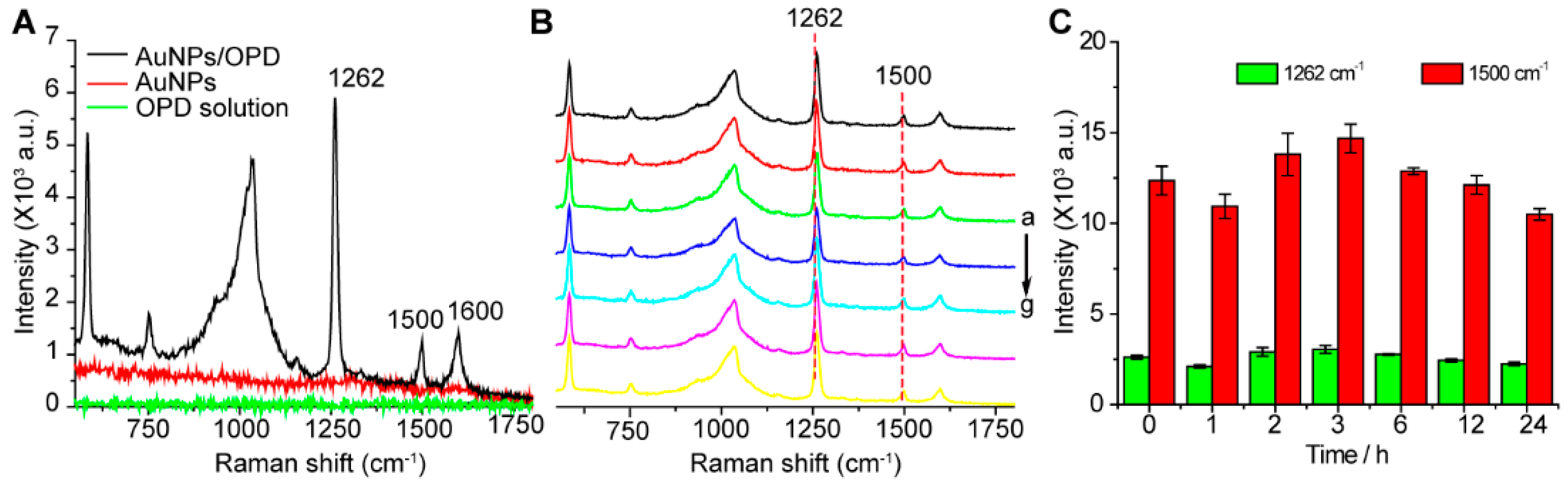
2.3. SERS Spectra of OPD
2.4. DFT Calculation
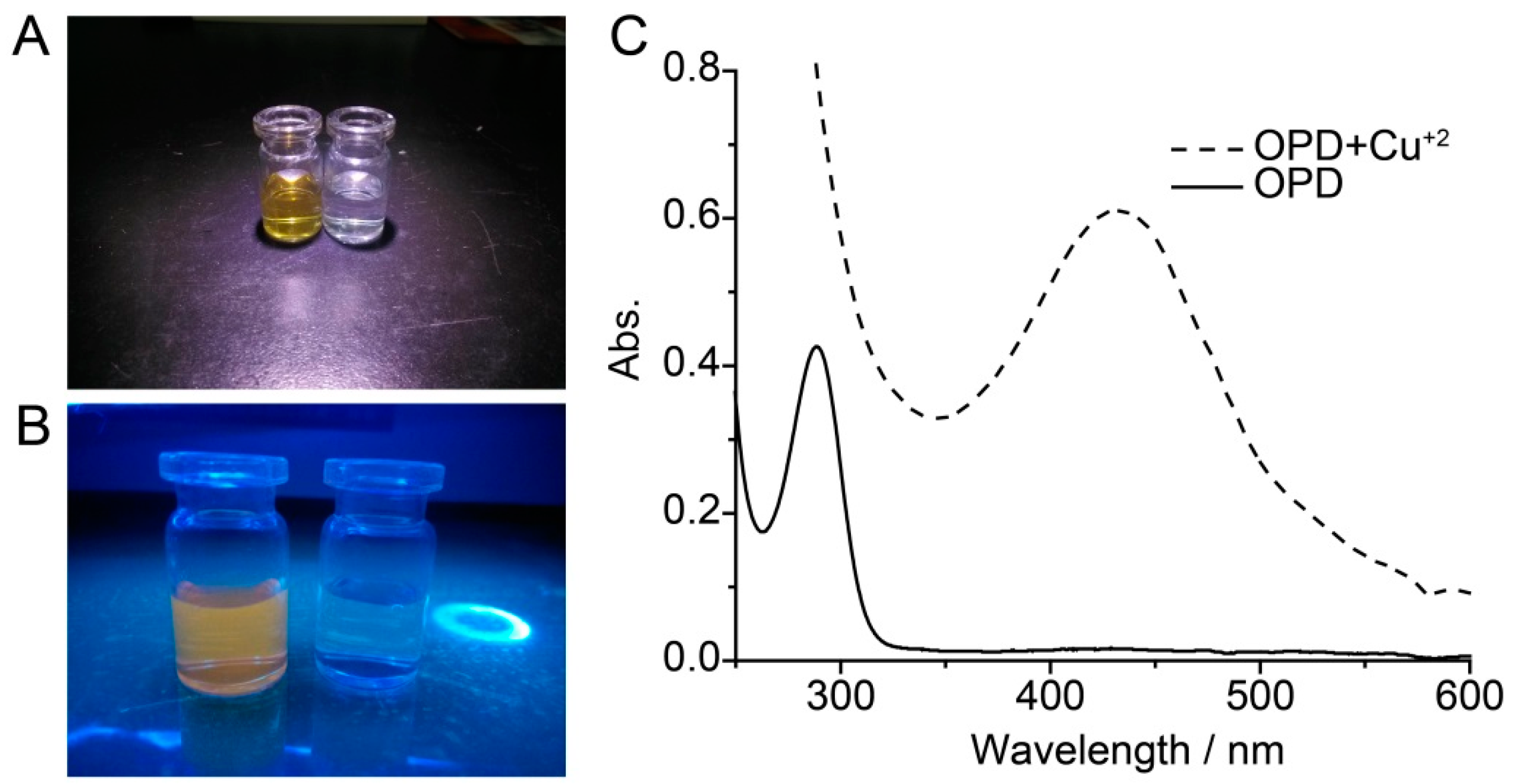
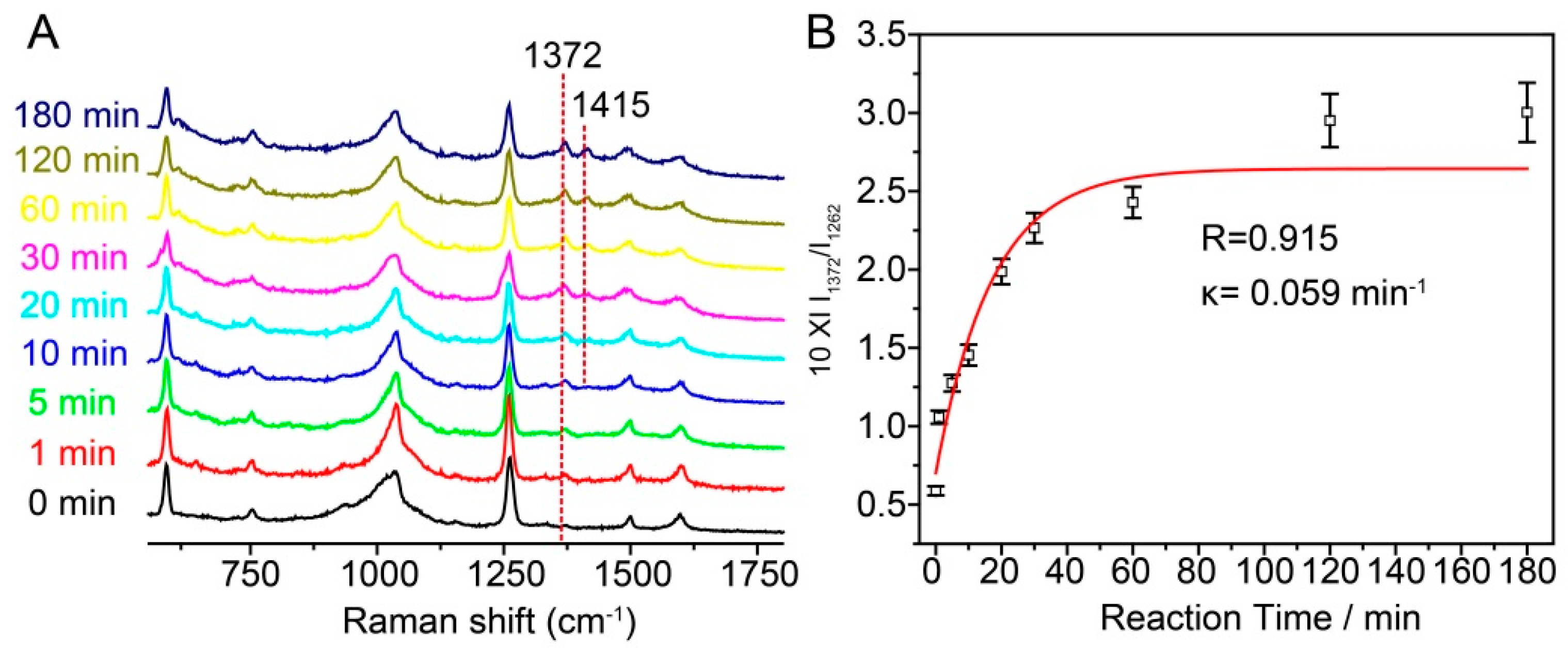
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shi, L.; Jing, C.; Ma, W.; Li, D.-W.; Halls, J.E.; Marken, F.; Long, Y.-T. Plasmon resonance scattering spectroscopy at the single-nanoparticle level: Real-time monitoring of a click reaction. Angew. Chem. Int. Ed. 2013, 52, 6011–6014. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, C.; Cheng, L.; Lee, S.-T.; Liu, Z. Noble metal coated single-walled carbon nanotubes for applications in surface enhanced Raman scattering imaging and photothermal therapy. J. Am. Chem. Soc. 2012, 134, 7414–7422. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chon, H.; Lee, J.; Ko, J.; Chung, B.H.; Lim, D.W.; Choo, J. Rapid and sensitive phenotypic marker detection on breast cancer cells using surface-enhanced Raman scattering (SERS) imaging. Biosens. Bioelectron. 2014, 51, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, X.; Zhang, Y.; Zhang, W.; Ren, T.; Chen, Z.; Wang, F.; Yang, H. Fabrication of intelligent poly(N-isopropylacrylamide)/silver nanoparticle composite films with dynamic surface-enhanced Raman scattering effect. RSC Adv. 2015, 5, 40437–40443. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Zhang, W.; Ren, T.; Wang, F.; Yang, H. Laser-induced plasmonic heating on silver nanoparticles/poly(N-isopropylacrylamide) mats for optimizing SERS detection. J. Raman Spectrosc. 2017, 48, 243–250. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, W.; Birke, R.L.; Lombardi, J.R. Detection of short-lived intermediates in electrochemical reactions using time-resolved surface-enhanced Raman spectroscopy. J. Phys. Chem. 1990, 94, 4766–4769. [Google Scholar] [CrossRef]
