The Facile Synthesis of Branch-Trunk Ag Hierarchical Nanostructures and Their Applications for High-Performance H2O2 Electrochemical Sensors
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Apparatus
2.3. Synthesis of Branch-Truck Ag Hierarchical Nanostructures
2.4. Fabrication of Branch-Truck Ag Hierarchical Nanosturture Sensor
3. Results and Discussion
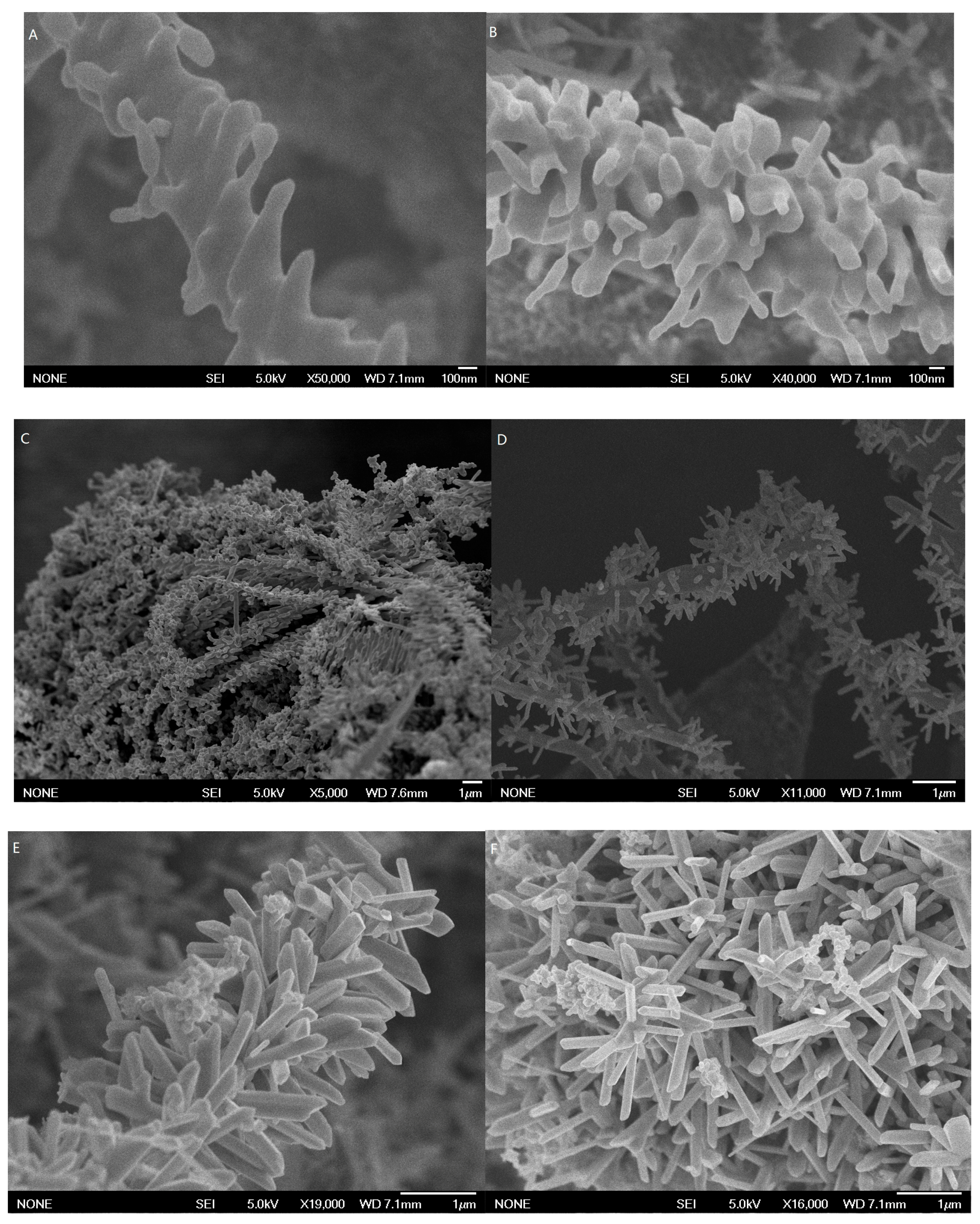
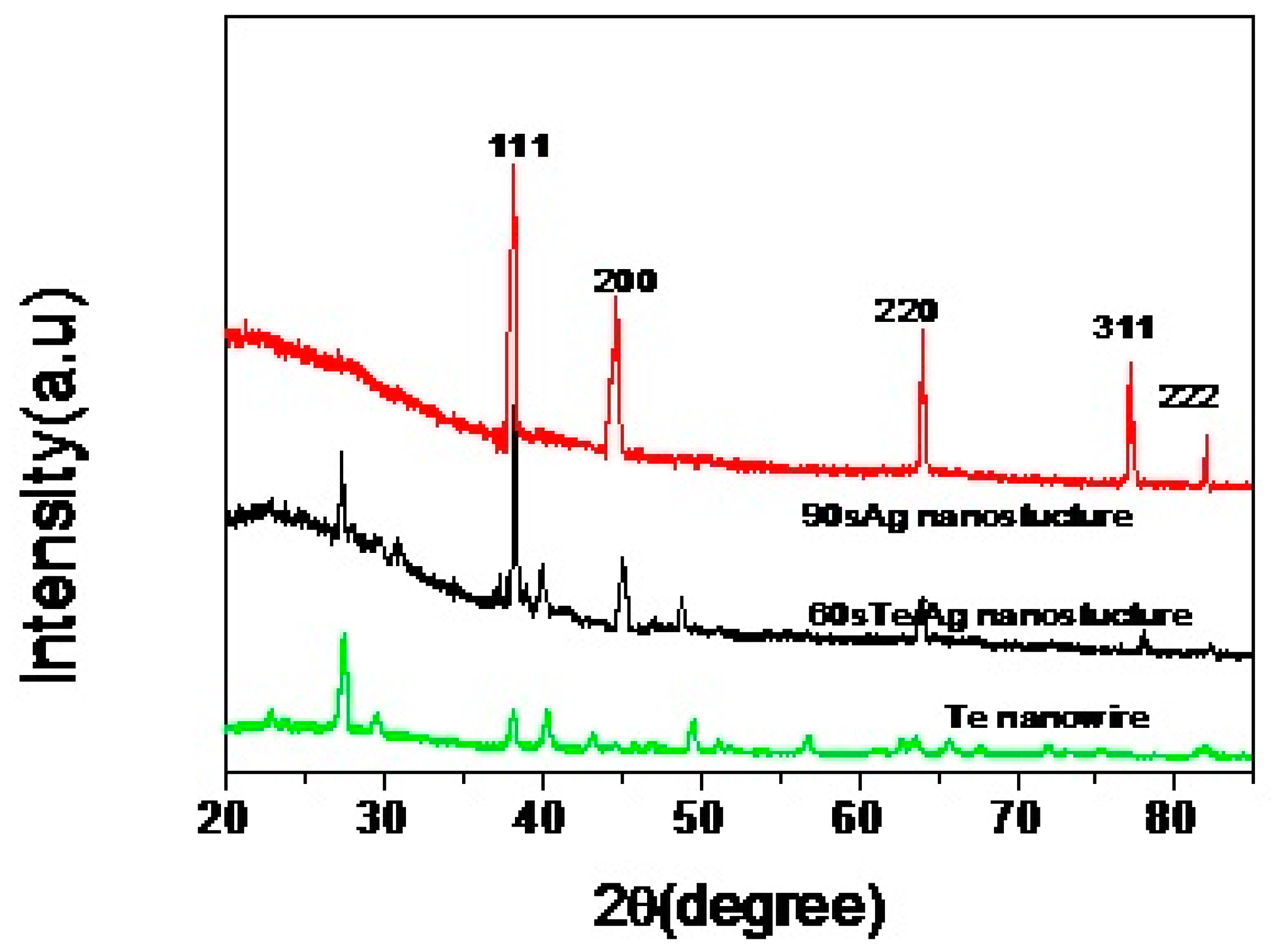
3.1. Characterization
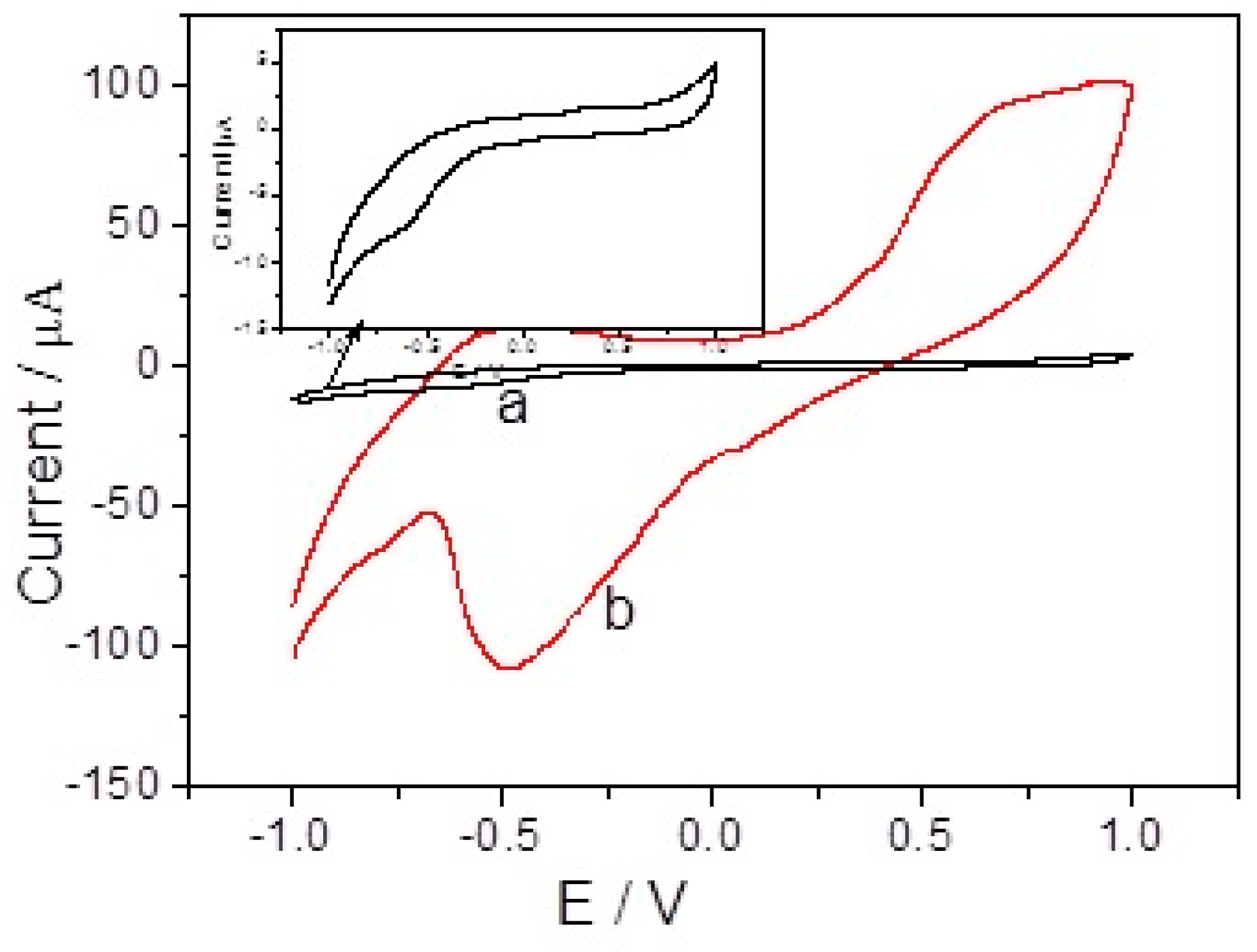
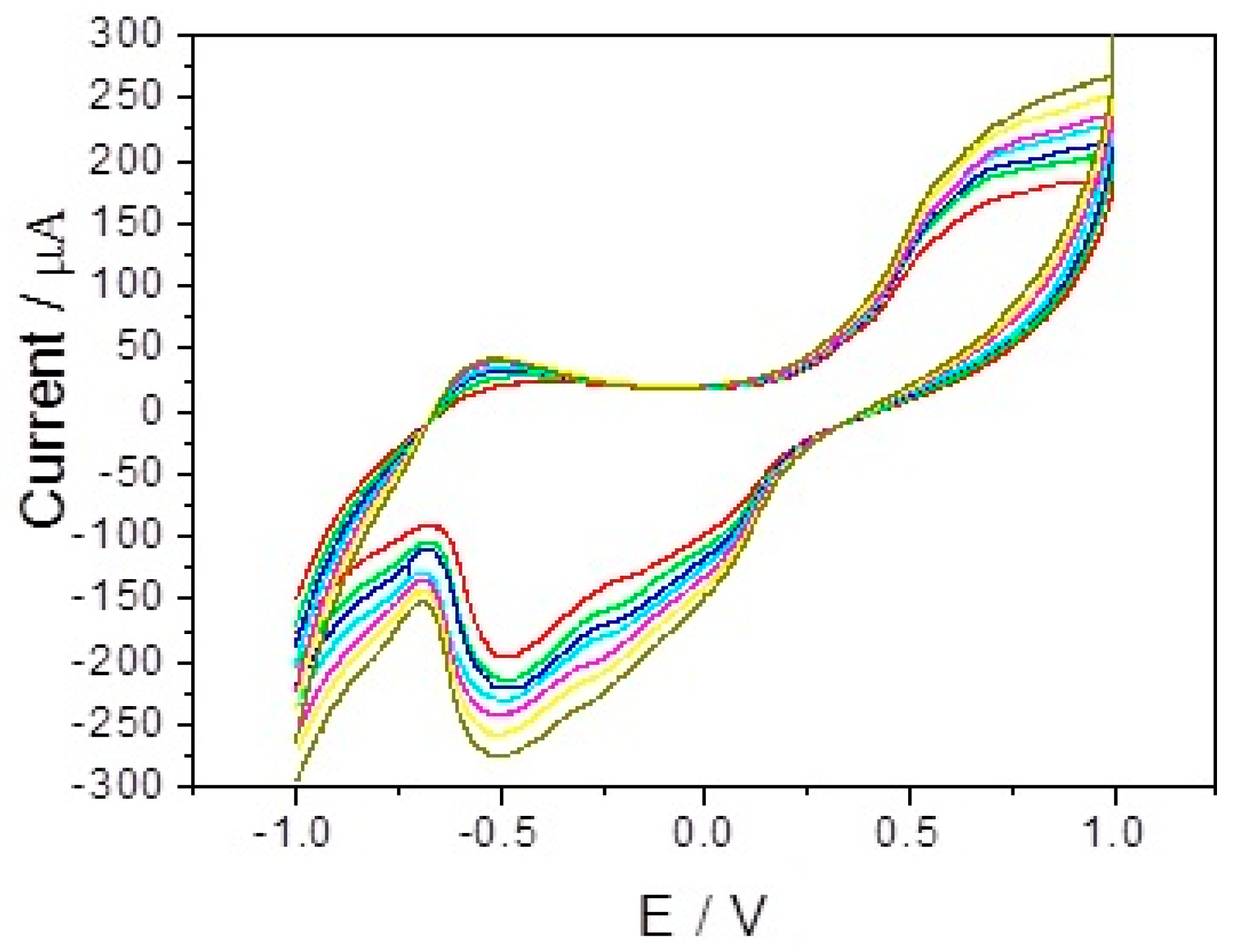
3.2. The Influence of Synthesis Condition:
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zeng, G.M.; Zhang, C.; Huang, D.L.; Lai, C.; Tang, L.; Zhou, Y.Y.; Xu, P.; Wang, H.; Qin, L.; Cheng, M. Practical and regenerable electrochemical aptasensor based on nanoporous gold and thymine-Hg2+-thymine base pairs for Hg2+ detection. Biosens. Bioelectron. 2017, 90, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.M.; Wang, M.G.; Yang, W.; Liu, S.T. Synthesis of surface layered hierarchical octahedral-like structured Zn2SnO4/SnO2 with excellent sensing properties toward HCHO. Sens. Actuators B Chem. 2017, 243, 1171–1180. [Google Scholar] [CrossRef]
- Venkadesh, A.; Radhakrishnan, S.; Mathiyarasu, J. Eco-friendly synthesis and morphology-dependent superior electrocatalytic properties of CuS nanostructures. Electrochim. Acta 2017, 246, 544–552. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Kim, H.Y.; Kim, B.S. A novel CuS microflower superstructure based sensitive and selective nonenzymatic glucose detection. Sens. Actuators B Chem. 2016, 233, 93–99. [Google Scholar] [CrossRef]
