Sensitive Bioanalysis Based on in-Situ Droplet Anodic Stripping Voltammetric Detection of CdS Quantum Dots Label after Enhanced Cathodic Preconcentration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus and Materials
2.2. Preparation of Ab2-CdS or Apt2-CdS Conjugates
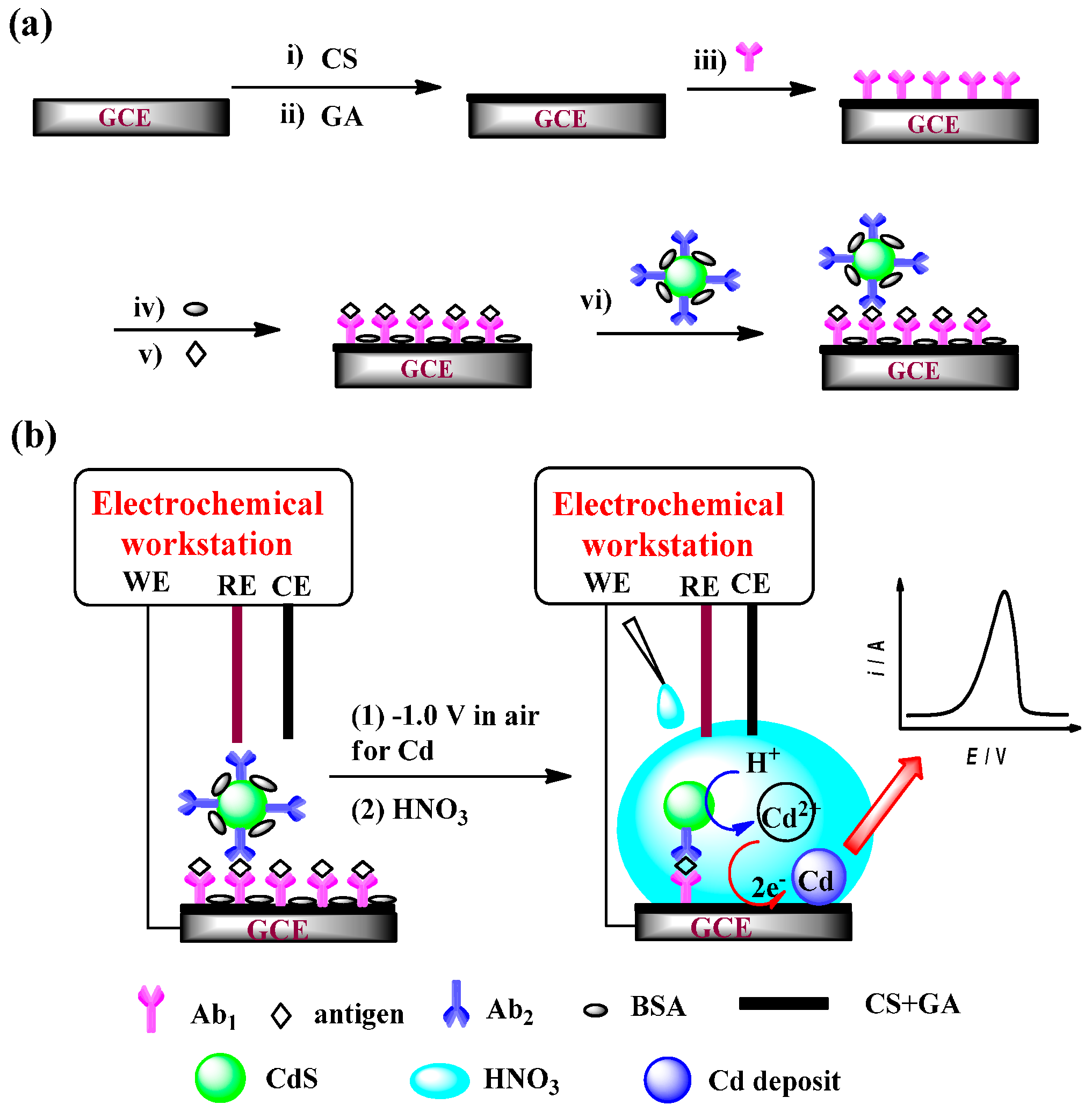
2.3. Preparation of Immunoelectrodes
2.4. Preparation of Aptamer-Electrodes
2.5. Conventional Cell Measurement Procedures
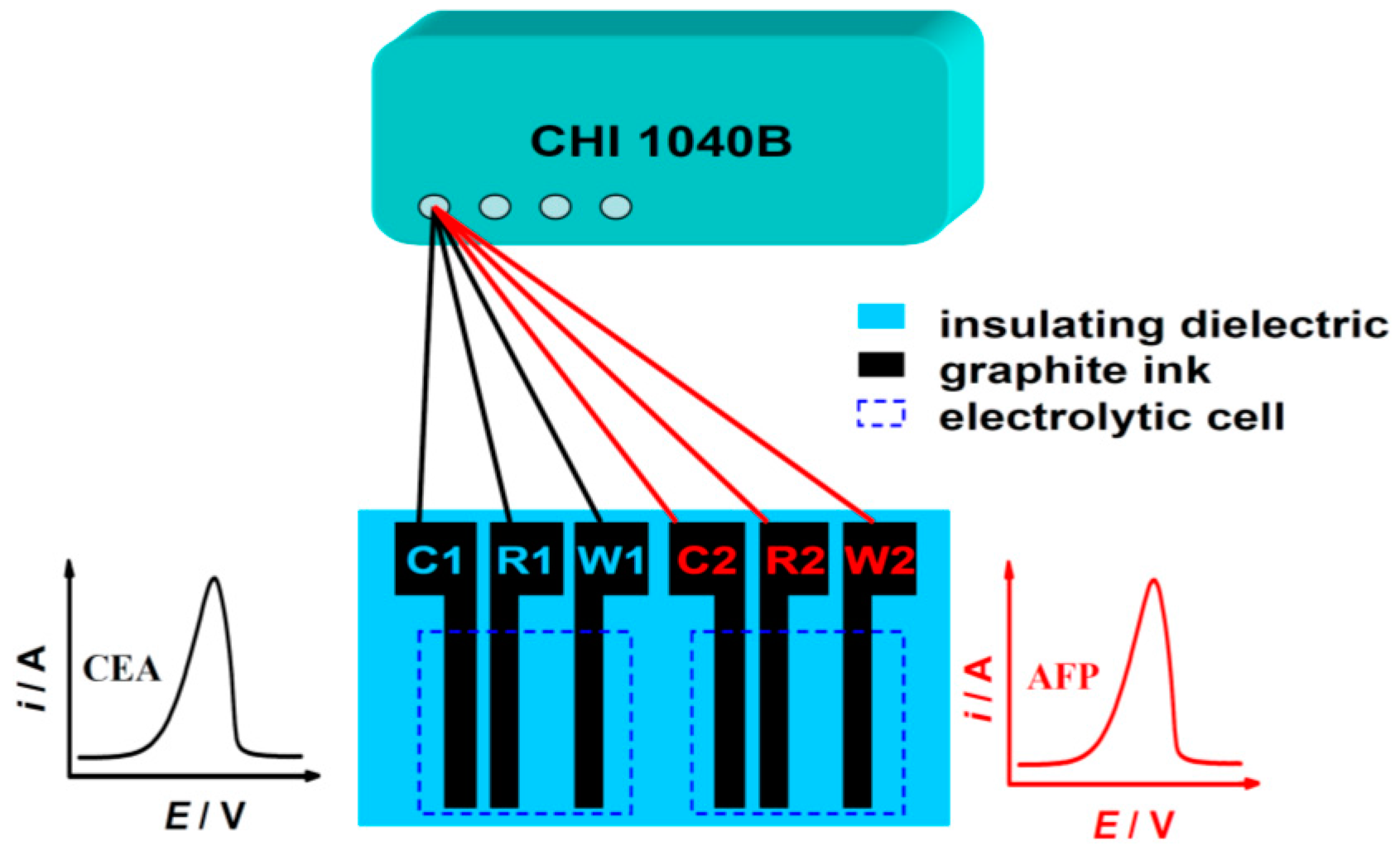
2.6. SPCE Measurements
3. Results
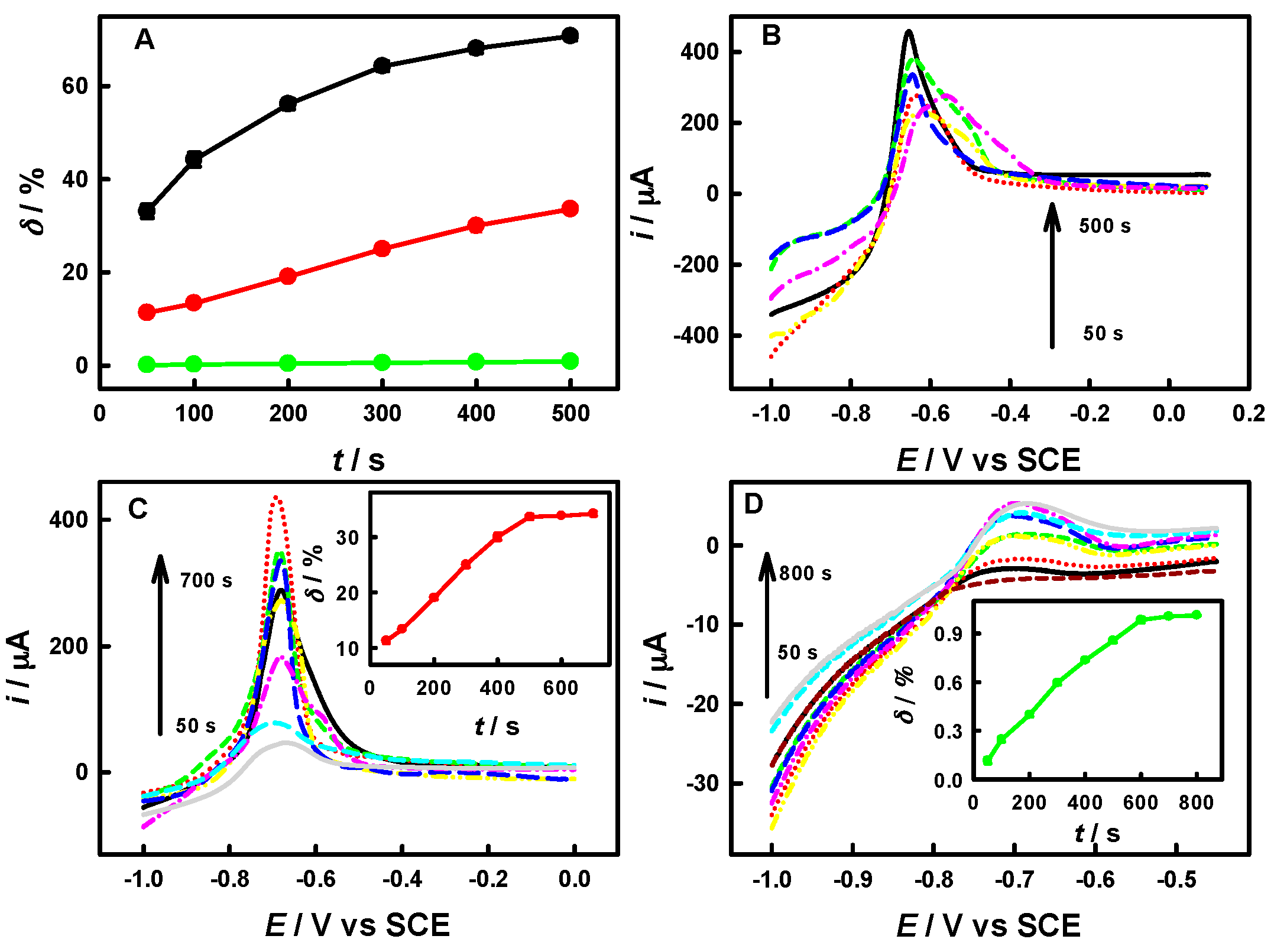
3.1. Simulated Experiments for Evaluating the Signaling Efficiency of our Protocol
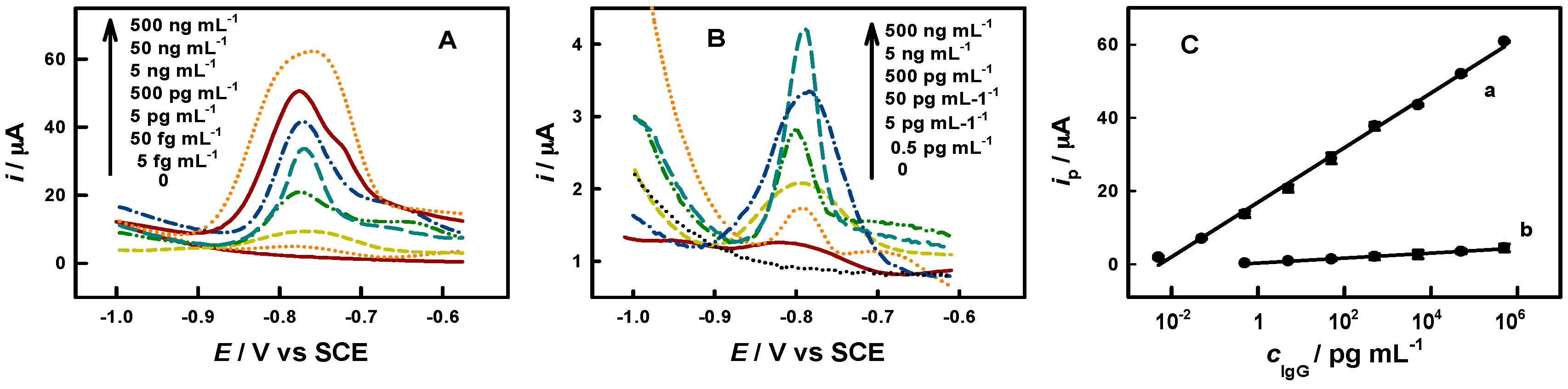
3.2. Immunoassay of IgG, CEA and AFP
3.3. Aptamer-Based Bioanalysis
3.4. Simultaneous Two-Target Immunoassay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Ab | Antibody |
| AFP | α-fetoprotein |
| Ag | Antigen |
| anti-IgG | Anti-human immunoglobulin G |
| ASV | Anodic stripping voltammetry |
| AuNPs | Gold nanoparticles |
| BSA | Bovine serum albumin |
| CE | Counter electrode |
| CEA | Carcinoembryonic antigen |
| CS | Chitosan |
| CV | Cyclic voltammetry |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide |
| GA | Glutaraldehyde |
| GCE | Glassy carbon electrode |
| IgG | Immunoglobulin G |
| LOD | Limit of detection |
| LSV | Linear sweep voltammetry |
| MLAB | Metal-labeled amperometric bioassay |
| NHS | N-hydroxysuccinimide |
| QDs | Quantum dots |
| RE | Reference electrode |
| RD | Relative deviation |
| RSD | Relative standard deviation |
| SCE | Saturated calomel electrode |
| WE | Working electrode |
References
- Hansen, J.A.; Wang, J.; Kawde, A.N.; Xiang, Y.; Gothelf, K.V.; Collins, G. Quantum-Dot/Aptamer-Based Ultrasensitive Multi-Analyte Electrochemical Biosensor. J. Am. Chem. Soc. 2006, 128, 2228–2229. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.J.; Zhang, Y.Q.; Bernard, P.E.; Reuben, J.M.; Ueno, N.T.; Arlinghaus, R.B.; Zu, Y.L.; Qin, L.D. Multiplexed volumetric bar-chart chip for point-of-care diagnostics. Nat. Commun. 