Electrochemical Oxidation of l-selenomethionine and Se-methylseleno-l-cysteine at a Thiol-Compound-Modified Gold Electrode: Its Application in a Flow-Through Voltammetric Sensor
Abstract
:1. Introduction
2. Experimental Section
2.1. Apparatus and Materials
2.2. Determining l-Selenomethionine Using Cyclic Voltammetry
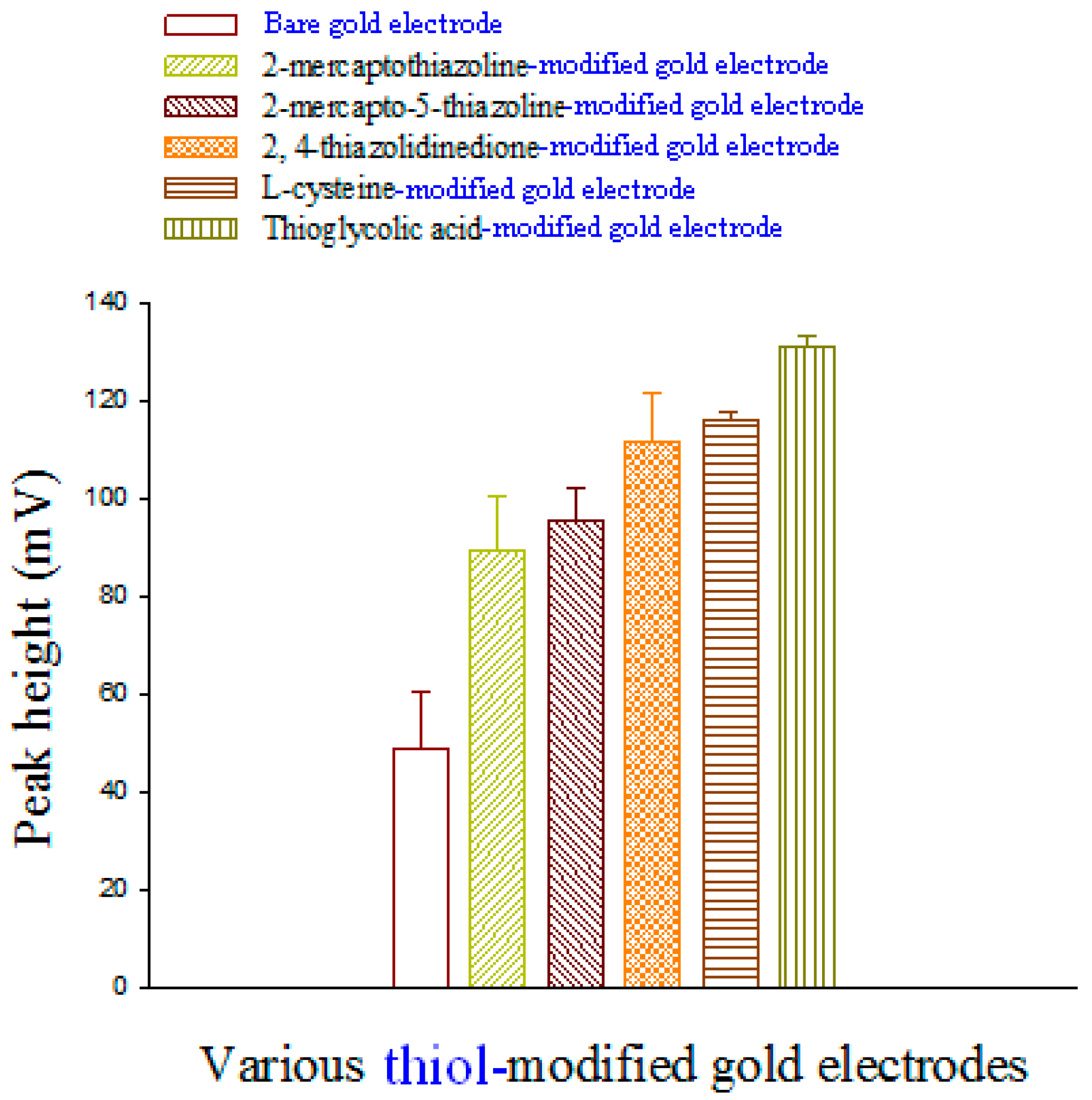
2.3. Preparing Thiol-Modified-Au Electrodes
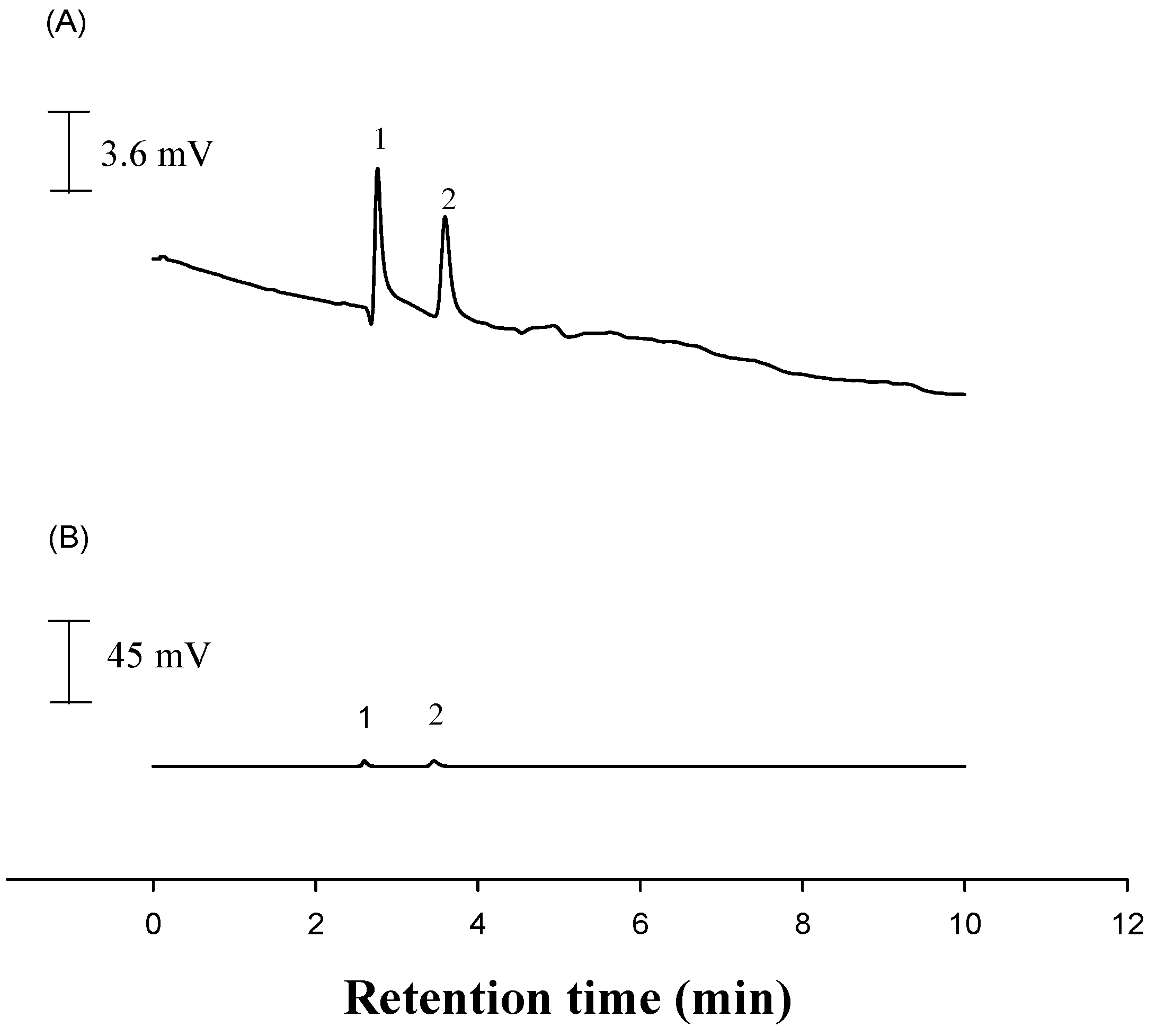
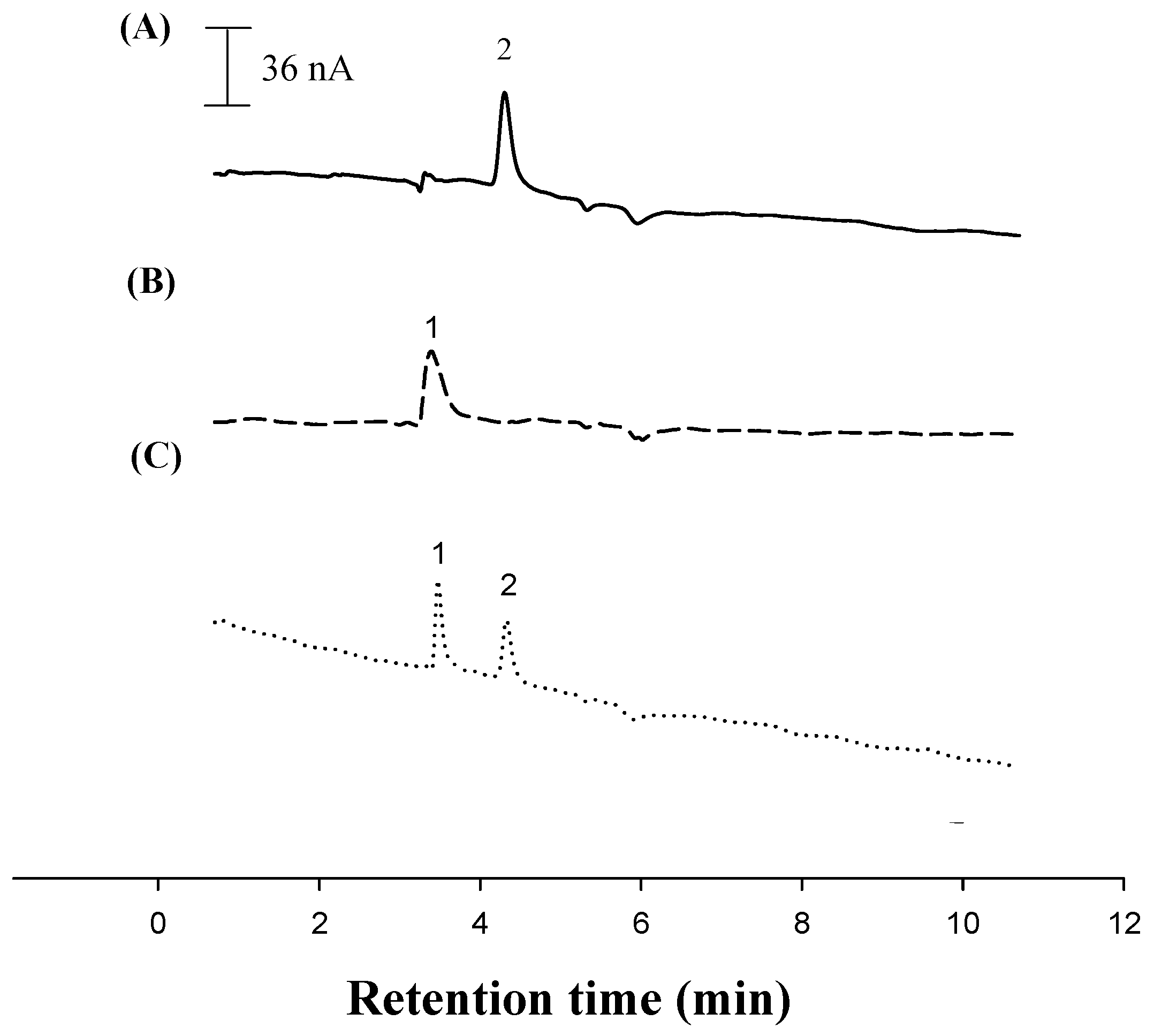
2.4. Construction of a Voltammetric Sensor for Liquid Chromatography
2.5. Application to Commercial Selenium Supplement Drugs
3. Results and Discussion
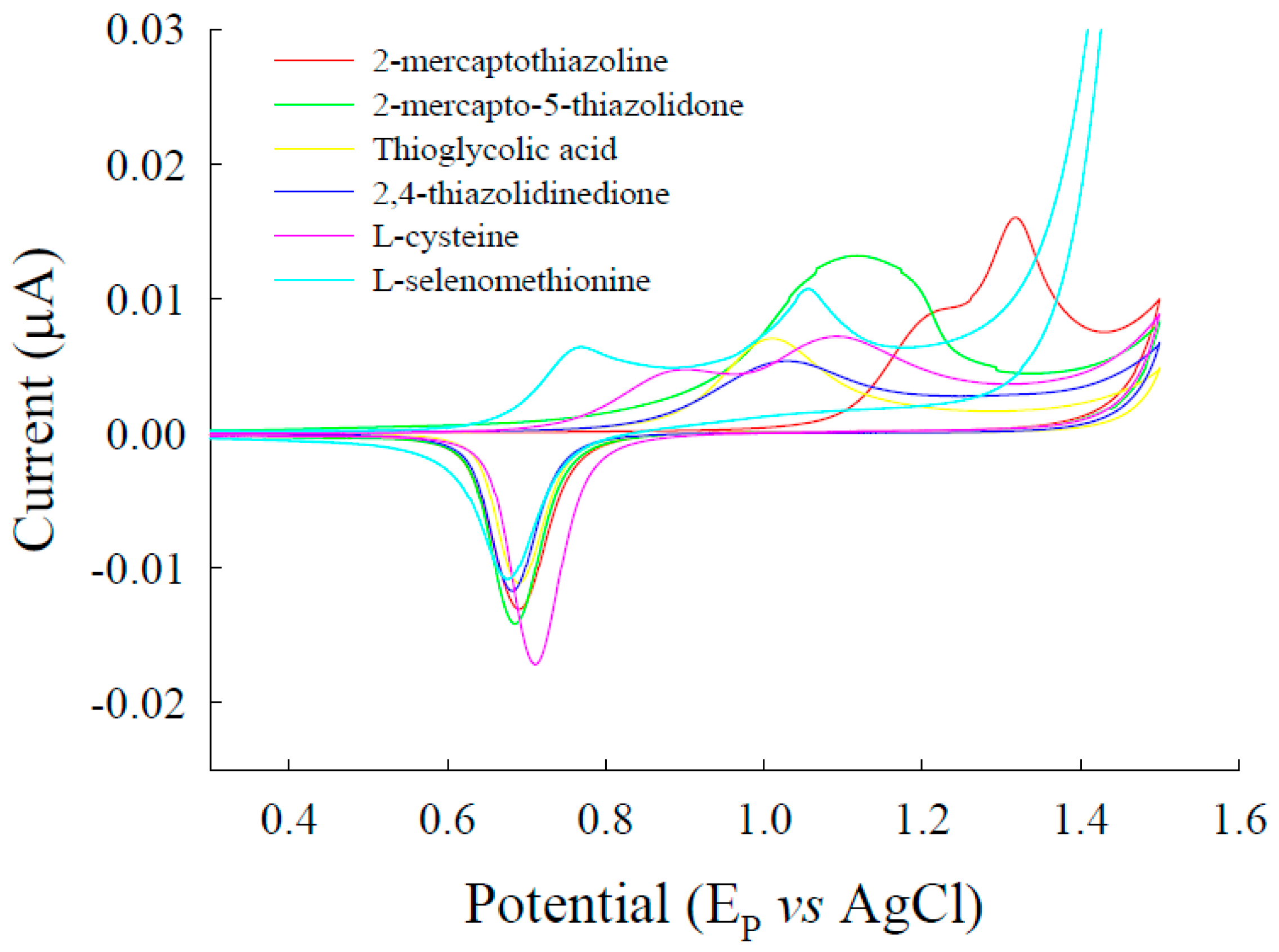
3.1. Voltammetric Behavior of l-Selenomethionine on Au Electrodes
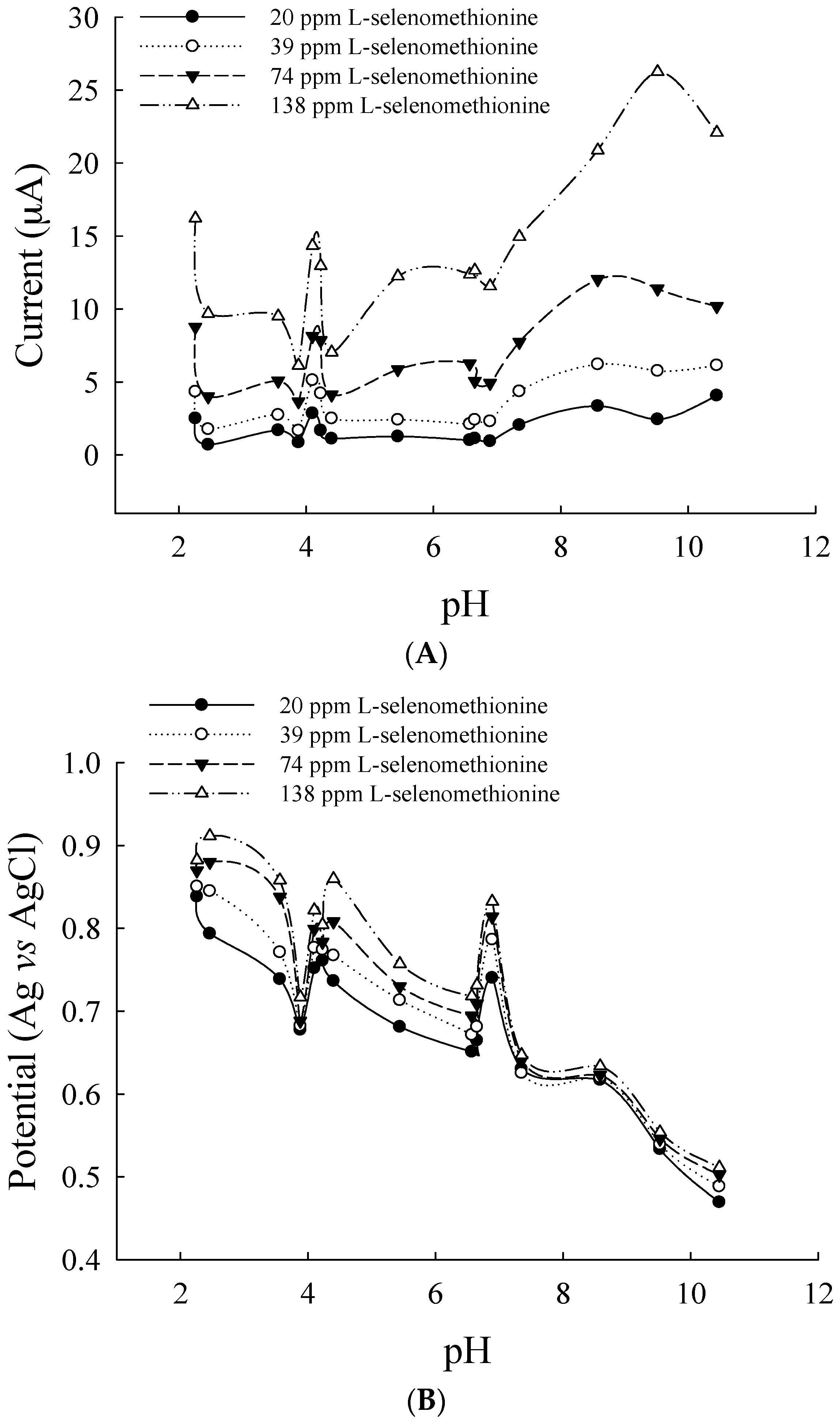
3.2. Optimal Conditions for a Flow-Through Voltammetric Detector
3.3. Accuracy and Precision
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rezvanfar, M.A.; Ahmadi, A.; Shojaei-Saadi, H.A.; Baeeri, M.; Abdollahi, M. Molecular mechanisms of a novel selenium-based complementary medicine which confers protection against hyperandrogenism-induced polycystic ovary. Theriogenology 2012, 78, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.Y.; Zhou, H.Y.; Sun, T.W.; Wu, Y.Y. Bacteriostasis experiment in vitro of Se-enriched herbal medicine compound prescription to Escherichia coli and Salmonella. J. Huber Inst. Natl. (Nat. Sci. Ed.) 2007, 25, 199–202. [Google Scholar]