- Her, Y.C.; Yeh, B.Y.; Huang, S.L. Vapor−Solid Growth of p-Te/n-SnO2 Hierarchical heterostructures and their enhanced room-temperature gas sensing properties. Appl. Mater. Interfaces 2014, 6, 9150–9159. [Google Scholar] [CrossRef] [PubMed]
- Khoang, N.Y.D.; Trung, D.D.; Duy, N.V.; Hoa, N.D.; Hieu, N.V. Design of SnO2/ZnO hierarchical nanostructures for enhanced ethanol gas-sensing performance. Sens. Actuators B Chem. 2012, 174, 594–601. [Google Scholar] [CrossRef]
- Usui, Y.; Sato, K.; Tanaka, M. Catalytic dihydroxylation of olefins with hydrogenperoxide: An organic-solvent- and metal-free system. Angew. Chem. Int. Ed. 2003, 42, 5623–5625. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.R.; Leung, C.; Whitley, D.; Zhu, Y.; Arnold, R.G.; Saez, A.E. Advanced oxidation of trace organics in water by hydrogen peroxide solar photolysis. Ind. Eng. Chem. Res. 2011, 50, 12479–12487. [Google Scholar] [CrossRef]
- Huang, X.; Atwood, C.S.; Hartshorn, M.A.; Multhaup, G.; Goldstein, L.E.; Scarpa, R.C.; Cuajungco, M.P.; Gray, D.N.; Lim, J.; Moir, R.D.; et al. The Aβ peptide of Alzheimer’s disease directly produces hydrogen peroxide throughmetal ion reduction. Biochemistry 1999, 38, 7609–7616. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Wang, M.J. Preparation of Microfibrillated Cellulose Composites for sustained release of H2O2 or O2 for biomedical applications. Sustain. Chem. Eng. 2013, 1, 1129–1134. [Google Scholar] [CrossRef]
- Haghighi, B.; Hamidi, H.; Gorton, L. Formation of a robust and stable film comprising ionic liquid and polyoxometalate on glassy carbon electrode modified with multiwalled carbon nanotubes: Toward sensitive and fast detection of hydrogen peroxide and iodate. Electrochim. Acta 2010, 55, 4750–4757. [Google Scholar] [CrossRef]