2012, 3, 1283. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Elbaz, J.; Teller, C.; Willner, I. Amplified Detection of DNA through an Autocatalytic and Catabolic DNAzyme-Mediated Process. Angew. Chem. Int. Ed. 2011, 50, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Saletti, G.; Çuburu, N.; Yang, J.S.; Dey, A.; Czerkinsky, C. Enzyme-linked immunospot assays for direct ex vivo measurement of vaccine-induced human humoral immune responses in blood. Nat. Protoc. 2013, 8, 1073–1087. [Google Scholar] [CrossRef]
- Kokkinos, C.; Angelopoulou, M.; Economou, A.; Prodromidis, M.; Florou, A.; Haasnoot, W.; Petrou, P.; Kakabakos, S. Lab-on-a-Membrane Foldable Devices for Duplex Drop-Volume Electrochemical Biosensing Using Quantum Dot Tags. Anal. Chem. 2016, 88, 6897–6904. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xin, Y.; Wu, W.; Fu, B.; Zhang, Z. Topotactic Conversion of Copper(I) Phosphide Nanowires for Sensitive Electrochemical Detection of H2O2 Release from Living Cells. Anal. Chem. 2016. [Google Scholar] [CrossRef]
- Munge, B.S.; Coffey, A.L.; Doucette, J.M.; Somba, B.K.; Malhotra, R.; Patel, V.; Gutkind, J.S.; Rusling, J.F. Nanostructured Immunosensor for Attomolar Detection of Cancer Biomarker Interleukin-8 Using Massively Labeled Superparamagnetic Particles. Angew. Chem. Int. Ed. 2011, 50, 7915–7918. [Google Scholar] [CrossRef]
- Krishnan, S.; Mani, V.; Wasalathanthri, D.; Kumar, C.V.; Rusling, J.F. Attomolar Detection of a Cancer Biomarker Protein in Serum by Surface Plasmon Resonance Using Superparamagnetic Particle Labels. Angew. Chem. Int. Ed. 2010, 49, 1–5. [Google Scholar]
- Zhang, L.B.; Zhu, J.B.; Guo, S.J.; Li, T.; Li, J.; Wang, E.K. Photoinduced Electron Transfer of DNA/Ag Nanoclusters Modulated by G-Quadruplex/Hemin Complex for the Construction of Versatile Biosensors. J. Am. Chem. Soc. 2013, 135, 2403–2406. [Google Scholar] [CrossRef] [PubMed]