- Shi, C.; Zbang, W.; Birke, R.L.; Cosser, D.K.; Lombardi, J.R. Time-resolved SERS, cyclic voltammetry, and digital simulation. J. Phys. Chem. 1991, 95, 6276–6285. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, W.; Lombardi, J.R.; Birke, R.R. Nanosecond time scale kinetics in the flavin mononucleotide on an electrode surface using time-resolved surface-enhanced Raman spectroscopy. J. Phys. Chem. 1992, 96, 10093–10096. [Google Scholar] [CrossRef]
- Zhang, W.; Vivoni, A.; Lombardi, J.R.; Birke, R.R. Time-resolved SERS study of direct photochemical charge transfer between FMN and a Ag electrode. J. Phys. Chem. 1995, 99, 12846–12857. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, W.; Birke, R.L.; Lombardi, J.R. SERS investigation of the adsorption and electroreduction of 4-cyanopyridine on a silver electrode. J. Electroanal. Chem. 1997, 423, 67–81. [Google Scholar] [CrossRef]
- Taylor, R.W.; Coulston, R.J.; Biedermann, F.; Mahajan, S.; Baumberg, J.J.; Scherman, O.A. In situ SERS monitoring of photochemistry within a nanojunction reactor. Nano Lett. 2013, 13, 5985–5990. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhu, Y.; Lin, M.; Wang, Q.; Zhao, L.; Yang, Y.; Yao, K.X.; Han, Y. Site-specific growth of Au–Pd alloy horns on Au nanorods: A platform for highly sensitive monitoring of catalytic reactions bysurface enhancement Raman spectroscopy. J. Am. Chem. Soc. 2013, 135, 8552–8561. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Walkenfort, B.; Schlücker, S. Label-free SERS monitoring of chemical reactions catalyzed by small gold nanoparticles using 3D plasmonic superstructures. J. Am. Chem. Soc. 2013, 135, 1657–1660. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Herrmann, C.; Kömpe, K.; Haase, M.; Schlücker, S. Synthesis of bifunctional Au/Pt/Au core/shell nanoraspberries for in situ SERS monitoring of platinum-catalyzed reactions. J. Am. Chem. Soc. 2011, 133, 19302–19305. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Cai, W.; Yang, L.; Liu, J. Monitoring plasmon-driven surface catalyzed reactions in situ using time-dependent surface-enhanced Raman spectroscopy on single particles of hierarchical peony-like silver microflowers. Nanoscale 2014, 6, 8612. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Tang, X.; Sun, B.; Yang, L. Highly sensitive in situ monitoring of catalytic reactions by surface enhancement Raman spectroscopy on multifunctional Fe3O4/C/Au NPs. Nanoscale 2014, 6, 7954–7958. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ma, B.; Yang, L.; Liu, J. Hybrid single nanoreactor for in situ SERS monitoring of plasmon-driven and small Au nanoparticles catalyzed reactions. Chem. Commun. 2015, 51, 11394–11397. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Li, D.W.; Cui, J.; Cao, Y.; Long, Y.T. In situ monitoring of palladacycle-mediated carbonylation by surface-enhanced Raman spectroscopy. RSC Adv. 2015, 5, 97734–97737. [Google Scholar] [CrossRef]
- Van Schrojenstein Lantman, E.M.; Deckert-Gaudig, T.; Mank, A.J.G.; Deckert, V.; Weckhuysen, B.M. Catalytic processes monitored at the nanoscale with tip-enhanced Raman spectroscopy. Nat. Nanotechnol. 2012, 7, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Trojanowicz, M.; Geschke, O.; Krawczyński vel Krawczyk, T.; Cammann, K. Biosensors based on oxidases immobilized in various conducting polymers. Sens. Actuators B Chem. 1995, 28, 191–199. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S.; Wang, E. Gram-scale, low-sost, rapid fabrication of high-quality width-controlled one-dimensional conducting polymer nanobelts. Chem. Mater. 2007, 19, 4621–4623. [Google Scholar] [CrossRef]