- Shan, J.; Wang, X.; Ding, L.; Wei, H.; Sun, H. Advances in studies on biological functions of selenium and its determination method in Chinese herbal medicine. Chin. Tradit. Herb. Drugs 2003, 34, 280–283. [Google Scholar]
- Xie, C.; Cui, J.; Fu, H. High-Energy Composite Peptide Selenoprotein Nutrient Solution, Preparation Method and Application Thereof PCT. U.S. Patent US20160009604 A1, 26 December 2013. [Google Scholar]
- Kikkert, J.; Hale, B.; Berkelaar, E. Selenium accumulation in durum wheat and spring canola as a function of amending soils with selenite, selenate and or sulphate. Plant Soil 2013, 372, 629–641. [Google Scholar] [CrossRef]
- Cheng, J.Z.; Yang, P.; Gui, R.Y. Research progress on speciation of selenium compounds in plants. J. Zhejiang A F Univ. 2012, 29, 288–295. [Google Scholar]
- Kolachi, N.F.; Kazi, T.G.; Afridi, H.I.; Khan, S.; Baig, J.A.; Wadhwa, S.K.; Shah, A.Q.; Shah, F. Development of extraction methods for speciation analysis of selenium in aqueous extracts of medicinal plants. J. AOAC Int. 2011, 94, 1069–1075. [Google Scholar] [PubMed]
- Kolachi, N.F.; Kazi, T.G.; Afridi, H.I.; Khan, S.; Wadhwa, S.K.; Shah, A.Q.; Shah, F.; Baig, J.A. Determination of selenium content in aqueous extract of medicinal plants used as herbal supplement for cancer patients. Food Chem. Toxicol. 2010, 48, 3327–3332. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Jiang, Q.; Wang, R.H.; Gong, H.; Han, Y. Determination of trace selenium in plants by hydride generation atomic fluorescence spectrometry with program temperature-controlled graphite digestion. Spectrosc. Spectr. Anal. 2014, 34, 235–240. [Google Scholar]