- Yalow, R.S.; Berson, S.A. Immunoassay of endogenous plasma insulin in man. J. Clin. Investig. 1960, 39, 1157–1175. [Google Scholar] [CrossRef] [PubMed]
- Pradelles, P.; Grassi, J.; Maclouf, J. Enzyme Immunoassays of Eicosanoids Using Acetylcholine Esterase as Label: An Alternative to Radioimmunoassay. Anal. Chem. 1985, 57, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Chen, C.L.; Liu, S.Q. Enzyme-Functionalized Silica Nanoparticles as Sensitive Labels in Biosensing. Anal. Chem. 2009, 81, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Kent, A.D.; Heemstra, J.M. Enzyme-Linked Small-Molecule Detection Using Split Aptamer Ligation. Anal. Chem. 2012, 84, 6104–6109. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.X.; Xu, C.X.; Yang, C.; Liu, S.Q.; Wang, M.L. ZnO quantum dot labeled immunosensor for carbohydrate antigen 19-9. Biosens. Bioelectron. 2011, 26, 2720–2723. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.W.; Ma, Z.Y.; Yu, P.P.; Dong, X.Y.; Xu, J.J.; Chen, H.Y. Highly Sensitive Photoelectrochemical Immunoassay with Enhanced Amplification Using Horseradish Peroxidase Induced Biocatalytic Precipitation on a CdS Quantum Dots Multilayer Electrode. Anal. Chem. 2012, 84, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dai, Q.; Austin, L.; Coutts, J.; Knowles, G.; Zou, J.H.; Chen, H.; Huo, Q. A One-Step Homogeneous Immunoassay for Cancer Biomarker Detection Using Gold Nanoparticle Probes Coupled with Dynamic Light Scattering. J. Am. Chem. Soc. 2008, 130, 2780–2782. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.; Zayats, M.; Willner, I. Semiconductor Quantum Dots for Bioanalysis. Angew. Chem. Int. Ed. 2008, 47, 7602–7625. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.K.; Bhansali, S. Lung Cancer and Its Early Detection Using Biomarker-Based Biosensors. Chem. Rev. 2011, 111, 6783–6809. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.P.F. Biosensors: Sense and Sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, D.W.; LeBlanc, G.; Meschievitz, M.E.; Cliffel, D.E. Electrochemical Sensors and Biosensors. Anal. Chem. 2012, 84, 685–707. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, X.; Wei, L.; Liu, M.; Chen, Y.; Li, G. Simple electrochemical sensing of attomolar proteins using fabricated complexes with enhanced surface binding avidity. Chem. Sci. 2015, 6, 4311–4317. [Google Scholar] [CrossRef]
- Das, J.; Ivanov, I.; Montermini, L.; Rak, J.; Sargent, E.H.; Kelley, S.O. An electrochemical clamp assay for direct, rapid analysis of circulating nucleic acids in serum. Nat. Chem. 2015, 7, 569–575. [Google Scholar] [CrossRef]
- Feng, L.N.; Bian, Z.P.; Peng, J.; Jiang, F.; Yang, G.H.; Zhu, Y.D.; Yang, D.; Jiang, L.P.; Zhu, J.J. Ultrasensitive Multianalyte Electrochemical Immunoassay Based on Metal Ion Functionalized Titanium Phosphate Nanospheres. Anal. Chem. 2012, 84, 7810–7815. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, A.; He, P.; Fang, Y. Cadmium sulfide nanocluster-based electrochemical stripping detection of DNA hybridization. Analyst 2003, 128, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Zhang, H.; Jiang, B.; Chai, Y.; Yuan, R. Quantum Dot Layer-by-Layer Assemblies as Signal Amplification Labels for Ultrasensitive Electronic Detection of Uropathogens. Anal. Chem. 2011, 83, 4302–4306. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; El Said, W.A.; Choi, J.W. Highly sensitive electrochemical detection of potential cytotoxicity of CdSe/ZnS quantum dots using neural cell chip. Biosens. Bioelectron. 2012, 32, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.L.; Wang, J.; Wang, L.M.; Du, D.; Timchalk, C.; Barry, R.; Lin, Y.H. A Novel Nanoparticle-Based Disposable Electrochemical Immunosensor for Diagnosis of Exposure to Toxic Organophosphorus Agents. Adv. Funct. Mater. 2011, 21, 4371–4378. [Google Scholar] [CrossRef]
