Synthesis of Graphene-Based Sensors and Application on Detecting SF6 Decomposing Products: A Review
Abstract
:1. Introduction
2. Synthesis and Analysis of Graphene-Based Materials
2.1. Synthesis of Graphene-Based Materials
2.2. Adsorption and Sensing Mechanism of Graphene-Based Materials
3. Application of Graphene-Based Sensors
3.1. Theoretical Calculations about Graphene-Based Sensors
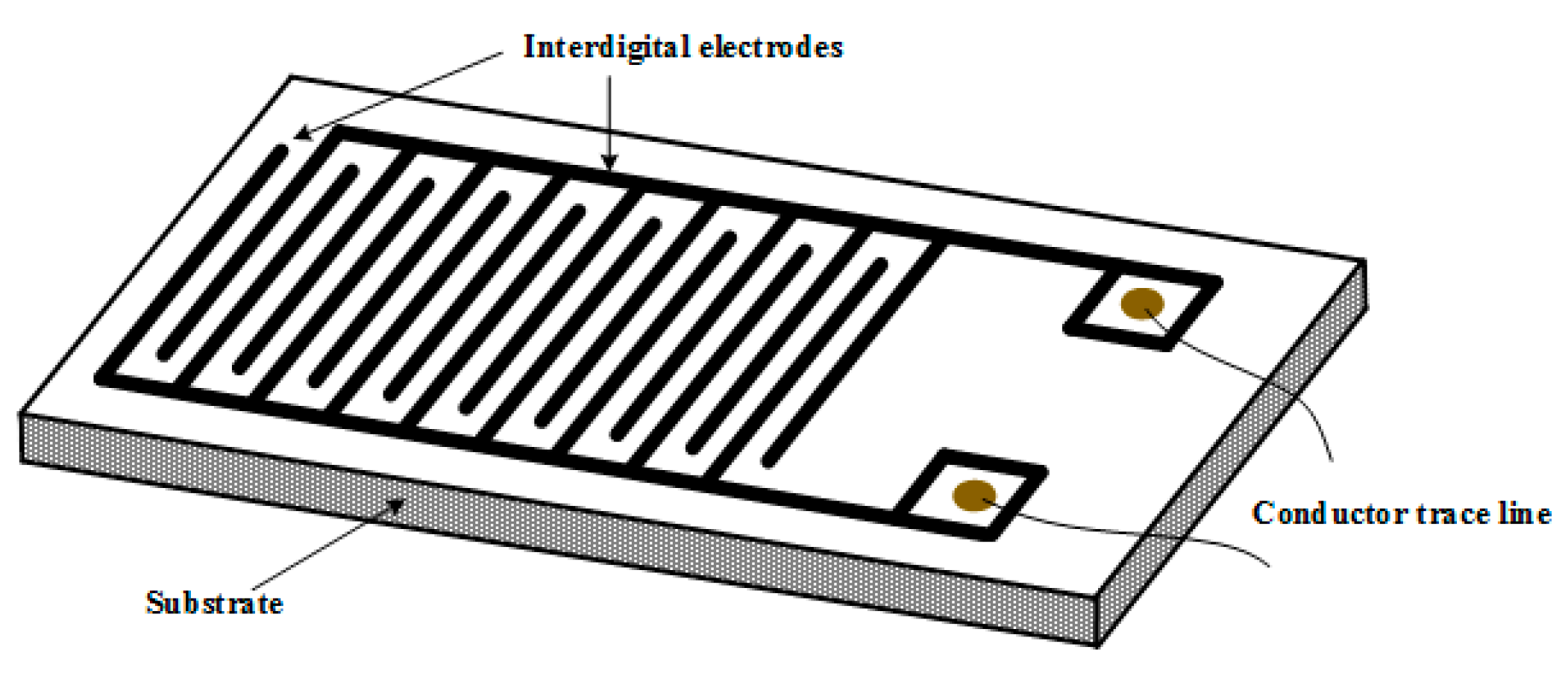
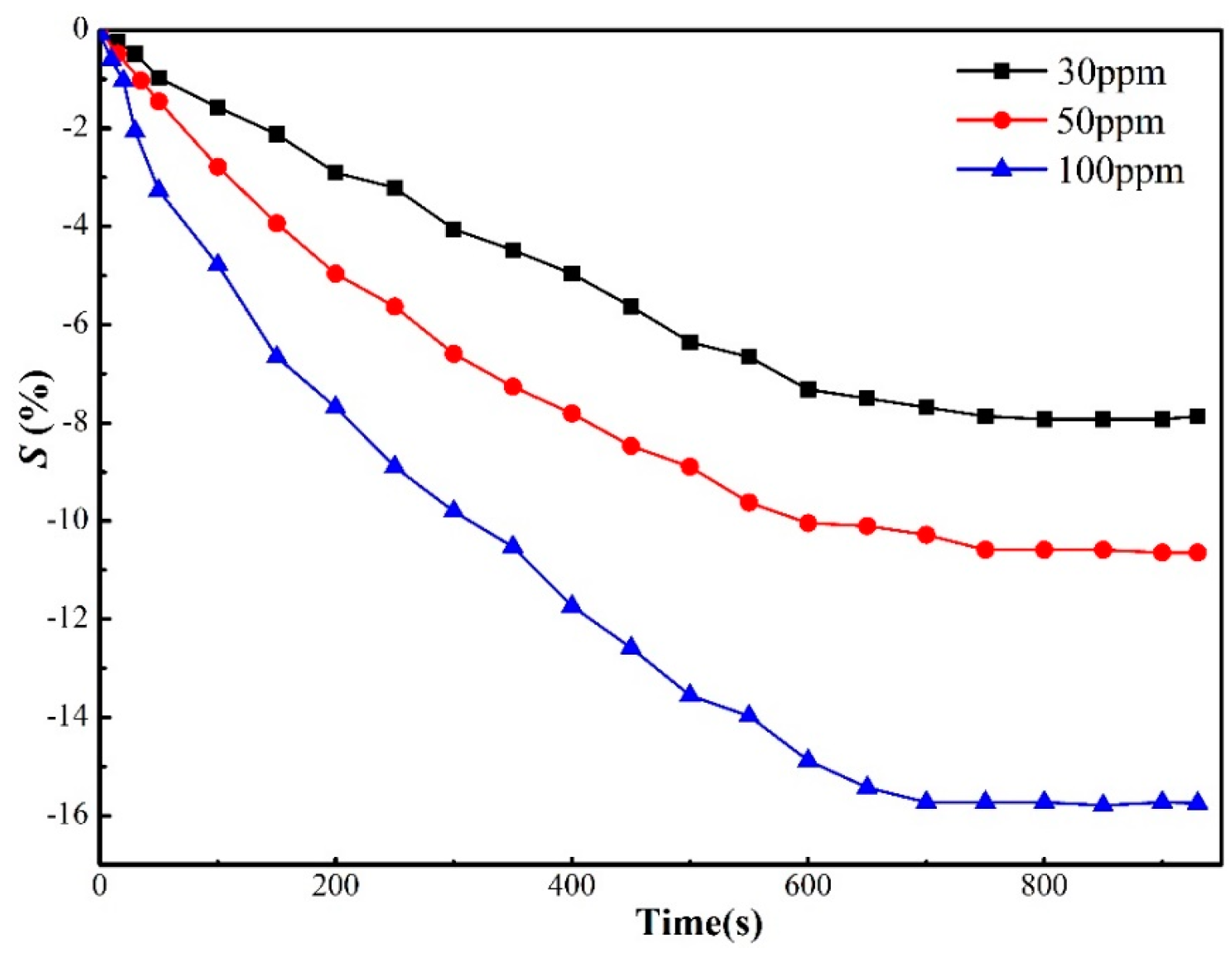
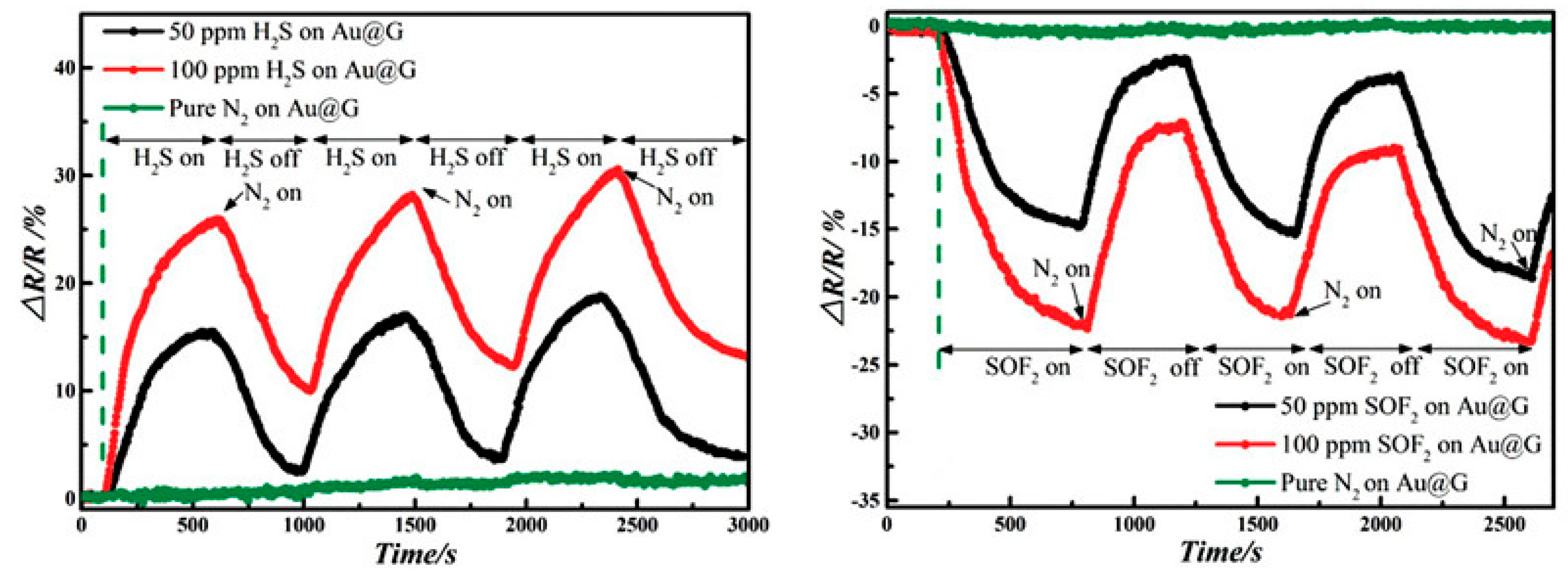
3.2. Experimental Analyses about Graphene-Based Sensors
4. Conclusions and Perspectives
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Wallace, P.R. The Band Theory of Graphite. Phys. Rev. 1947, 71, 622–634. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Falko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Abergel, D.S.L.; Apalkov, V.; Berashevich, J.; Ziegler, K.; Chakraborty, T. Properties of graphene: A theoretical perspective. Adv. Phys. 2010, 59, 261–482. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, B.; Liu, G.; Zhuang, X.; Kang, E.-T. Graphene and its derivatives: Switching ON and OFF. Chem. Soc. Rev. 2012, 41, 4688–4707. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Qu, X. Extraordinary physical properties of functionalized graphene. Small 2012, 8, 2138–2151. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611–622. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Weiss, N.O.; Zhou, H.; Lei, L.; Yuan, L.; Shan, J.; Yu, H.; Duan, X. Graphene: An emerging electronic material. Adv. Mater. 2012, 24, 5782–5825. [Google Scholar] [CrossRef] [PubMed]
- Yavari, F.; Koratkar, N. Graphene-Based Chemical Sensors. J. Phys. Chem. Lett. 2012, 3, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Arellano, J.S.; Molina, L.M.; Rubio, A.; Alonso, J.A. Density functional study of adsorption of molecular hydrogen on graphene layers. J. Chem. Phys. 2000, 112, 8114–8119. [Google Scholar] [CrossRef]
- Ataca, C.; Akturk, E.; Ciraci, S. Hydrogen storage of calcium atoms adsorbed on graphene: First-principles plane wave calculations. Phys. Rev. B Condens. Matter 2009, 79, 041406. [Google Scholar] [CrossRef]
- Casolo, S.; Løvvik, O.M.; Martinazzo, R.; Tantardini, G.F. Understanding adsorption of hydrogen atoms on graphene. J. Chem. Phys. 2009, 130, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Yuan, J.; Giannozzi, P. Gas adsorption on graphene doped with B, N, Al, and S: A theoretical study. Appl. Phys. Lett. 2009, 95, 232105. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. B 2008, 77, 125416. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Suehiro, J.; Zhou, G.B.; Hara, M. Detection of partial discharge in SF6 gas using a carbon nanotube-based gas sensor. Sens. Actuators B Chem. 2005, 105, 164–169. [Google Scholar] [CrossRef]
- Ph, R.-J.; Yousfi, M. New Breakdown Electric Field Calculation for SF6 High Voltage Circuit Breaker Applications. Plasma Sci. Technol. 2007, 9, 690. [Google Scholar]