- Deng, G.C.; Hou, S.M.; Mei, T.; Zhang, X.; Meng, W.; Yv, G.Y.; Zang, S.L. Speciation analysis of selenium in Cordyceps militaris by fluorescence spectrophotometry. J. Anal. Sci. 2006, 22, 21–24. [Google Scholar]
- Zhou, X.L.; Yu, Z.; Zhen, S.; Lin, A.Q.; Chang, S. Microscopic identification and the content of selenium in Gentiana rhodantha. North. Horticulture 2014, 2, 156–158. [Google Scholar]
- Kolachi, N.F.; Kazi, T.G.; Baig, J.A.; Kandhro, G.A.; Khan, S.; Afridi, H.I.; Kumar, S.; Shah, A.Q. Microwave-assisted acid extraction of selenium from medicinal plants followed by electrothermal atomic absorption spectrometric determination. J. AOAC Int. 2010, 93, 694–702. [Google Scholar] [PubMed]
- Zhang, Y.; Frankenberger, W.T. Speciation of selenium in plant water extracts by ion exchange chromatography-hydride generation atomic absorption spectrometry. Sci. Total Environ. 2001, 269, 39–47. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, K.; Liu, E.; Li, Y. Elemental fingerprint of herbal medicines formed by inductively coupled plasma atomic emission spectroscopy (ICP-AES). J. Eng. Sci. Technol. Rev. 2013, 6, 111–114. [Google Scholar]
- Yan, Q.; Yang, L.; Zhang, H.; Niu, L. Determination of mineral elements in fifteen medicinal herbs and their infusions by inductively coupled plasma atomic emission spectrometry. Asian J. Chem. 2012, 24, 899–902. [Google Scholar]