- Recio, E.; Alvarez-Rodriguez, M.L.; Rumbero, A.; Garzon, E.; Coque, J.J.R. Destruction of chloroanisoles by using a hydrogen peroxide activated method and itsapplication to remove chloroanisoles from cork stoppers. J. Agric. Food Chem. 2011, 59, 12589–12597. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Nishioka, M.; Sadakata, M. Sterilization by H2O2 droplets undercorona discharge. J. Electrostat. 2002, 55, 173–187. [Google Scholar] [CrossRef]
- Choudhury, N.A.; Raman, R.K.; Sampath, S.; Shukla, A.K. An alkaline direct borohydride fuel cell with hydrogen peroxide as oxidant. J. Power Sources 2005, 143, 1–8. [Google Scholar] [CrossRef]
- Hasegawa, S.; Shimotani, K.; Kishi, K.; Watanabe, H. Electricity generation from decomposition of hydrogen peroxide. Electrochem. Solid State Lett. 2005, 8, A119–A121. [Google Scholar] [CrossRef]
- Thome-Duret, V.; Reach, G.; Gangnerau, M.N.; Lemonnier, F.; Klein, J.C.; Zhang, Y.; Hu, Y.; Wilson, G.S. Use of a subcutaneous glucose sensor to detect decreases in glucose concentration prior to observation in blood. Anal. Chem. 1996, 68, 3822–3826. [Google Scholar] [CrossRef] [PubMed]
- Duff, D.G.; Baiker, A.; Edwards, P.P. A new hydrosol of gold clusters. 1. Formation and particle size variation. Langmuir 1993, 9, 2301–2309. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Zhu, R.R.; Du, H.; Li, H.; Yang, Y.T.; Jia, Q.Y.; Bian, B. Higher catalyticactivity of porphyrin functionalized Co3O4 nanostructures for visual and colorimetric detection of H2O2 and glucose. Mater. Sci. Eng. C 2014, 43, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, B.C.; Chang, C.J. A targetable fluorescent probe for imaging hydrogen peroxide in the mitochondria of living cells. J. Am. Chem. Soc. 2008, 130, 9638–9639. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.F. Quantitative determination of trace levels of hydrogen peroxide incrospovidone and a pharmaceutical product using high performance liquid chromatography with coulometric detection. Int. J. Pharm. 2009, 375, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.D.; Lin, Y.H. Amperometric glucose biosensor based on self-assembling glucose oxidase on carbon nanotubes. Electrochem. Commun. 2006, 8, 251–256. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical Biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Kim, S.J. An enzymatic biosensor for hydrogen peroxide based on one-pot preparation of CeO 2-reduced graphene oxide nanocomposite. RSC Adv. 2015, 5, 44346–44352. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Kim, S.J. Facile fabrication of NiS and a reduced grapheme oxide hybrid film for nonenzymatic detection of glucose. RSC Adv. 2015, 5, 12937–12943. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Liu, P.; Chen, M.Q.; Cai, Z.Q.; Cheng, F.L. Template Control Preparation of High-Density and Large-Area Ag Nanowire Array and H2O2 Determination. Int. J. Electrochem. Sci. 2015, 10, 4314–4323. [Google Scholar]
- Li, D.; Meng, L.Y.; Dang, S.C.; Jiang, D.L.; Shi, W.D. Hydrogen peroxide sensing using Cu2O nanocubes decorated by Ag-Au alloy nanoparticles. J. Alloys Compd. 2017, 690, 1–7. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, J.H.; Na, J.Y.; Kim, D.H.; Kang, D.; Kim, S.K.; Seong, T.Y. Ag nanowire-based electrodes for improving the output power of ultraviolet AlGaN-based light-emitting diodes. J. Alloys Compd. 2017, 703, 198–203. [Google Scholar] [CrossRef]