- Irawan, R.; Scelsi, G.B.; Woolsey, G.A. Optical Fiber Sensing of SF6 Degradation in High-Voltage Switchgear. J. Nonlinear Opt. Phys. Mater. 2012, 10, 181–195. [Google Scholar] [CrossRef]
- Minagawa, T.; Kawada, M.; Yamauchi, S.; Kamei, M.; Nishida, C. Development of SF6 decomposition gas sensor. Surf. Coat. Technol. 2003, 169, 643–645. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, B.; Liu, W.; Zhang, J. Detection of Partial Discharge in SF6 Decomposition Gas Based on Modified Carbon Nanotubes Sensors. Procedia Eng. 2012, 29, 4107–4111. [Google Scholar] [CrossRef]
- Ju, T.; Min, F.; Tan, Z.; Sun, C. Crossover Response Processing Technology of Photoacoustic Spectroscopy Signal of SF6 Decomposition Components under Partial Discharge. High Volt. Eng. 2013, 39, 257–264. [Google Scholar]
- Tang, J.; Zeng, F.; Pan, J.; Zhang, X.; Yao, Q.; He, J.; Hou, X. Correlation analysis between formation process of SF6 decomposed components and partial discharge qualities. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 864–875. [Google Scholar] [CrossRef]
- Beyer, C.; Jenett, H.; Klockow, D. Influence of reactive SFx gases on electrode surfaces after electrical discharges under SF6 atmosphere. IEEE Trans. Dielectr. Electr. Insul. 2000, 7, 234–240. [Google Scholar] [CrossRef]
- Braun, J.M.; Chu, F.Y.; Seethapathy, R. Characterization of GIS Spacers Exposed to SF6 Decomposition Products. IEEE Trans. Electr. Insul. 1987, 22, 187–193. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, A.D.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, S.; Fang, Y.; Dong, S. Reducing sugar: new functional molecules for the green synthesis of graphene nanosheets. ACS Nano 2010, 4, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Kumar, R.; Mazher, J.; Balan, A. Graphene made easy: High quality, large-area samples. Solid State Commun. 2009, 149, 718–721. [Google Scholar] [CrossRef]
- Martín-García, B.; Velázquez, M.M.; Rossella, F.; Bellani, V.; Diez, E.; Fierro, J.L.G.; Pérez-Hernández, J.A.; Hernández-Toro, J.; Claramunt, S.; Cirera, A. Functionalization of reduced graphite oxide sheets with a zwitterionic surfactant. Chemphyschem 2012, 13, 3682–3690. [Google Scholar] [CrossRef] [PubMed]
- Obraztsov, A.N.; Obraztsova, E.A.; Tyurnina, A.V.; Zolotukhin, A.A. Chemical vapor deposition of thin graphite films of nanometer thickness. Carbon 2007, 45, 2017–2021. [Google Scholar] [CrossRef]
- Obraztsov, A.N.; Zolotukhin, A.A.; Ustinov, A.O.; Volkov, A.P.; Svirko, Y.; Jefimovs, K. DC discharge plasma studies for nanostructured carbon CVD. Diam. Relat. Mater. 2003, 12, 917–920. [Google Scholar] [CrossRef]
- Gadipelli, S.; Zheng, X.G. Graphene-based materials: Synthesis and gas sorption, storage and separation. Prog. Mater. Sci. 2015, 69, 1–60. [Google Scholar] [CrossRef]
- Yang, R.T. Adsorbents: Adsorbents: Fundamentals and Applications; Wiley-Interscience: Hoboken, NJ, USA, 2003. [Google Scholar]
- Au, H. Molecular Dynamics Simulation of Nanoporous Graphene for Selective Gas Separation; Massachusetts Institute of Technology: Cambridge, MA, USA, 2013. [Google Scholar]