- Montes-Bayon, M.; Diaz, M.; Maria, J.; Gonzalez, E.B.; Sanz-Medel, A. Evaluation of different sample extraction strategies for selenium determination in selenium-enriched plants (Allium sativum and Brassica juncea) and Se speciation by HPLC-ICP-MS. Talanta 2006, 68, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Chan, Q.; Afton, S.E.; Caruso, J.A. Selenium speciation profiles in selenite-enriched soybean (Glycine Max) by HPLC-ICPMS and ESI-ITMS. Metallomics 2010, 2, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Afton, S.; Kubachka, K.; Catron, B.; Caruso, J.A. Simultaneous characterization of selenium and arsenic analytes via ion-pairing reversed phase chromatography with inductively coupled plasma and electrospray ionization ion trap mass spectrometry for detection applications to river water, plant extract and urine matrices. J. Chromatogr. A 2008, 1208, 156–163. [Google Scholar] [PubMed]
- Małgorzata, A.B.; Amund, M. Determination of selenium and its compounds in marine organisms. J. Trace Elem. Med. Biol. 2015, 29, 91–98. [Google Scholar]
- Avila, F.W.; Yang, Y.; Faquin, V.; Ramos, S.J.; Guilherme, L.R.; Thannhauser, T.W.; Li, L. Impact of selenium supply on Se-methylselenocysteine and glucosinolate accumulation in selenium-biofortified Brassica sprouts. Food Chem. 2014, 165, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Gusmao, R.; Diaz-Cruz, J.M.; Arino, C.; Esteban, M. Chemometric analysis of voltammetric data on metal ion binding by selenocystine. J. Phys. Chem. A 2012, 116, 6526–6531. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, T.; Liu, Y.; Zheng, W. Electrochemical oxidation of selenocystine and selenomethionine. Colloids Surf. B 2009, 74, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Yan, X.; Zheng, W.; Li, R.; Cheng, T.; Ruan, X. The