- Zuo, X.; Song, S.; Zhang, J.; Pan, D.; Wang, L.; Fan, C. A Target-Responsive Electrochemical Aptamer Switch (TREAS) for Reagentless Detection of Nanomolar ATP. J. Am. Chem. Soc. 2007, 129, 1042–1043. [Google Scholar] [CrossRef] [PubMed]
- Barfidokht, A.; Ciampi, S.; Luais, E.; Darwish, N.; Gooding, J.J. Distance-Dependent Electron Transfer at Passivated Electrodes Decorated by Gold Nanoparticles. Anal. Chem. 2013, 85, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Xu, A.; Liu, L.; Deng, W.; Chen, C.; Tan, Y.; Fu, Y.; Xie, Q.; Yao, S. Ultrasensitive Electrochemical Immunoassay of Proteins based on in Situ Duple Amplification of Gold Nanoparticle Biolabel Signals. Chem. Commun. 2015, 51, 8540–8543. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Liu, L.; Xu, A.; Wang, L.; Tan, Y.; Chen, C.; Xie, Q. Ultrasensitive Immunoassay of Proteins Based on Gold Label/Silver Staining, Galvanic Replacement Reaction Enlargement, and In Situ Microliter-Droplet Anodic Stripping Voltammetry. J. Phys. Chem. C 2016, 120, 2855–2865. [Google Scholar] [CrossRef]
- Qin, X.; Xu, A.; Wang, L.; Liu, L.; Chao, L.; He, F.; Tan, Y.; Chen, C.; Xie, Q. In situ microliter-droplet anodic stripping voltammetry of copper stained on the gold label after galvanic replacement reaction enlargement for ultrasensitive immunoassay of proteins. Biosens. Bioelectron. 2016, 79, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Zhao, S.; Huang, Y.; Jiang, J.; Ye, F. Magnetic Bead-Sensing-Platform-Based Chemiluminescence Resonance Energy Transfer and Its Immunoassay Application. Anal. Chem. 2012, 84, 2708–2712. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, P.; Bu, L.; Wang, T.; Xie, Q.; Xu, X.; Lei, L.; Zou, C.; Yao, S. Chemical/Biochemical Preparation of New Polymeric Bionanocomposites with Enzyme Labels Immobilized at High Load and Activity for High-Performance Electrochemical Immunoassay. J. Phys. Chem. C 2010, 114, 1472–1480. [Google Scholar] [CrossRef]
