Synergistic Use of Gold Nanoparticles (AuNPs) and “Capillary Enzyme-Linked Immunosorbent Assay (ELISA)” for High Sensitivity and Fast Assays
Abstract
1. Introduction
2. Experimental
2.1. Materials and Chemical Reagents
2.2. Preparation of AuNPs Conjugated with antiCRP-HRP (AuNP•antiCRP-HRP)
2.3. Immobilization of the Capture antiCRP Inside a Capillary Tube
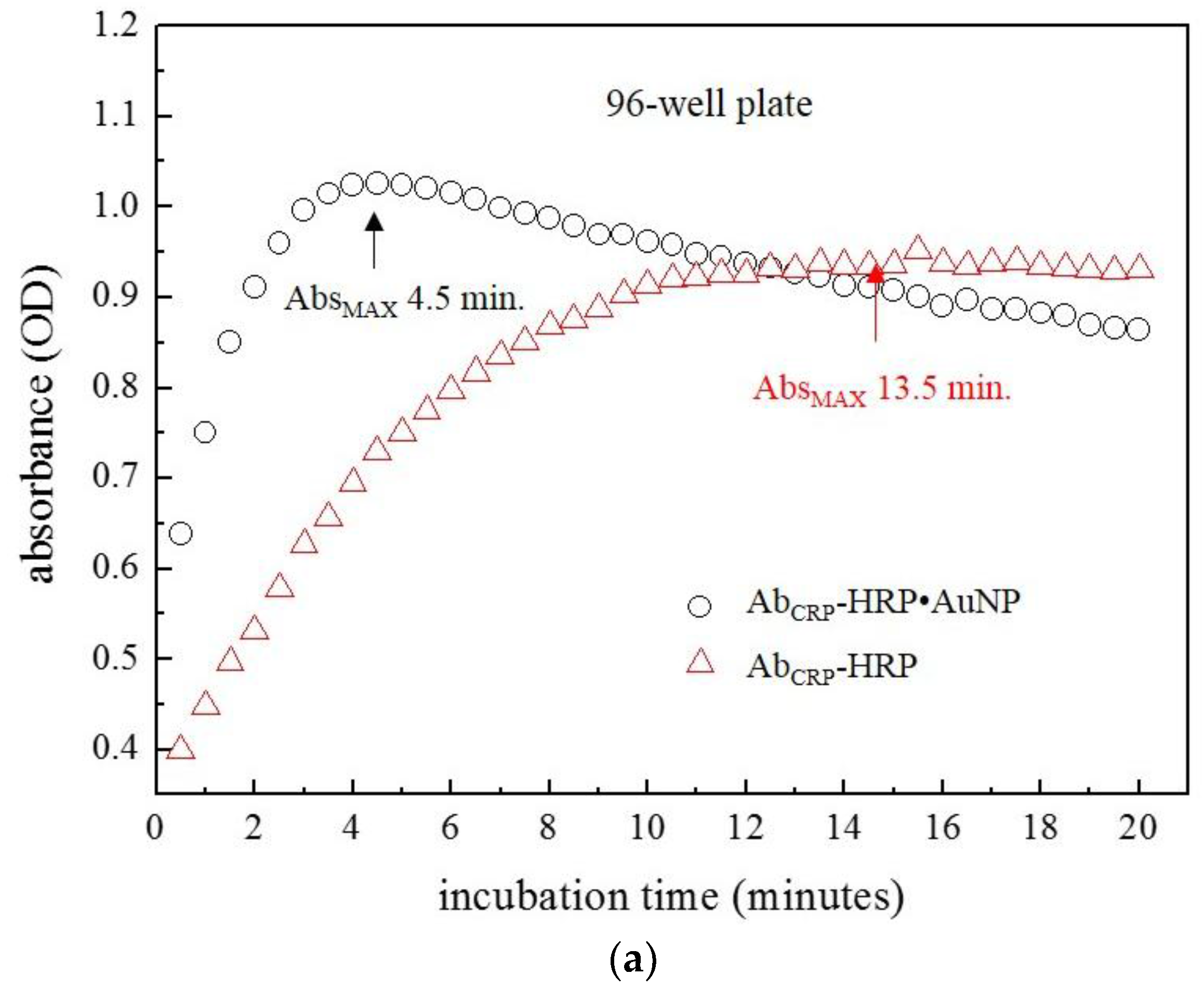
2.4. Immunoassays on a 96 Well-Plate
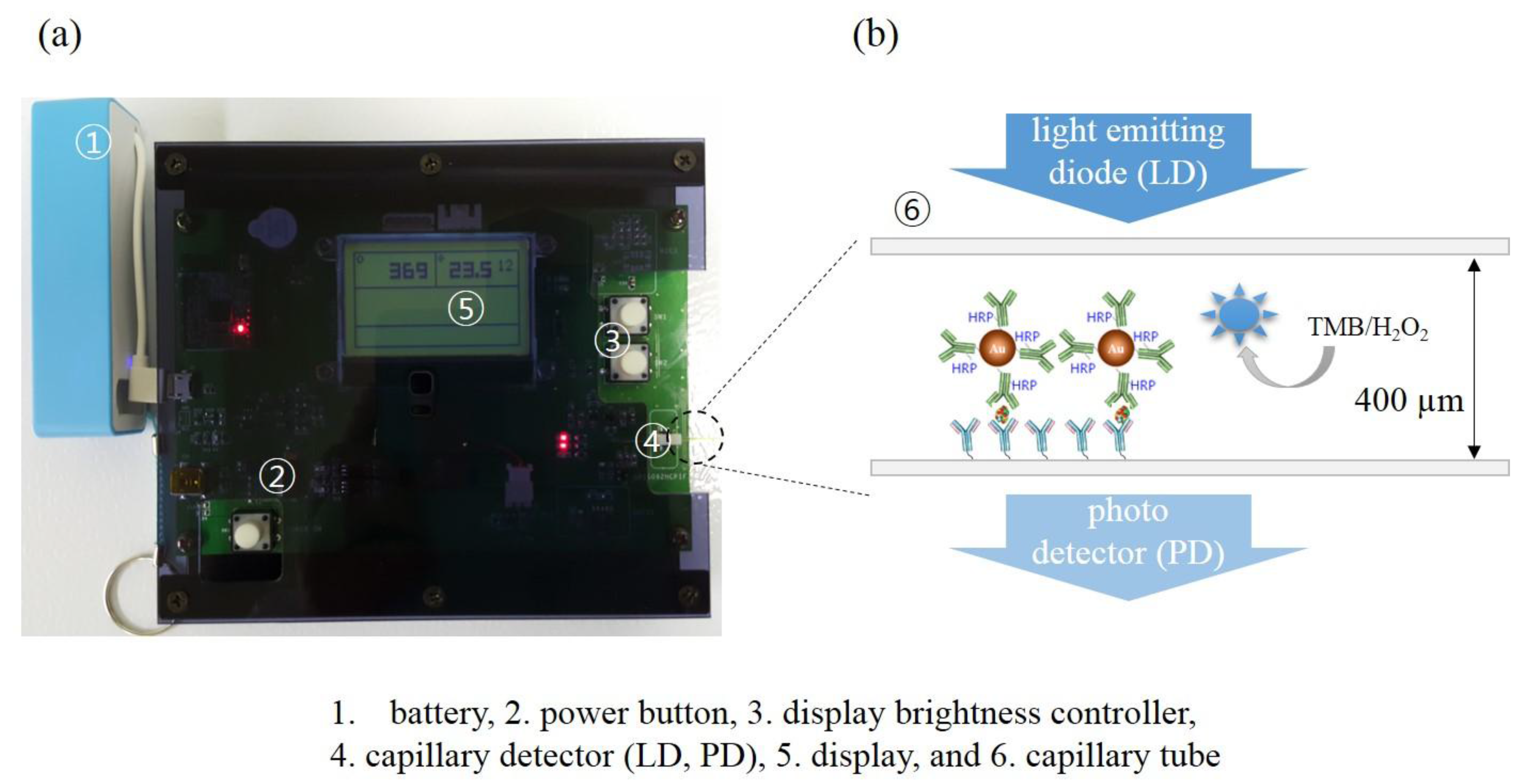
2.5. Home-Made Optical Detector
2.6. Immunoassays in Capillary Tubes
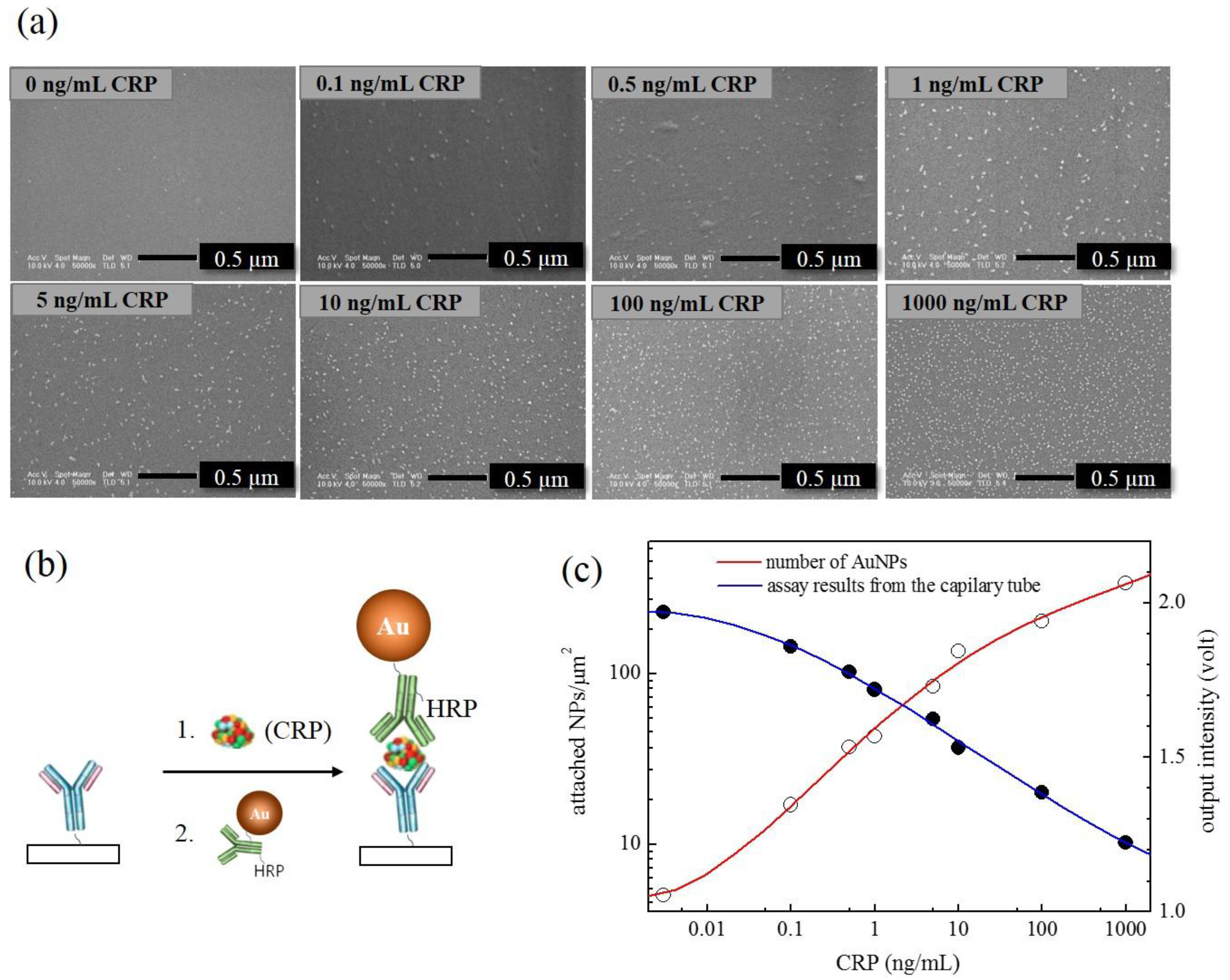
2.7. SEM Analysis of the “AuNP Capillary ELISA”
3. Results and Discussion
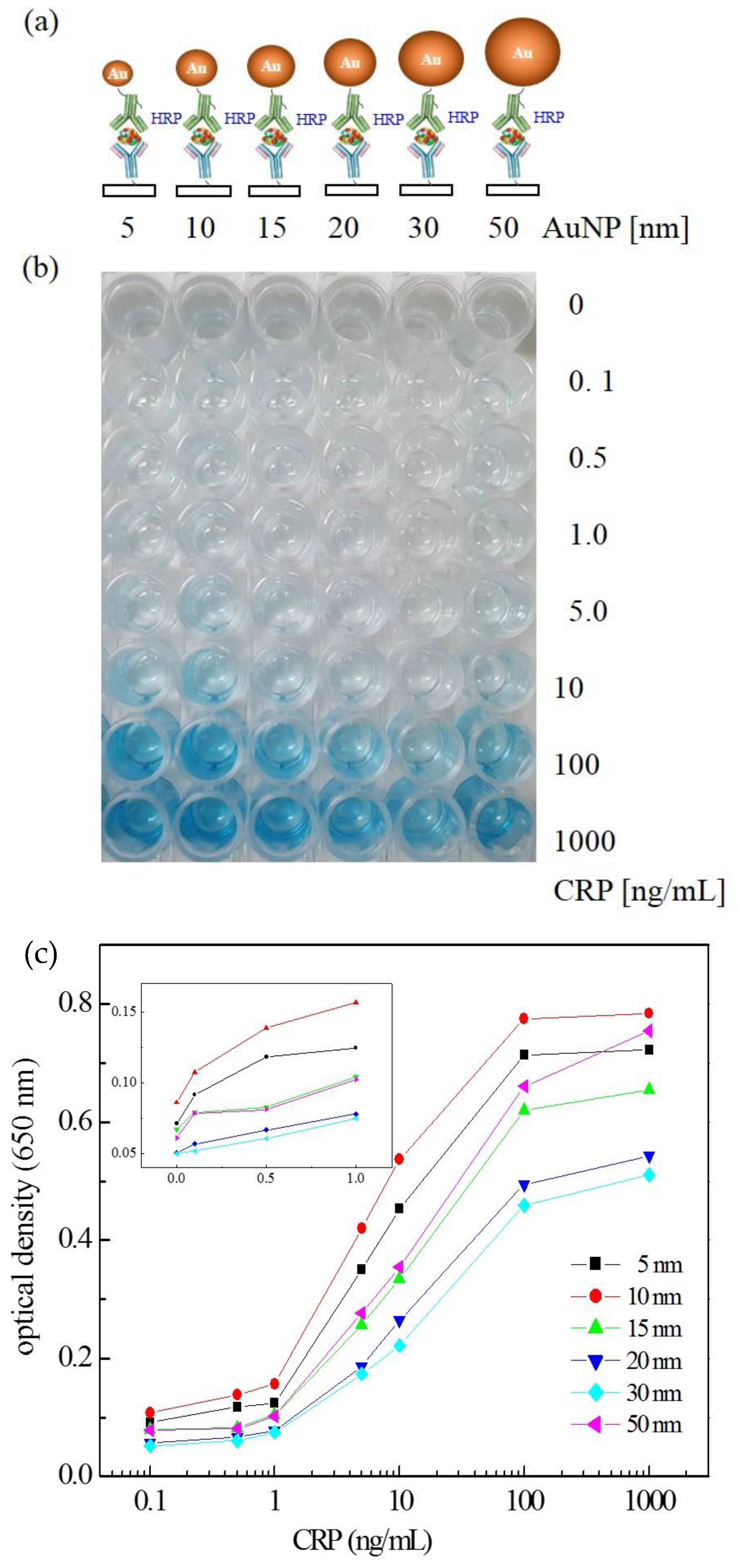
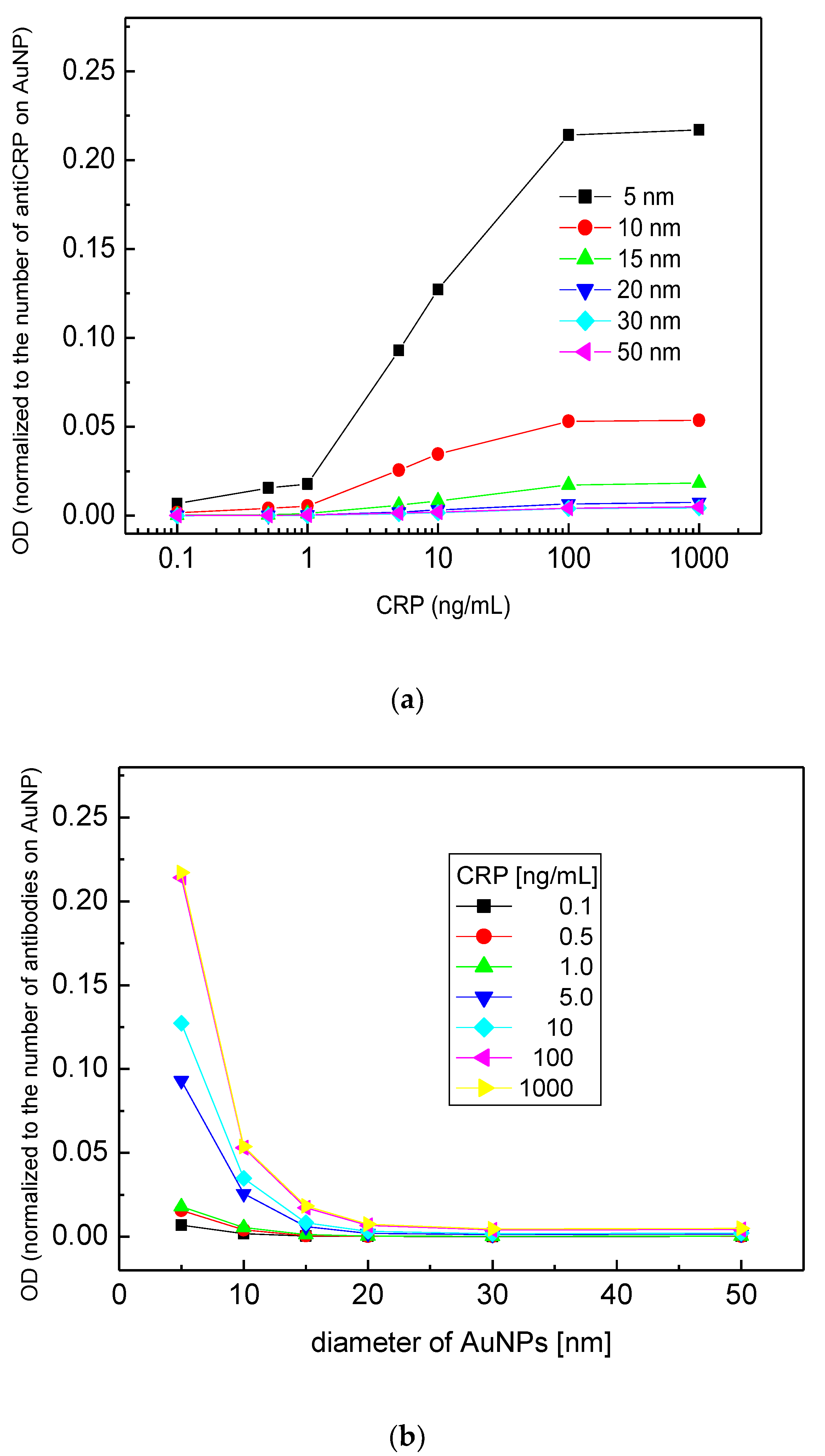
3.1. Size Effect of AuNPs on ELISA
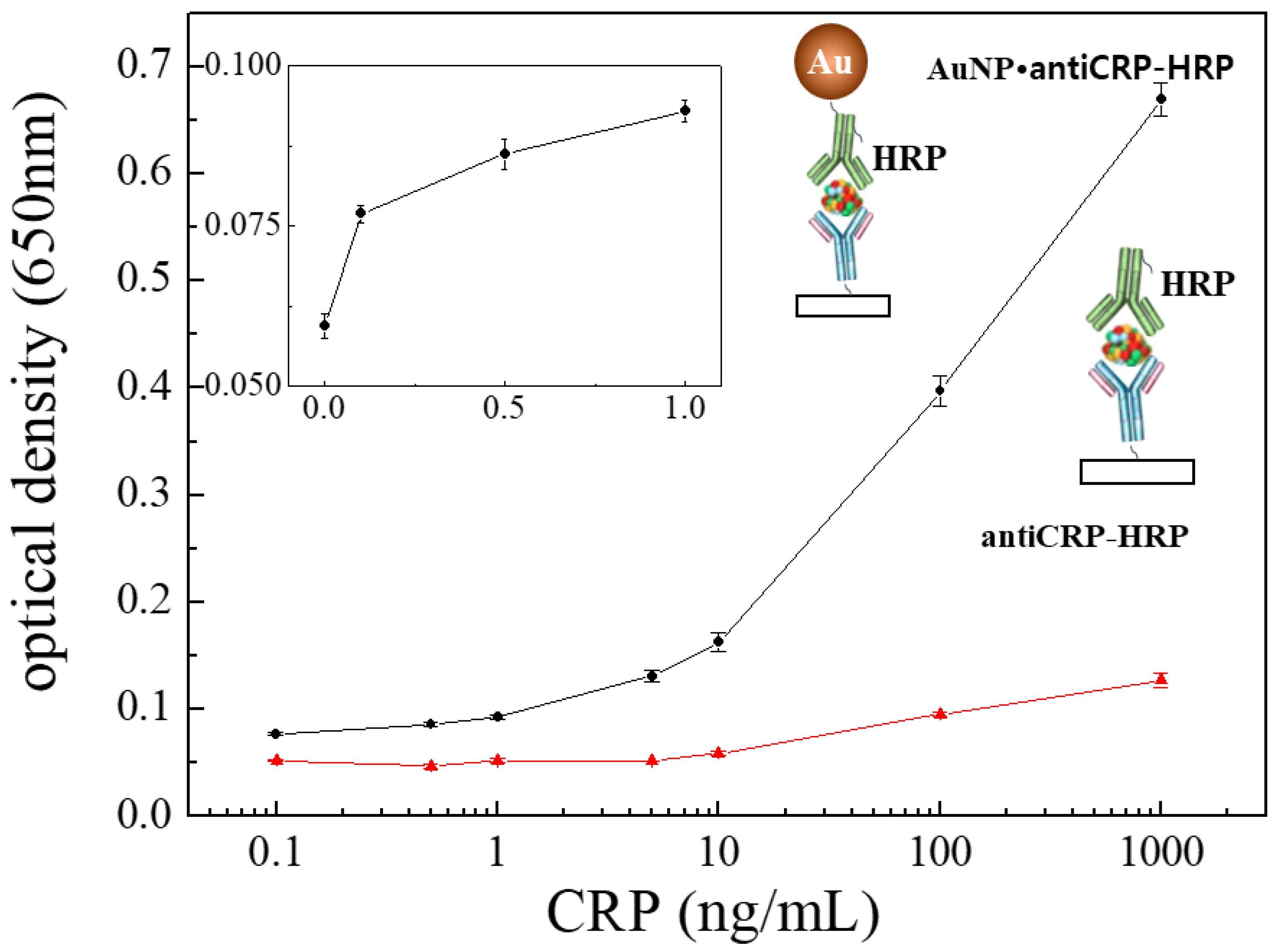
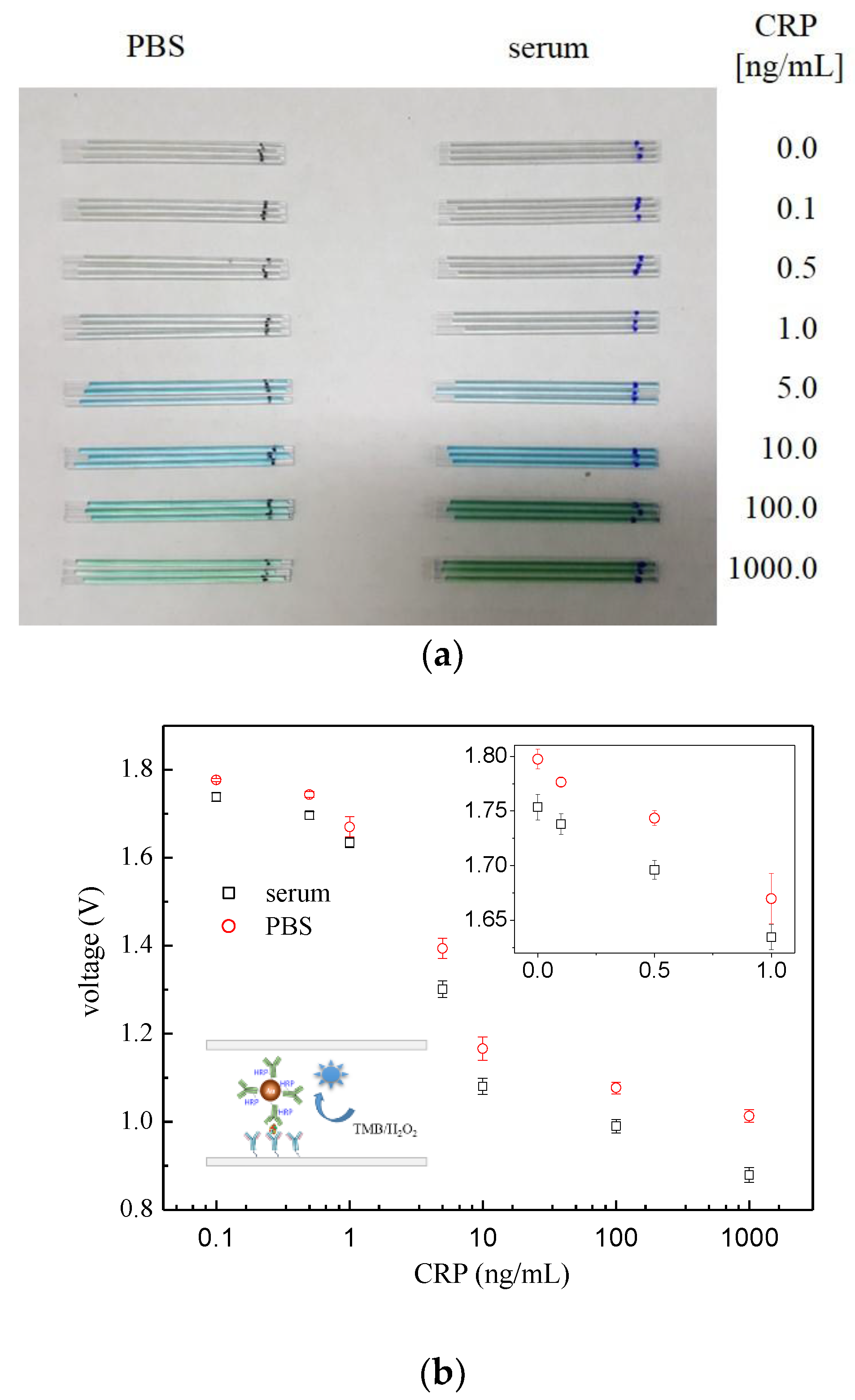
3.2. “Capillary ELISA” Using AuNP•antiCRP-HRP