electrochemical reaction mechanism of selenocystine on selenium-gold film modified glassy carbon electrode. Colloids Surf. B 2006, 49, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Shu, Y.; Wang, Z.; Wan, Q. Preparation of 3-mercaptopropionic acid self-assembled gold electrode and its electrochemical investigations for UA. J. Wuhan Inst. Technol. 2011, 33, 20–26. [Google Scholar]
- Phares, N.; White, R.J.; Plaxco, K.W. Improving the stability and sensing of electrochemical biosensors by employing trithiol-anchoring groups in a six-carbon self-assembled monolayer. Anal. Chem. 2009, 81, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, X.J.; Guo, L.R.; Jin, B.; Xia, X.H.; Zheng, L.M. Direct electrochemistry of cytochrome c on a phosphonic acid terminated self-assembled monolayers. Talanta 2009, 78, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Chang, B.Y.; Nam, H.; Park, S.M. Selective electrochemical sensing of glycated hemoglobin (HbA1c) on thiophene-3-boronic acid self-assembled monolayer covered gold electrodes. Anal. Chem. 2008, 80, 8035–8044. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, I.; Stobiecka, M.; Orlewska, C.; Rohand, T.; Janssen, D.; Dehaen, W.; Radecka, H. Electroactive dipyrromethene-Cu(II) self-assembled monolayers: Complexation reaction on the surface of gold electrodes. Langmuir 2008, 24, 11239–11245. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, A.; Laboria, N.; Latta, D.; O’Sullivan, C.K. Electron permeable self-assembled monolayers of dithiolated aromatic scaffolds on gold for biosensor applications. Anal. Chem. 2008, 80, 2256–2563. [Google Scholar] [CrossRef] [PubMed]