- Liscio, A.; Veronese, G.P.; Treossi, E.; Suriano, F.; Rossella, F.; Bellani, V.; Rizzoli, R.; Samorì, P.; Palermo, V. Charge transport in graphene–polythiophene blends as studied by Kelvin Probe Force Microscopy and transistor characterization. J. Mater. Chem. 2011, 21, 2924–2931. [Google Scholar] [CrossRef]
- Esfandiar, A.; Ghasemi, S.; Irajizad, A.; Akhavan, O.; Gholami, M.R. The decoration of TiO2/reduced graphene oxide by Pd and Pt nanoparticles for hydrogen gas sensing. Int. J. Hydr. Energy 2012, 37, 15423–15432. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, R.; Song, G.; Li, Y.; Chen, Z.; Hu, J. Highly aligned SnO2 nanorods on graphene sheets for gas sensors. J. Mater. Chem. 2011, 21, 17360–17365. [Google Scholar] [CrossRef]
- Congcong, M.A.; Shao, X.H.; Cao, D.P. Nitrogen-doped graphene as an excellent candidate for selective gas sensing. Sci. China Chem. 2014, 57, 911–917. [Google Scholar]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.D.; Allen, M.J.; Tung, V.C.; Yang, Y.; Kaner, R.B.; Weiller, B.H. Practical chemical sensors from chemically derived graphene. ACS Nano 2009, 3, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, R.S.; Navarro, C.G.; Balasubramanian, K.; Burghard, M.; Kern, K. Electrochemical Modification of Graphene. Adv. Mater. 2008, 20, 3050–3053. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, F.S.; Ren, J.B.; Ju, T.; Bing, Y. Simulation on the B-doped Single-walled Carbon Nanotubes Detecting the Partial Discharge of SF6. High Volt. Eng. 2011, 37, 1689–1694. [Google Scholar]
- Zhou, Z.; Gao, X.; Yan, J.; Song, D. Doping effects of B and N on hydrogen adsorption in single-walled carbon nanotubes through density functional calculations. Carbon 2006, 44, 939–947. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, Z.; Meng, F.; Qiu, Y.; Ju, T. DFT Calculations on the Adsorption of Components of SF6 Decomposed Under Partial Discharge Onto Carboxyl Carbon Nanotubes. Proc. Chin. Soc. Electr. Eng. 2012, 32, 85–91. [Google Scholar]
- Zhang, X.; Gui, Y.; Dai, Z. A simulation of Pd-doped SWCNTs used to detect SF6 decomposition components under partial discharge. Appl. Surf. Sci. 2014, 315, 196–202. [Google Scholar] [CrossRef]
- Zhang, X.; Gui, Y.; Xiao, H.; Zhang, Y. Analysis of adsorption properties of typical partial discharge gases on Ni-SWCNTs using density functional theory. Appl. Surf. Sci. 2016, 379, 47–54. [Google Scholar] [CrossRef]
- Ganji, M.D.; Sharifi, N.; Ardjmand, M.; Ahangari, M.G. Pt-decorated graphene as superior media for H2S adsorption: A first-principles study. Appl. Surf. Sci. 2012, 261, 697–704. [Google Scholar] [CrossRef]
- Cuong, N.T.; Sugiyama, A.; Fujiwara, A.; Mitani, T.; Chi, D.H. Density functional study of Pt(4) clusters adsorbed on a carbon nanotube support. Phys. Rev. B 2009, 79, 1377–1381. [Google Scholar] [CrossRef]
- Berahman, M.; Asad, M.; Sheikhi, M.H.; Zarifkar, A.; Gebauer, R.; Taheri, M. H2S Gas Sensor Based on Thin Film Graphene Nanoribbons Decorated with Copper: A First Principles Study. In Proceedings of the Ultrafine Grained and Nano-Structured Materials, Tehran, Iran, 5–6 November 2013.