- Chen, Z.B.; Zhang, C.M.; Wu, Q.; Li, K.; Tan, L.L. A Novel application of triangular silver nanoplates forcolorimetric detection of H2O2. Sens. Actuators B Chem. 2015, 220, 314–317. [Google Scholar] [CrossRef]
- Yan, T.J.; Yan, X.Y.; Guo, R.R.; Zhang, W.N.; Li, W.J.; You, J.M. Ag/AgBr/BiOBr hollow hierarchical microspheres with enhanced activity and stability for RhB degradation under visible light irradiation. Catal. Commun. 2013, 42, 30–34. [Google Scholar] [CrossRef]
- Wang, X.L.; Yin, H.Y.; Nie, Q.L.; Wu, W.W.; Zhang, Y.; Yuan, Q.L. Hierarchical Ag/AgCl-TiO2 hollow spheres with enhanced visible-light photocatalytic activity. Mater. Chem. Phys. 2017, 185, 143–151. [Google Scholar] [CrossRef]
- Qian, H.S.; Yu, S.H.; Gong, J.Y.; Luo, L.B.; Fei, L.F. High-Quality Luminescent Tellurium Nanowires of Several Nanometers in Diameter and High Aspect Ratio Synthesized by a Poly(Vinyl Pyrrolidone) -Assisted Hydrothermal Process. Langmuir 2006, 22, 3830–3835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.H.; Yu, S.J.; Jiang, Y.M.; Jia, L.P.; Xia, X.H.; Ye, W.H.; Wang, C.M. A novel Pt@Te-reduced graphene oxide/polyimide composite catalyst for hydrogen evolution. Int. J. Hydrogen Energy 2015, 40, 16238–16247. [Google Scholar] [CrossRef]
- Keuleyan, S.; Wang, M.J.; Chung, F.R.; Commons, J.; Koski, K.J. A Silicon-Based Two-Dimensional Chalcogenide: Growth of Si2Te3 Nanoribbons and Nanoplates. Nano Lett. 2005, 15, 2285–2290. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.G.; Mayers, B.; Herricks, T.; Xia, Y.N. Polyol Synthesis of Uniform Silver Nanowires: A Plausible Growth Mechanism and the Supporting Evidence. Nano Lett. 2003, 3, 955–960. [Google Scholar] [CrossRef]