- Cui, R.J.; Pan, H.C.; Zhu, J.J.; Chen, H.Y. Versatile Immunosensor Using CdTe Quantum Dots as Electrochemical and Fluorescent Labels. Anal. Chem. 2007, 79, 8494–8501. [Google Scholar] [CrossRef] [PubMed]
- Dequaire, M.; Degrand, C.; Limoges, B.T. An Electrochemical Metalloimmunoassay Based on a Colloidal Gold Label. Anal. Chem. 2000, 72, 5521–5528. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.; Yan, F.; Wu, J.; Leng, C.; Ju, H. Ultrasensitive Multiplexed Immunoassay with Electrochemical Stripping Analysis of Silver Nanoparticles Catalytically Deposited by Gold Nanoparticles and Enzymatic Reaction. Anal. Chem. 2011, 83, 2726–2732. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Zou, Z.; Shin, Y.; Wang, J.; Wu, H.; Engelhard, M.H.; Liu, J.; Aksay, I.A.; Lin, Y. Sensitive Immunosensor for Cancer Biomarker Based on Dual Signal Amplification Strategy of Graphene Sheets and Multienzyme Functionalized Carbon Nanospheres. Anal. Chem. 2010, 82, 2989–2995. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, M.; Zhu, J. Dual-Signal Amplification Strategy for Ultrasensitive Photoelectrochemical Immunosensing of α-Fetoprotein. Anal. Chem. 2012, 84, 10492–10499. [Google Scholar] [CrossRef] [PubMed]
- Chun, C.; Joo, J.; Kwon, D.; Kim, C.S.; Cha, H.J.; Chung, M.S.; Jeon, S. A facile and sensitive immunoassay for the detection of alpha-fetoprotein using gold-coated magnetic nanoparticle clusters and dynamic light scattering. Chem. Commum. 2011, 47, 11047–11049. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.Y.; Wang, H.F.; Chen, J.T.; Yan, X.P. Fluorescence Resonance Energy Transfer Inhibition Assay for α-Fetoprotein Excreted during Cancer Cell Growth Using Functionalized Persistent Luminescence Nanoparticles. J. Am. Chem. Soc. 2011, 133, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Yang, C.; Tong, Y.; Xu, G.; Ma, X.; Lin, Y.; Chen, G. Label-free electrochemiluminescent immunosensor for α-fetoprotein: Performance of Nafion–carbon nanodots nanocomposite films as antibody carriers. Chem. Commum. 