- Chen, C.; Xu, K.; Ji, X.; Miao, L.; Jiang, J. Enhanced adsorption of acidic gases (CO2, NO2 and SO2) on light metal decorated graphene oxide. Phys. Chem. Chem. Phys. 2014, 16, 11031–11036. [Google Scholar] [CrossRef] [PubMed]
- Rad, A.S.; Esfahanian, M.; Maleki, S.; Gharati, G. Application of carbon nanostructures toward SO2 and SO3 adsorption: A comparison between pristine graphene and N-doped graphene by DFT calculations. J. Sulfur Chem. 2016, 37, 176–188. [Google Scholar]
- Rad, A.S.; Shabestari, S.S.; Mohseni, S.; Aghouzi, S.A. Study on the adsorption properties of O3, SO2, and SO3 on B-doped graphene using DFT calculations. J. Solid State Chem. 2016, 237, 204–210. [Google Scholar] [CrossRef]
- Rad, A.S.; Zareyee, D. Adsorption properties of SO2 and O3 molecules on Pt-decorated graphene: A theoretical study. Vacuum 2016, 130, 113–118. [Google Scholar]
- Zhang, X.; Yu, L.; Gui, Y.; Hu, W. First-principles study of SF6 decomposed gas adsorbed on Au-decorated graphene. Appl. Surf. Sci. 2016, 367, 259–269. [Google Scholar] [CrossRef]
- Castillo, R.M.D.; Sansores, L.E. Study of the electronic structure of Ag, Au, Pt and Pd clusters adsorption on graphene and their effect on conductivity. Eur. Phys. J. B 2015, 88, 1–13. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, R.; Gui, Y.; Zeng, H. Gas Sensing Analysis of Ag-Decorated Graphene for Sulfur Hexafluoride Decomposition Products Based on the Density Functional Theory. Sensors 2016, 16, 1830. [Google Scholar] [CrossRef] [PubMed]
- Mabayoje, O.; Seredych, M.; Bandosz, T.J. Cobalt (hydr)oxide/graphite oxide composites: Importance of surface chemical heterogeneity for reactive adsorption of hydrogen sulfide. J. Colloid Interface Sci. 2012, 378, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mabayoje, O.; Seredych, M.; Bandosz, T.J. Enhanced reactive adsorption of hydrogen sulfide on the composites of graphene/graphite oxide with copper (hydr)oxychlorides. ACS Appl. Mater. Interfaces 2012, 4, 3316–3324. [Google Scholar] [CrossRef] [PubMed]
- Seredych, M.; Bandosz, T.J. Adsorption of hydrogen sulfide on graphite derived materials modified by incorporation of nitrogen. Mater. Chem. Phys. 2009, 113, 946–952. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Reactive adsorption of hydrogen sulfide on graphite oxide/Zr(OH)4 composites. Chem. Eng. J. 2011, 166, 1032–1038. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Effects of Surface Features on Adsorption of SO2 on Graphite Oxide/Zr(OH)4 Composites. J. Phys. Chem. C 2010, 114, 14552–14560. [Google Scholar] [CrossRef]
- Babu, D.J.; Kühl, F.G.; Yadav, S.; Markert, D.; Bruns, M.; Hampe, M.J.; Schneider, J.J. Adsorption of pure SO2 on nanoscaled graphene oxide. RSC Adv. 2016, 6, 36834–36839. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, X.; Tang, J. The sensitive characteristics study of SF6 decomposed gases using a graphene sensor. In Proceedings of the 2014 International Conference on High Voltage Engineering and Application, Poznan, Poland, 8–11 September 2014.