- Zeng, X.P.; Zhou, B.P.; Gao, Y.B.; Wang, C.; Li, B.; Yeung, C.Y.; Wen, W.J. Structural dependence of silver nanowires on polyvinyl pyrrolidone (PVP) chain length. Nanotechnology 2014, 25, 495601–495606. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.P.; Qiao, X.L.; Qiu, X.L.; Chen, J.G.; Jiang, R.Z. Convenient, rapid synthesis of silver nanocubes and nanowires via a microwave-assisted polyol method. Nanotechnology 2010, 21, 025607–025614. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Kodera, T.; Kita, H. Electrochemical behavior of H2O2 at Ag in HClO4 aqueous solution. Electrochim. Acta 1986, 31, 377–383. [Google Scholar] [CrossRef]
- Downard, A.J. Electrochemically assisted covalent modification of carbon electrodes. Electroanalysis 2000, 12, 1085–1096. [Google Scholar] [CrossRef]
- Šljukić, B.; Banks, C.E.; Compton, R.G. An overview of the electrochemical reduction of oxygen at carbon-based modified electrodes. J. Iran. Chem. Soc. 2005, 2, 1–25. [Google Scholar] [CrossRef]
- Wang, M.Y.; Shen, T.; Wang, M.; Zhang, D.G.; Chen, J. One-pot green synthesis of Ag nanoparticles-decorated reduced graphene oxide for efficient nonenzymatic H2O2 biosensor. Mater. Lett. 2013, 107, 311–314. [Google Scholar] [CrossRef]
- Ramezani, H.; Azizi, S.N.; Hosseini, S.R. NaY zeolite as a platform for preparation of Ag nanoparticles arrays in order to construction of H2O2 sensor. Sens. Actuators B Chem. 2017, 248, 571–579. [Google Scholar] [CrossRef]