- Shamsipur, M.; Kazemi, S.H.; Alizadeh, A.; Mousavi, M.F.; Workentin, M.S. Self-assembled monolayers of a hydroquinone-terminated alkanethiol onto gold surface. Interfacial electrochemistry and Michael-addition reaction with glutathione. J. Electroanal. Chem. 2007, 610, 218–226. [Google Scholar] [CrossRef]
- Feng, G.; Niu, T.; You, X.; Wan, Z.; Kong, Q.; Bi, S. Studies on the effect of electrode pretreatment on the coverage of self-assembled monolayers of dodecanethiol on gold by electrochemical reductive desorption determination. Analyst 2011, 136, 5058–5063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Peng, T.; Yang, C.F. A piezoelectric gene-sensor using actinomycin D-functionalized nano-microspheres as amplifying probes. J. Electroanal. Chem. 2002, 522, 152–157. [Google Scholar] [CrossRef]
- Baldrich, E.; Laczka, O.; Del Campo, F.J.; Munoz, F.X. Gold immuno-functionalisation via self-assembled monolayers: Study of critical parameters and comparative performance for protein and bacteria detection. J. Immun. Methods 2008, 336, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Shaporenko, A.; Muller, J.; Weidner, J.; Terfort, A.; Zhamikov, M. Balance of Structure-Building Forces in Selenium-Based Self-Assembled Monolayers. J. Am. Chem. Soc. 2007, 129, 2232–2233. [Google Scholar] [CrossRef] [PubMed]