- Zilli, D.; Bonelli, P.R.; Cukierman, A.L. Room temperature hydrogen gas sensor nanocomposite based on Pd-decorated multi-walled carbon nanotubes thin films. Sens. Actuators B Chem. 2011, 157, 169–176. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Wu, X.; Hu, W. Experimental Sensing and Density Functional Theory Study of H2S and SOF2 Adsorption on Au-Modified Graphene. Adv. Sci. 2015, 2, 1500101. [Google Scholar] [CrossRef] [PubMed]
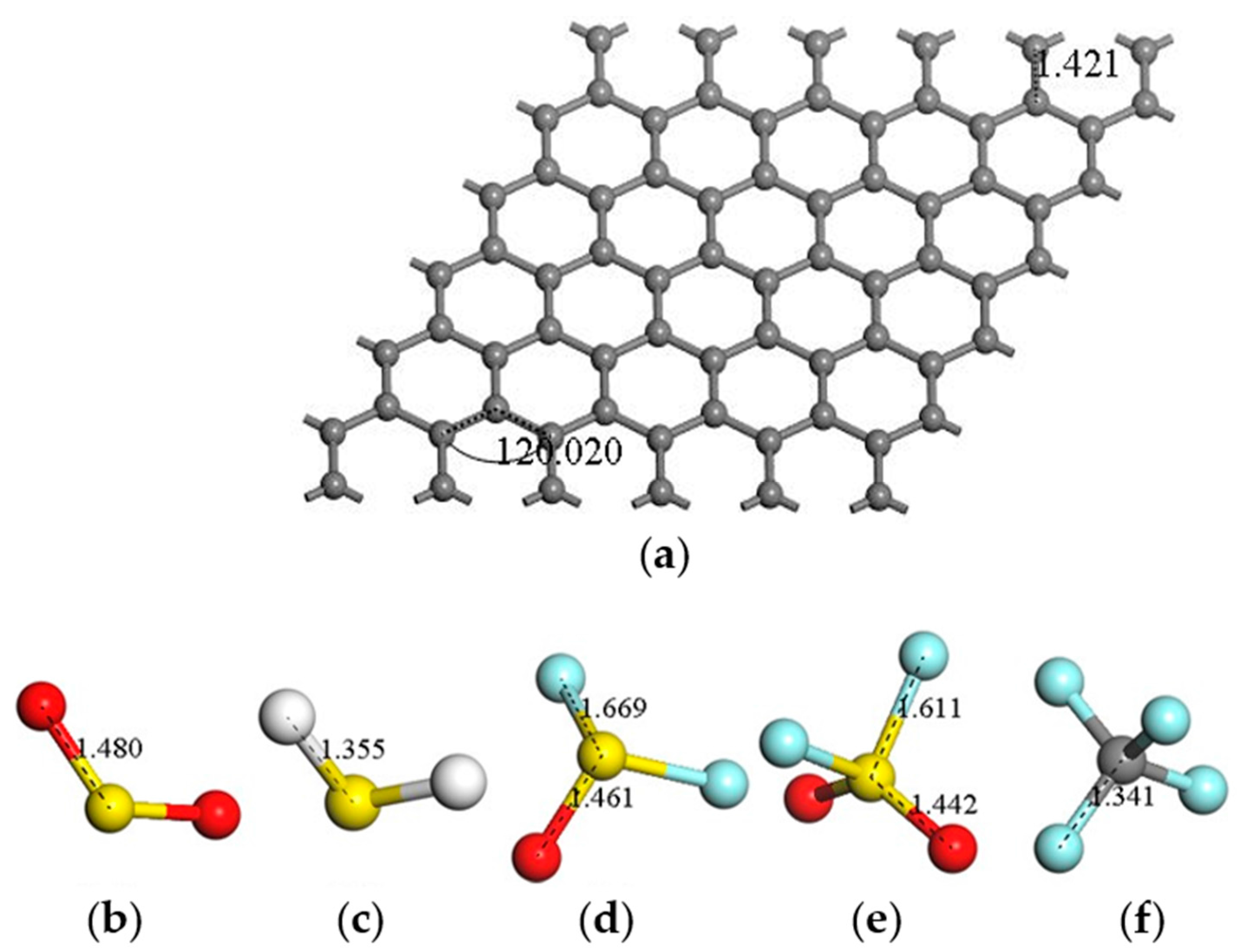
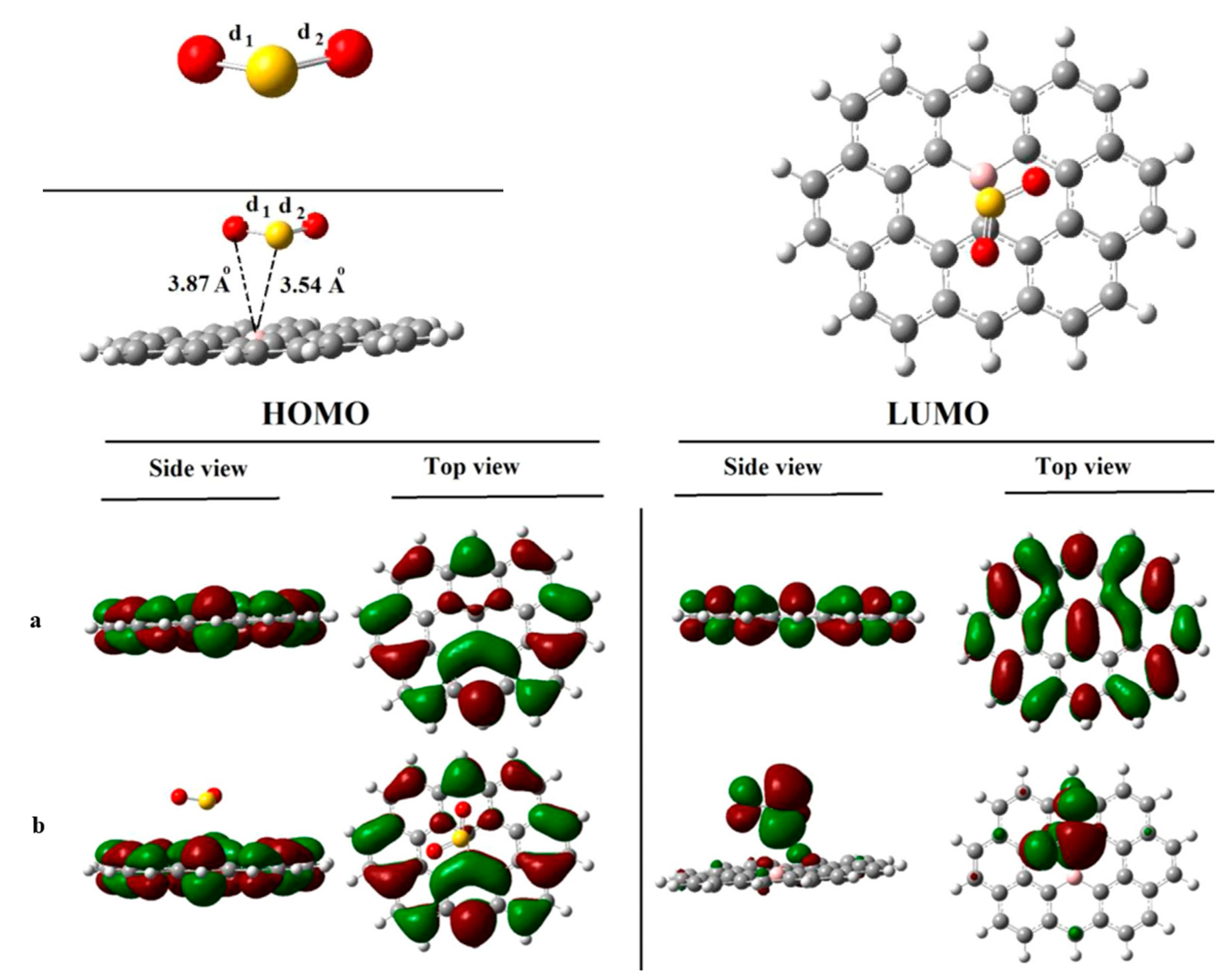