- Nia, P.M.; Lorestani, F.; Meng, W.P.; Alias, Y. A novel non-enzymatic H2O2 sensor based on polypyrrole nanofibers–silver nanoparticles decorated reduced graphene oxide nanocomposites. Appl. Surf. Sci. 2015, 332, 648–656. [Google Scholar]
- Azizia, S.N.; Ghasemi, S.; Samadi-Maybodi, A.; Ranjbar-Azad, M. A new modified electrode based on Ag-doped mesoporous SBA-16 nanoparticles as non-enzymatic sensor for hydrogen peroxide. Sens. Actuators B Chem. 2015, 216, 271–278. [Google Scholar] [CrossRef]
- Hao, H.; Sheng, Q.L.; Zheng, J.B. One-step synthesis of Ag@SiO2@Ag nanomaterial and its applicationas hydrogen peroxide sensor. Colloids Surf. A 2017, 518, 124–129. [Google Scholar] [CrossRef]
- Azizi, S.N.; Ghasemi, S.; Kavian, S. Synthesis and characterization of NaX nanozeolite using stem sweep as silica source and application of Ag-modified nanozeolite in electrocatalytic reduction of H2O2. Biosens. Bioelectron. 2014, 62, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.L.; Zhang, H.F.; Zheng, J.B. A non-enzymatic electrochemical hydrogen peroxide sensor based on Ag decorated boehmite nanotubes/reduced grapheme oxide nanocomposites. J. Electroanal. Chem. 2017, 784, 55–61. [Google Scholar] [CrossRef]

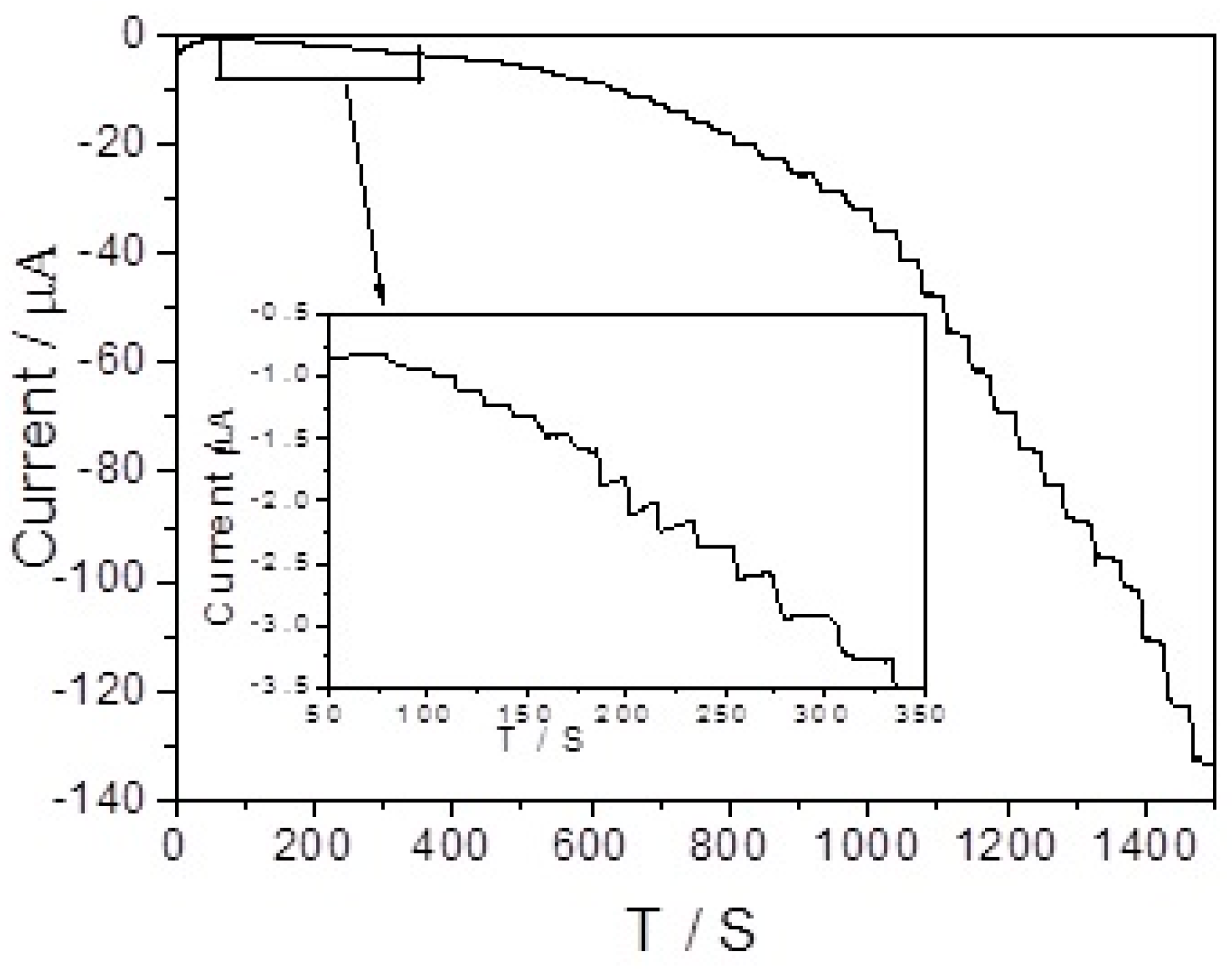
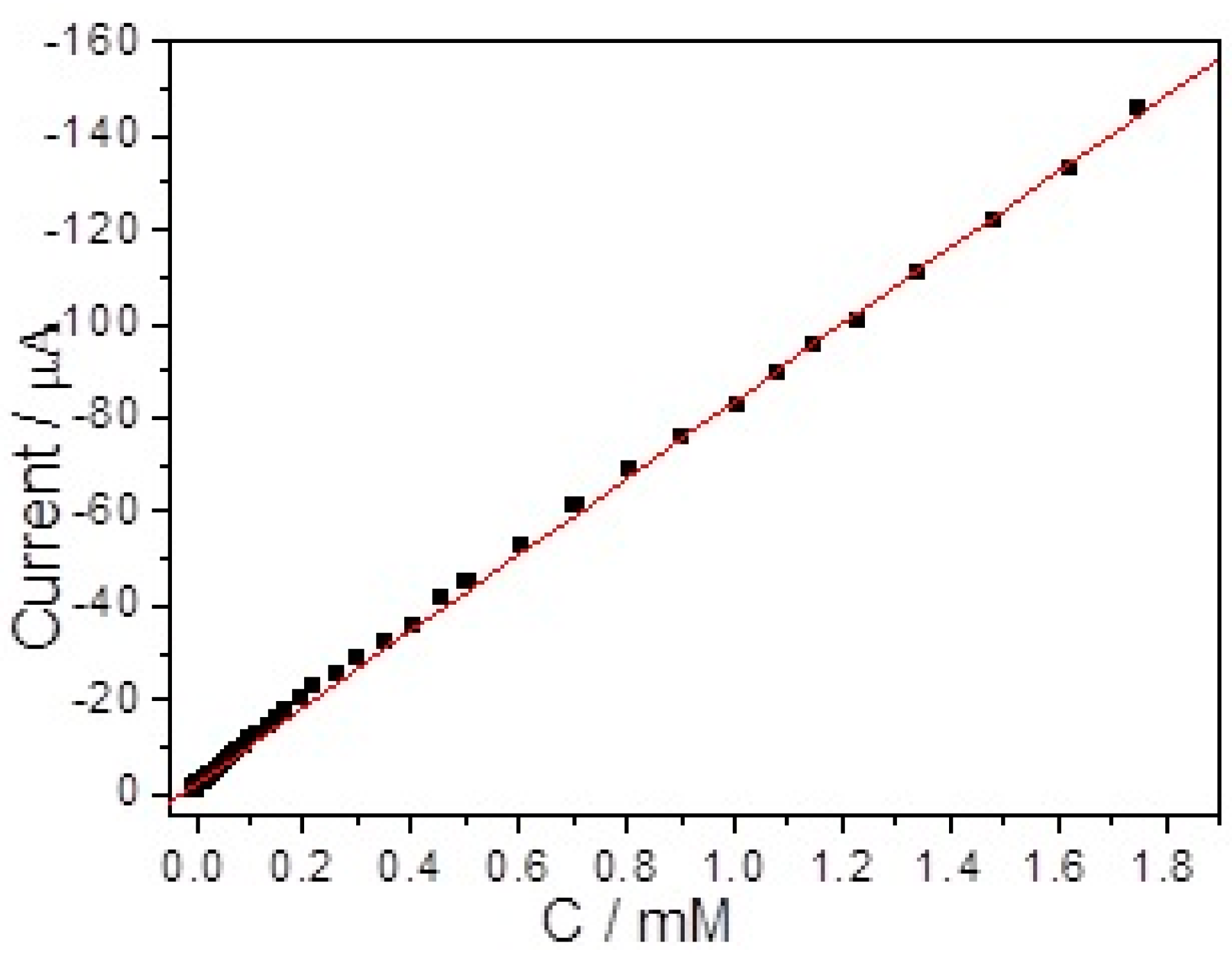


| Electrode | Sensitivity (μA mM−1 cm−2) | Linear Range (μM) | LOD (μM) | Ref |
|---|---|---|---|---|
| Ag NPs/rGO/GCE | 225 | 50–5000 | 10 | [40] |
| Ag Y/CPE | 650.7 | 20–5000 | 1.4 | [41] |
| Ag NPs/rGO/GCE | --- | 10–6000 | 1.8 | [42] |
| Ag-Au@Cu2O NWs/GCE | --- | 100–9000 | 1.1 | [43] |
| AgNPs/SBA-16/GCE | 816.6 | 20–8000 | 2.95 | [44] |