- Llave, E.; Scherlis, D. Selenium-Based Self-Assembled Monolayers: The Nature of Adsorbate-Surface Interactions. Langmuir 2010, 26, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Sampath, S. Enhanced stability of short- and long-chain diselenide self-assembled monolayers on gold probed by electrochemistry, spectroscopy, and microscopy. J. Colloids Interface Sci. 2007, 312, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Li, W.J. Studies on the Electrochemical Behavior of Thiazolidine and Its Applications Using a Flow–Through Chronoamperometric Sensor Based on a Gold Electrode. Molecules 2011, 16, 7608–7620. [Google Scholar] [CrossRef] [PubMed]
- Maria, O.P.; Fotis, T. Speciation analysis of selenium using voltammetric techniques. Anal. Chim. Acta 2002, 467, 167–178. [Google Scholar]
- Walker, L.M.; Karnaukh, E.A.; Dewan, F.; Buzzeo, M.C. Voltammetric behavior of selenocystine at modified gold substrates. Electrochem. Commun. 2016, 69, 28–31. [Google Scholar] [CrossRef]

| Samples | Added (mg·L−1) | Found a(mg·L−1) Mean ± SD b | Recovered (%) |
|---|---|---|---|
| l-selenomethionine | |||
| Sample 1 | 0.5 | 0.486 ± 0.007 | 97.3 |
| 1.0 | 1.014 ± 0.018 | 101 | |
| 2.0 | 1.985 ± 0.024 | 99.2 | |
| Sample 2 | 0.5 | 0.488 ± 0.046 | 97.6 |
| 1.0 | 1.045 ± 0.069 | 104 | |
| 2.0 | 2.002 ± 0.040 | 100 | |
| Sample 3 | 0.5 | 0.507 ± 0.068 | 101 |
| 1.0 | 1.036 ± 0.064 | 103 | |
| 2.0 | 1.993 ± 0.022 | 99.6 | |
| Se-methylseleno-l-cysteine b | |||
| Sample 4 | 0.5 | 0.499 ± 0.023 | 99.8 |
| 1.0 | 1.011 ± 0.031 | 101 | |
| 2.0 | 1.995 ± 0.013 | 99.7 | |
| Sample 5 | 0.5 | 0.549 ± 0.023 | 105 |
| 1.0 | 1.031 ± 0.014 | 103 | |
| 2.0 | 2.034 ± 0.032 | 103 | |
| Samples | l-selenomethionine | Se-methylseleno-l-cysteine b | ||