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, J.; Xianyu, Y.; Jiang, X. Point-of-care biochemical assays using gold nanoparticle-implemented microfluidics. Chem. Soc. Rev. 2014, 43, 6239–6253. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, S.; Wang, L.; Li, F.; Pingguan-Murphy, B.; Lu, T.J.; Xu, F. Advances in paper-based point-of-care diagnostics. Biosens. Bioelectron. 2014, 54, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S. Recent developments in paper-based microfluidic devices. Anal. Chem. 2015, 87, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Funano, S.; Henares, T.G.; Kurata, M.; Sueyoshi, K.; Endo, T.; Hisamoto, H. Capillary-based enzyme-linked immunosorbent assay for highly sensitive detection of thrombin-cleaved osteopontin in plasma. Anal. Biochem. 2013, 440, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-J.; Hyun, S.H.; Cho, H.Y.; Byun, S.; Kim, B.K.; Huh, C.; Chung, K.H.; Kim, Y.J. Sensitive “capillary elisa” via vapor-phase surface modification. Sens. Actuators B Chem. 2016, 233, 281–288. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Desmulliez, M.P.Y. Autonomous capillary microfluidic system with embedded optics for improved troponin I cardiac biomarker detection. Biosens. Bioelectron. 2014, 61, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Funano, S.-I.; Sugahara, M.; Henares, T.G.; Sueyoshi, K.; Endo, T.; Hisamoto, H. A single-step enzyme immunoassay capillary sensor composed of functional multilayer coatings for the diagnosis of marker proteins. Analyst 2015, 140, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Wakayama, H.; Henares, T.G.; Jigawa, K.; Funano, S.