| Ag@SiO2@Ag/GCE | 56.07 | 5–24,000 | 1.7 | [45] |
| AgNPs/X-CPE | 60.6 | 20–11,760 | 9.1 | [26] |
| Ag/boehmite NTs/rGO/GCE | 80.14 | 0.5–10,000 | 0.17 | [46] |
| branch-truck Ag HN/GCE | 325.52 | 0.05–1925 | 0.013 | our report |
| S. No | Added Concentration (mM) | Found Concentration (mM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 0.0100 | 0.01012 | 101.2 | 3.1 |
| 2 | 0.0500 | 0.05005 | 100.5 | 1.6 |
| 3 | 0.1000 | 0.09987 | 99.87 | 3.2 |
| 4 | 0.1500 | 0.15015 | 100.1 | 5.6 |
| 5 | 0.2000 | 0.01996 | 99.8 | 1.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, M.; Cai, Z.; Zhang, M.; Liu, P.; Cheng, F. The Facile Synthesis of Branch-Trunk Ag Hierarchical Nanostructures and Their Applications for High-Performance H2O2 Electrochemical Sensors. Sensors 2017, 17, 2896. https://doi.org/10.3390/s17122896
Zhang Y, Chen M, Cai Z, Zhang M, Liu P, Cheng F. The Facile Synthesis of Branch-Trunk Ag Hierarchical Nanostructures and Their Applications for High-Performance H2O2 Electrochemical Sensors. Sensors. 2017; 17(12):2896. https://doi.org/10.3390/s17122896
Chicago/Turabian StyleZhang, Yan, Meiqiong Chen, Zhiquan Cai, Min Zhang, Peng Liu, and Faliang Cheng. 2017. "The Facile Synthesis of Branch-Trunk Ag Hierarchical Nanostructures and Their Applications for High-Performance H2O2 Electrochemical Sensors" Sensors 17, no. 12: 2896. https://doi.org/10.3390/s17122896
APA StyleZhang, Y., Chen, M., Cai, Z., Zhang, M., Liu, P., & Cheng, F. (2017). The Facile Synthesis of Branch-Trunk Ag Hierarchical Nanostructures and Their Applications for High-Performance H2O2 Electrochemical Sensors. Sensors, 17(12), 2896. https://doi.org/10.3390/s17122896