- Yan, S.; Yang, S.; He, L.; Ye, C.; Song, X.; Liao, F. Quantum size effect of poly(o-phenylenediamine) quantum dots: From controllable fabrication to tunable photoluminescence properties. Synth. Metals 2014, 198, 142–149. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, green, and blue luminescence by carbon dots: Full-color emission tuning and multicolor cellular imaging. Angew. Chem. Int. Ed. 2015, 54, 5360–5363. [Google Scholar] [CrossRef] [PubMed]
- Malitesta, C.; Palmisano, F.; Torsi, L.; Zambonin, P.G. Glucose fast-response amperometric sensor based on glucose oxidase immobilized in an electropolymerized poly(o-phenylenediamine) film. Anal. Chem. 1990, 62, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Broere, D.L.J.; Plessius, R.; van der Vlugt, J.I. New avenues for ligand-mediated processes—Expanding metal reactivity by the use of redox-active catechol, o-aminophenol and o-phenylenediamine ligands. Chem. Soc. Rev. 2015, 44, 7011. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Song, J.F.; Zhao, M.Z.; Wang, J.X. Electrochemical immunoassay based on catalytic conversion of substrate by labeled metal ion and polarographic detection of the product generated. Anal. Biochem. 1998, 259, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.M.; Liaw, D.S. Cu(II) ion catalytic oxidation of o-phenylenediamine and diaminomaleonitrile and the crystal structure of the final products (C12N4H11)(ClO4)H2O and [Cu5(CN)6(dmf)4]. Inorg. Chim. Acta 1986, 113, L11–L12. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cancès, E. The IEF version of the PCM solvation method: An overview of a new method addressed to study molecular solutes at the QM ab initio level. J. Mol. Struct. Theochem. 1999, 464, 211–226. [Google Scholar] [CrossRef]