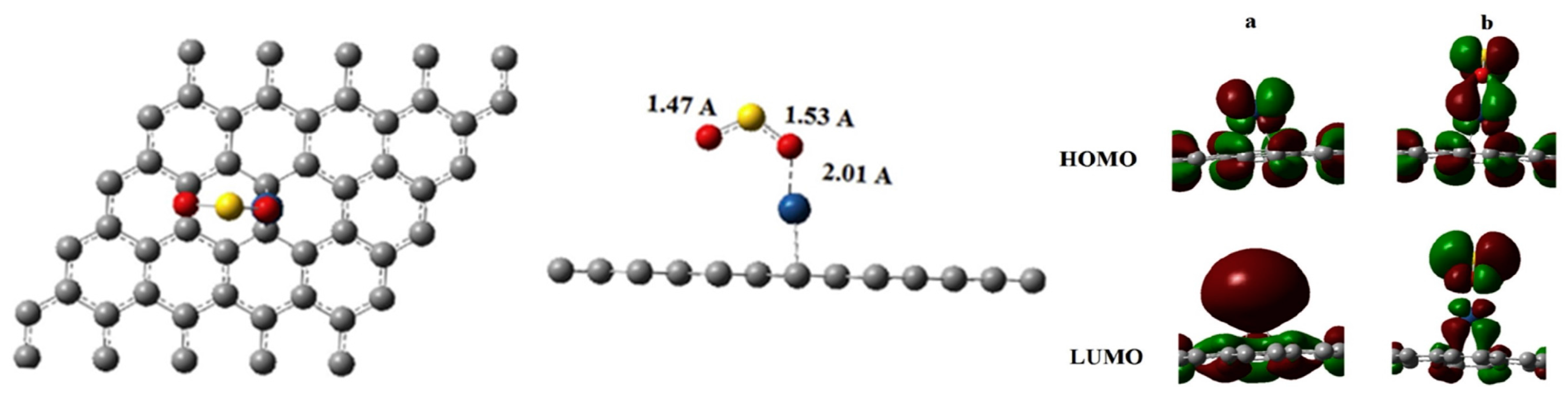
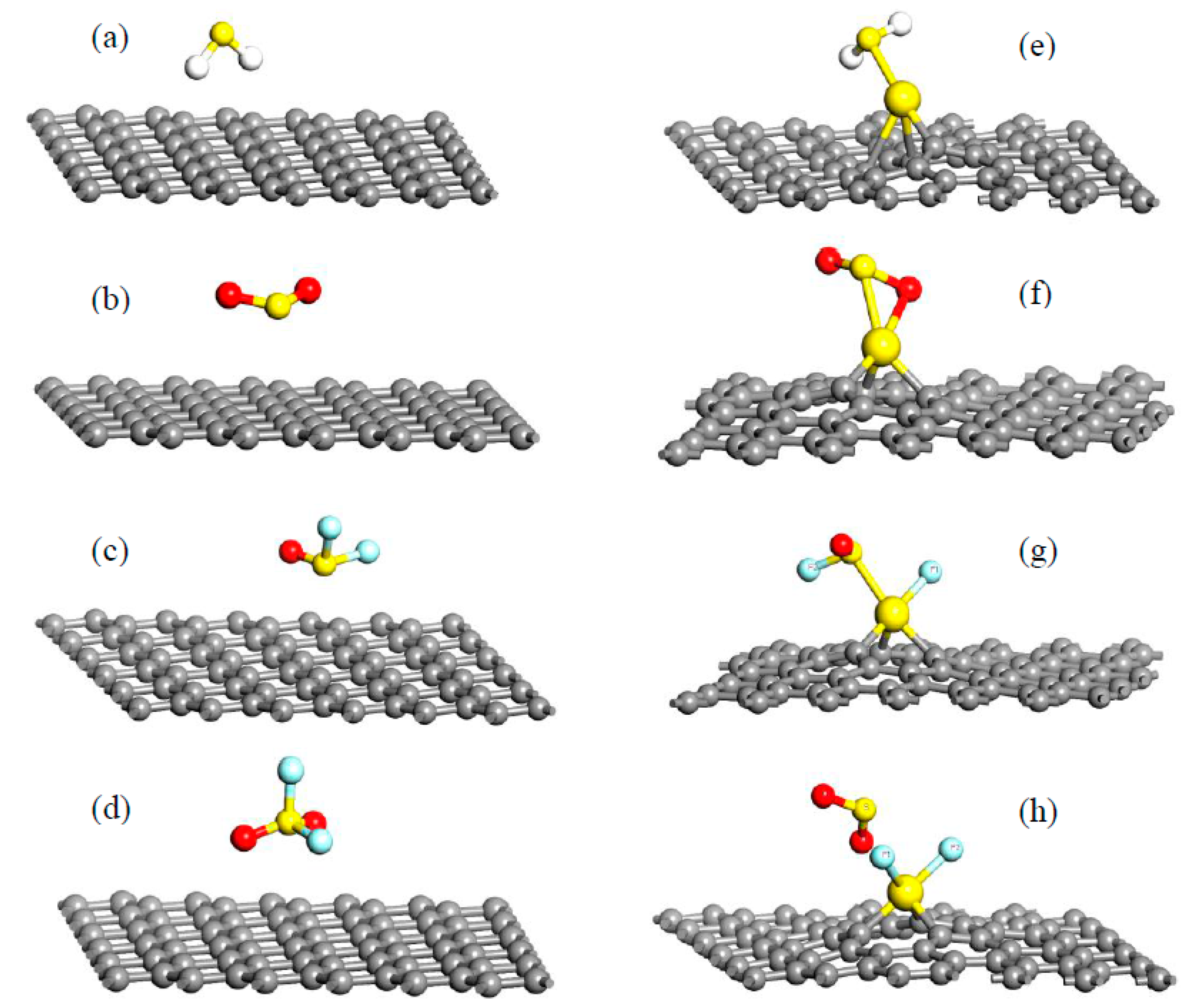
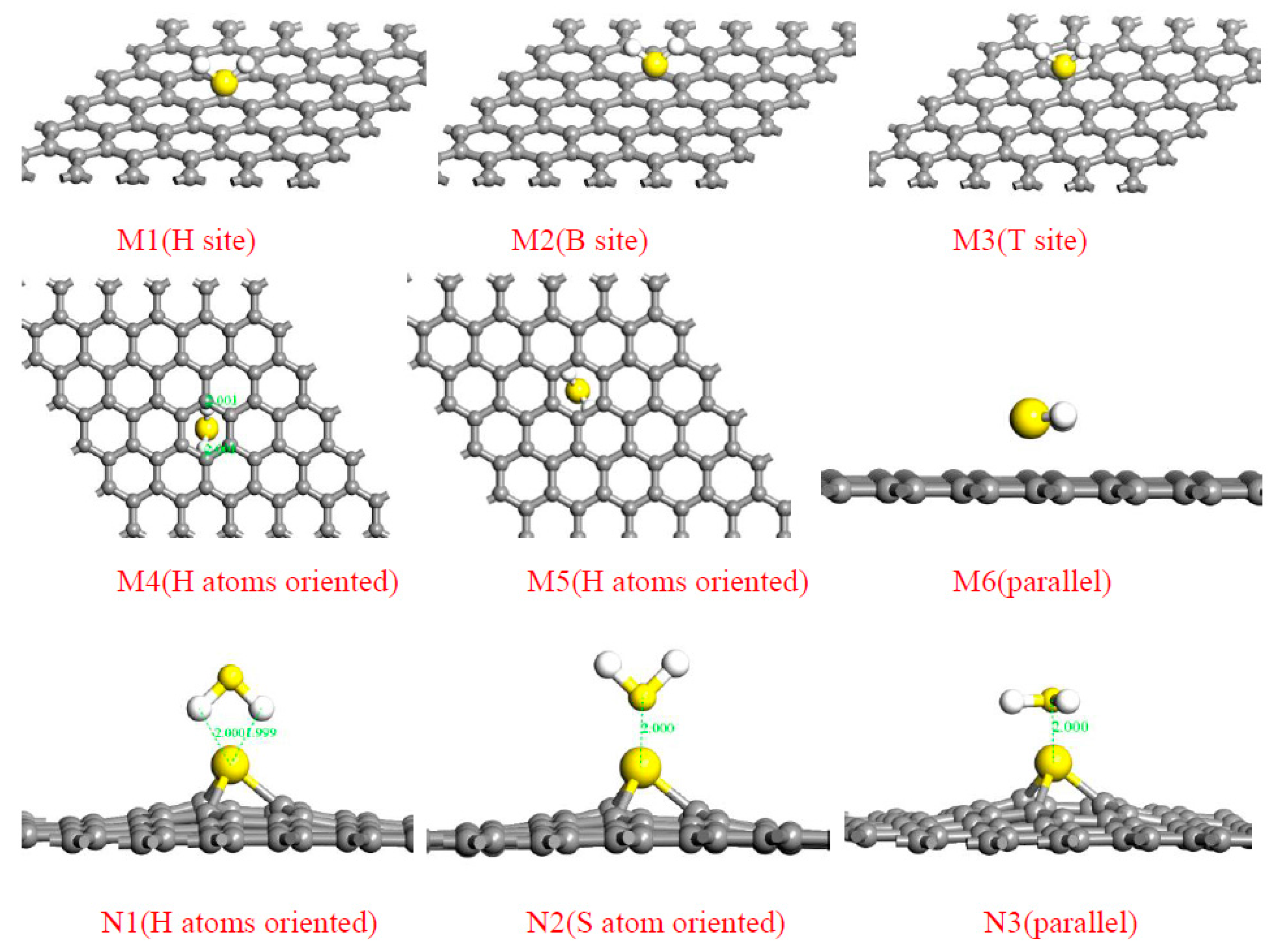
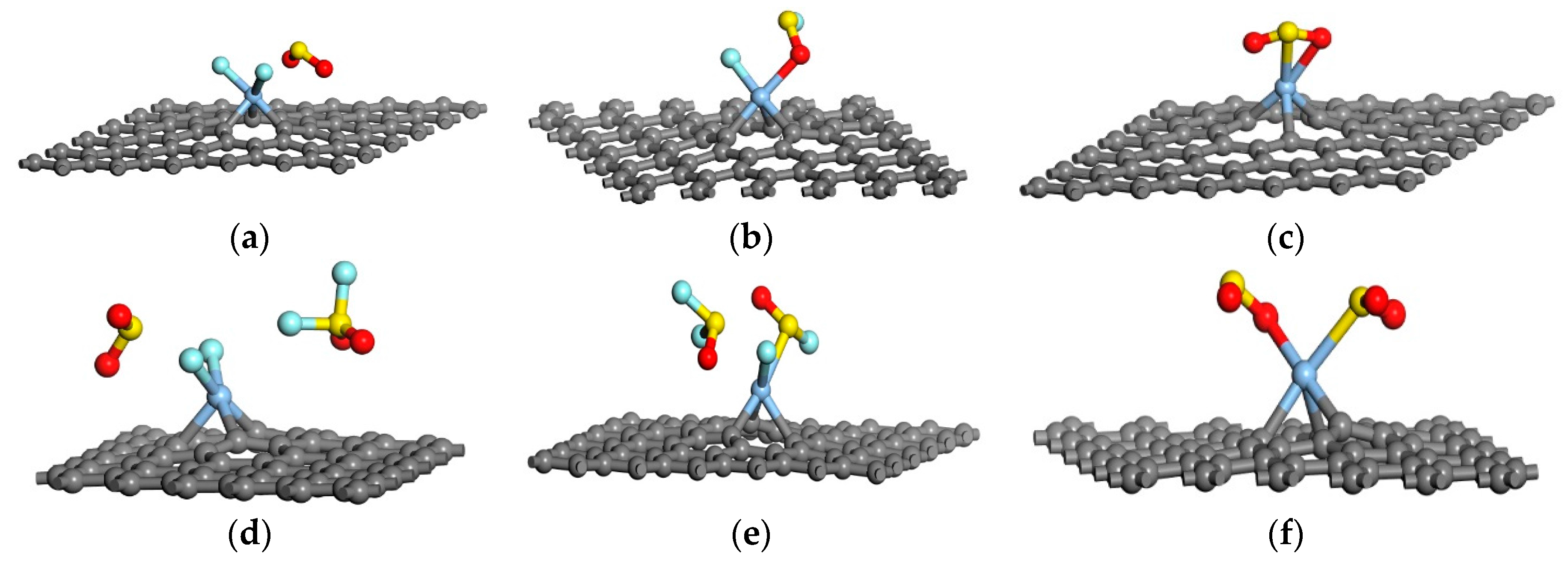
| Sites | M1 | M2 | M3 | M4 | M5 | M6 | N1 | N2 | N3 |
|---|---|---|---|---|---|---|---|---|---|
| Ead (H2S) (eV) | −0.308 | −0.322 | −0.367 | −0.521 | −0.617 | −0.287 | −0.551 | −0.9 | −0.543 |
| System | D (Å) | Ead (eV) | Qt (e) |
|---|---|---|---|
| Ag-graphene/SO2F2 | 2.013 | −1.448 | −1.084 |
| Ag-graphene/SOF2 | 2.029 | −0.678 | −0.153 |