|---|---|---|---|---|
| Concentration (%, w/w) N = 3 a | Concentration (%, w/w) N = 3 a | |||
| LC-ECD | LC-UV | LC-ECD | LC-UV | |
| Sample 1 | 0.035 ± (3.4) | 0.036 ± (2.8) | - c | - c |
| Sample 2 | 0.101 ± (1.4) | 0.102 ± (2.0) | - c | - c |
| Sample 3 | 0.131 ± (3.0) | 0.133 ± (3.1) | - c | - c |
| Sample 4 | - c | - c | 0.138 ± (2.5) | - c |
| Sample 5 | - c | - c | 0.875 ± (0.4) | - c |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-H.; Zhang, Y.-H. Electrochemical Oxidation of l-selenomethionine and Se-methylseleno-l-cysteine at a Thiol-Compound-Modified Gold Electrode: Its Application in a Flow-Through Voltammetric Sensor. Sensors 2017, 17, 383. https://doi.org/10.3390/s17020383
Wang L-H, Zhang Y-H. Electrochemical Oxidation of l-selenomethionine and Se-methylseleno-l-cysteine at a Thiol-Compound-Modified Gold Electrode: Its Application in a Flow-Through Voltammetric Sensor. Sensors. 2017; 17(2):383. https://doi.org/10.3390/s17020383
Chicago/Turabian StyleWang, Lai-Hao, and Yu-Han Zhang. 2017. "Electrochemical Oxidation of l-selenomethionine and Se-methylseleno-l-cysteine at a Thiol-Compound-Modified Gold Electrode: Its Application in a Flow-Through Voltammetric Sensor" Sensors 17, no. 2: 383. https://doi.org/10.3390/s17020383
APA StyleWang, L.-H., & Zhang, Y.-H. (2017). Electrochemical Oxidation of l-selenomethionine and Se-methylseleno-l-cysteine at a Thiol-Compound-Modified Gold Electrode: Its Application in a Flow-Through Voltammetric Sensor. Sensors, 17(2), 383. https://doi.org/10.3390/s17020383