- Computational Chemistry Comparison and Benchmark Database. Available online: http://cccbdb.nist.gov (accessed on 18 October 2016).
- Yuan, C.; Liu, X.; Jia, M.; Luo, Z.; Yao, J. Facile preparation of N- and O-doped hollow carbon spheres derived from poly(o-phenylenediamine) for supercapacitors. J. Mater. Chem. A 2015, 3, 3409–3415. [Google Scholar] [CrossRef]
- Baptista, P.; Doria, G.; Henriques, D.; Pereira, E.; Franco, R. Colorimetric detection of eukaryotic gene expression with DNA-derivatized gold nanoparticles. J. Biotechnol. 2005, 119, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Li, D.; Zhu, L.; Yang, W.; Tang, H. A new plasmonic Pickering emulsion based SERS sensor for in situ reaction monitoring and kinetic study. J. Mater. Chem. C 2016, 4, 736–744. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, R.-J.; Sun, J.-J.; Song, H.; Tian, J.-Z.; Li, D.-W.; Long, Y.-T. Real-Time Sensing of O-Phenylenediamine Oxidation on Gold Nanoparticles. Sensors 2017, 17, 530. https://doi.org/10.3390/s17030530
Yu R-J, Sun J-J, Song H, Tian J-Z, Li D-W, Long Y-T. Real-Time Sensing of O-Phenylenediamine Oxidation on Gold Nanoparticles. Sensors. 2017; 17(3):530. https://doi.org/10.3390/s17030530
Chicago/Turabian StyleYu, Ru-Jia, Jia-Jia Sun, Heng Song, Jing-Zhi Tian, Da-Wei Li, and Yi-Tao Long. 2017. "Real-Time Sensing of O-Phenylenediamine Oxidation on Gold Nanoparticles" Sensors 17, no. 3: 530. https://doi.org/10.3390/s17030530
APA StyleYu, R.-J., Sun, J.-J., Song, H., Tian, J.-Z., Li, D.-W., & Long, Y.-T. (2017). Real-Time Sensing of O-Phenylenediamine Oxidation on Gold Nanoparticles. Sensors, 17(3), 530. https://doi.org/10.3390/s17030530