| Ag-graphene/SO2 | 2.210 | −1.075 | −0.350 |
| Ag-graphene/2SO2F2 | 2.004 | −1.365 | −1.113 |
| Ag-graphene/2SOF2 | 2.080 | −0.503 | −0.203 |
| Ag-graphene/2SO2 | 2.210 | −1.465 | −0.701 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Cui, H.; Gui, Y. Synthesis of Graphene-Based Sensors and Application on Detecting SF6 Decomposing Products: A Review. Sensors 2017, 17, 363. https://doi.org/10.3390/s17020363
Zhang X, Cui H, Gui Y. Synthesis of Graphene-Based Sensors and Application on Detecting SF6 Decomposing Products: A Review. Sensors. 2017; 17(2):363. https://doi.org/10.3390/s17020363
Chicago/Turabian StyleZhang, Xiaoxing, Hao Cui, and Yingang Gui. 2017. "Synthesis of Graphene-Based Sensors and Application on Detecting SF6 Decomposing Products: A Review" Sensors 17, no. 2: 363. https://doi.org/10.3390/s17020363
APA StyleZhang, X., Cui, H., & Gui, Y. (2017). Synthesis of Graphene-Based Sensors and Application on Detecting SF6 Decomposing Products: A Review. Sensors, 17(2), 363. https://doi.org/10.3390/s17020363