-I.; Sueyoshi, K.; Endo, T.; Hisamoto, H. Design of a single-step immunoassay principle based on the combination of an enzyme-labeled antibody release coating and a hydrogel copolymerized with a fluorescent enzyme substrate in a microfluidic capillary device. Lab Chip 2013, 13, 4304–4307. [Google Scholar] [CrossRef] [PubMed]
- Henares, T.G.; Shirai, A.; Sueyoshi, K.; Endo, T.; Hisamoto, H. Fabrication and packaging of a mass-producible capillary-assembled microchip for simple and multiplexed bioassay. Sens. Actuators B Chem. 2015, 218, 245–252. [Google Scholar] [CrossRef]
- Edwards, A.D.; Reis, N.M.; Slater, N.K.H.; Mackley, M.R. A simple device for multiplex ELISA made from melt-extruded plastic microcapillary film. Lab Chip 2011, 11, 4267–4273. [Google Scholar] [CrossRef] [PubMed]
- Comotti, M.; Della Pina, C.; Matarrese, R.; Rossi, M. The catalytic activity of “naked” gold particles. Angew. Chem. Int. Ed. 2004, 43, 5812–5815. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Airò, F.; Merkoçi, A. Enhanced gold nanoparticle based ELISA for a breast cancer biomarker. Anal. Chem. 2010, 82, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, B.; Zhang, H.; Sun, Y.; Wang, J.; Zhang, J.; Fu, Y. BSA-stabilized Pt nanozyme for peroxidase mimetics and its application on colorimetric detection of mercury(II) ions. Biosens. Bioelectron. 2015, 66, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene oxide: Intrinsic peroxidase catalytic activity and its application to glucose detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Pais de Lacerda, A. High-sensitivity C-reactive protein as a biomarker of risk in coronary artery disease. Rev. Port. Cardiol. 2012, 31, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Yeh, E.T.H.; Willerson, J.T. Coming of age of C-reactive protein. Using Inflamm. Mark. Cardiol. 2003, 107, 370–371. [Google Scholar]
- Mishra, S.; Saadat, D.; Kwon, O.; Lee, Y.; Choi, W.-S.; Kim, J.-H.; Yeo, W.-H. Recent advances in salivary cancer diagnostics enabled by biosensors and bioelectronics. Biosens. Bioelectron. 2016, 81, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, J.M.; Schafer, C.A.; Schafer, J.J.; Farrell, J.J.; Paster, B.J.; Wong, D.T.W. Salivary biomarkers: Toward future clinical and diagnostic utilities. Clin. Microbiol. Rev. 2013, 26, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Punyadeera, C.; Dimeski, G.; Kostner, K.; Beyerlein, P.; Cooper-White, J. One-step homogeneous C-reactive protein assay for saliva. J. Immunol. Methods 2011, 373, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-J.; Cho, H.Y.; Kim, B.K.; Huh, C.; Chung, K.H.; Ahn, C.-G.; Kim, Y.J.; Kim, A. Highly sensitive detection of cardiac troponin I in human serum using gold nanoparticle-based enhanced sandwich immunoassay. Sens. Actuators B Chem. 2015, 221, 537–543. [Google Scholar] [CrossRef]
- Fenger, R.; Fertitta, E.; Kirmse, H.; Thunemann, A.F.; Rademann, K. Size dependent catalysis with CTAB-stabilized gold nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 9343–9349. [Google Scholar] [CrossRef] [PubMed]
- Bell, N.C.; Minelli, C.; Shard, A.G. Quantitation of IgG protein adsorption to gold nanoparticles using particle size measurement. Anal. Methods 2013, 5, 4591–4601. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Li, M.; Chong, Y.; Zeng, M.; Lo, Y.M.; Yin, J.-J. Size-dependent tuning of horseradish peroxidase bioreactivity by gold nanoparticles. Nanoscale 2015, 7, 4505–4513. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.-J.; Cho, H.Y.; Jeong, B.; Byun, S.; Huh, J.; Kim, Y.J. Synergistic Use of Gold Nanoparticles (AuNPs) and “Capillary Enzyme-Linked Immunosorbent Assay (ELISA)” for High Sensitivity and Fast Assays. Sensors 2018, 18, 55. https://doi.org/10.3390/s18010055
Kim W-J, Cho HY, Jeong B, Byun S, Huh J, Kim YJ. Synergistic Use of Gold Nanoparticles (AuNPs) and “Capillary Enzyme-Linked Immunosorbent Assay (ELISA)” for High Sensitivity and Fast Assays. Sensors. 2018; 18(1):55. https://doi.org/10.3390/s18010055
Chicago/Turabian StyleKim, Wan-Joong, Hyo Young Cho, Bongjin Jeong, Sangwon Byun, JaeDoo Huh, and Young Jun Kim. 2018. "Synergistic Use of Gold Nanoparticles (AuNPs) and “Capillary Enzyme-Linked Immunosorbent Assay (ELISA)” for High Sensitivity and Fast Assays" Sensors 18, no. 1: 55. https://doi.org/10.3390/s18010055
APA StyleKim, W.-J., Cho, H. Y., Jeong, B., Byun, S., Huh, J., & Kim, Y. J. (2018). Synergistic Use of Gold Nanoparticles (AuNPs) and “Capillary Enzyme-Linked Immunosorbent Assay (ELISA)” for High Sensitivity and Fast Assays. Sensors, 18(1), 55. https://doi.org/10.3390/s18010055