2012, 48, 3055–3057. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.Y.; Xu, B.Y.; Du, Y.; Xu, J.J.; Chen, H.Y. A branched electrode based electrochemical platform: Towards new label-free and reagentless simultaneous detection of two biomarkers. Chem. Commum. 2013, 49, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ma, Z. Highly stable electrochemical immunosensor for carcinoembryonic antigen. Biosens. Bioelectron. 2012, 35, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhan, X.; Liu, K.; Lv, H.; Duan, Y. Plasma-Enhanced Antibody Immobilization for the Development of a Capillary-Based Carcinoembryonic Antigen Immunosensor Using Laser-Induced Fluorescence Spectroscopy. Anal. Chem. 2013, 85, 4578–4585. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Liu, H.; Ju, H. Flow-Through Multianalyte Chemiluminescent Immunosensing System with Designed Substrate Zone-Resolved Technique for Sequential Detection of Tumor Markers. Anal. Chem. 2006, 78, 6999–7005. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Ge, L.; Gao, W.; Yu, J.; Song, X.; Ge, S.; Jia, Z.; Chu, C. Electrogenerated Chemiluminescence from a Phenyleneethynylene Derivative and its Ultrasensitive Immunosensing Application Using a Nanotubular Mesoporous Pt-Ag Alloy for Signal Amplification. Adv. Funct. Mater. 2012, 22, 3899–3906. [Google Scholar] [CrossRef]
- Lin, J.; Wei, Z.; Mao, C. A label-free immunosensor based on modified mesoporous silica for simultaneous determination of tumor markers. Biosens. Bioelectron. 2011, 29, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Jie, G.F.; Yuan, J.X. Novel Magnetic Fe3O4@CdSe Composite Quantum Dot-Based Electrochemiluminescence Detection of Thrombin by a Multiple DNA Cycle Amplification Strategy. Anal. Chem. 2012, 84, 2811–2817. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Li, D.; Ren, W.; Liu, Z.J.; Dong, S.J.; Wang, E.K. Ultrasensitive colorimetric detection of protein by aptamer-Au nanoparticles conjugates based on a dot-blot assay. Chem. Commum. 2008, 22, 2520–2522. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.; Xiao, Y.; Shlyahovsky, B.; Willner, I. Aptamer-Functionalized Au Nanoparticles for the Amplified Optical Detection of Thrombin. J. Am. Chem. Soc. 2004, 126, 11768–11769. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Shen, L.H.; Zhang, D.D.; Qi, H.L.; Gao, Q.; Ma, F.; Zhang, C.X. Electrochemical impedance spectroscopy for study of aptamer-thrombin interfacial interactions. Biosens. Bioelectron. 2008, 23, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.F.; Feng, F.; Zhao, L.; Wang, C.Y.; Wang, H.Y.; Tian, M.Z.; Qin, J.; Duan, Y.L.; He, X.X. Aptamer/thrombin/aptamer-AuNPs sandwich enhanced surface plasmon resonance sensor for the detection of subnanomolar thrombin. Biosens. Bioelectron. 2013, 47, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Zhang, Y.; Li, H.; Zhang, Y.; Tan, P.; Tang, H.; Yao, S. A novel method for the detection of point mutation in DNA using single-base-coded CdS nanoprobes. Biosens. Bioelectron. 2009, 24, 2339–2345. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Corn, R.M. DNAzyme Footprinting: Detecting Protein-Aptamer Complexation on Surfaces by Blocking DNAzyme Cleavage Activity. J. Am. Chem. Soc. 2013, 135, 2072–2075. [Google Scholar] [CrossRef] [PubMed]
- Willner, I.; Zayats, M. Electronic Aptamer-Based Sensors. Angew. Chem. Int. Ed. 2007, 46, 6408–6418. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Lubin, A.A.; Heeger, A.J.; Plaxco, K.W. Label-Free Electronic Detection of Thrombin in Blood Serum by Using an Aptamer-Based Sensor. Angew. Chem. Int. Ed. 2005, 44, 5456–5459. [Google Scholar] [CrossRef] [PubMed]
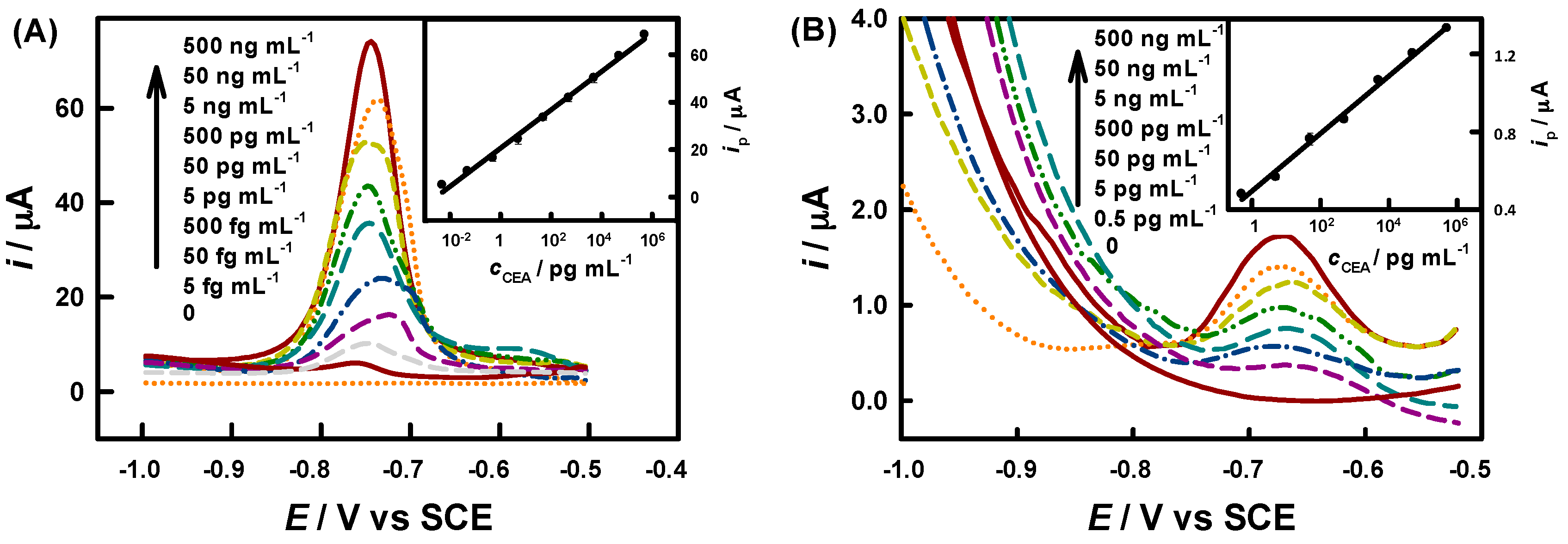

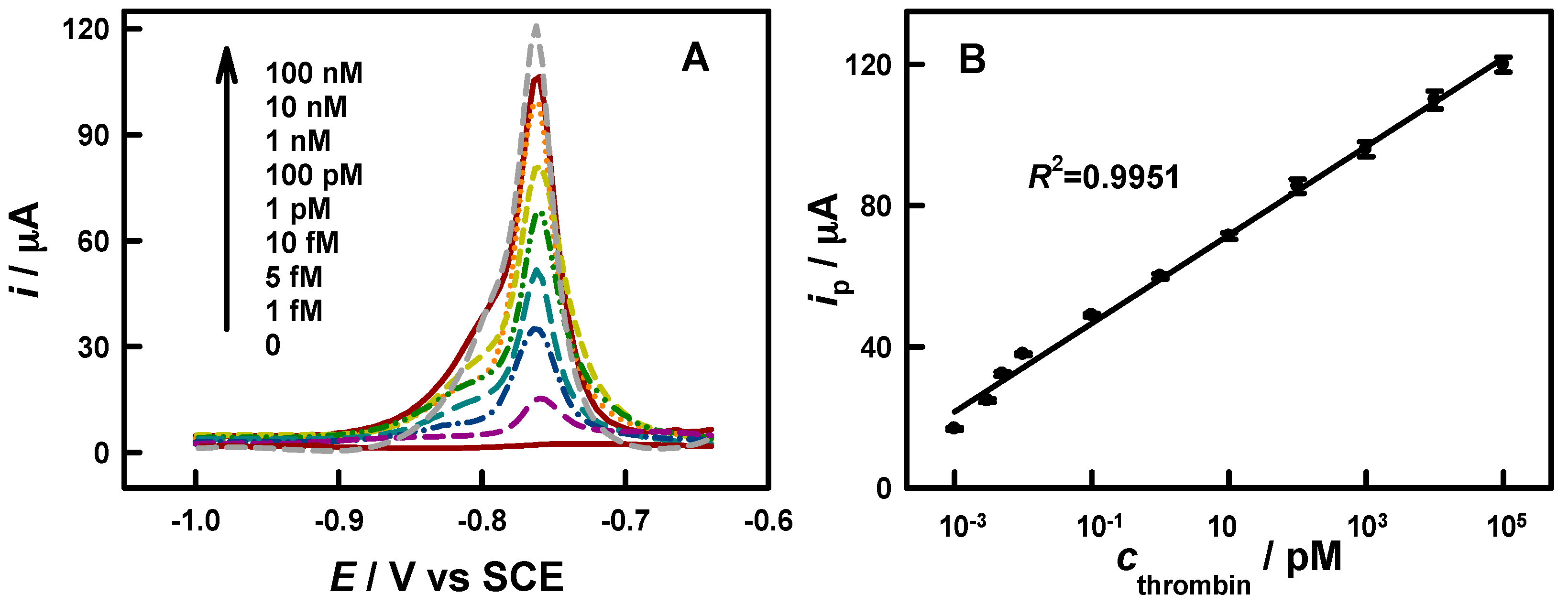
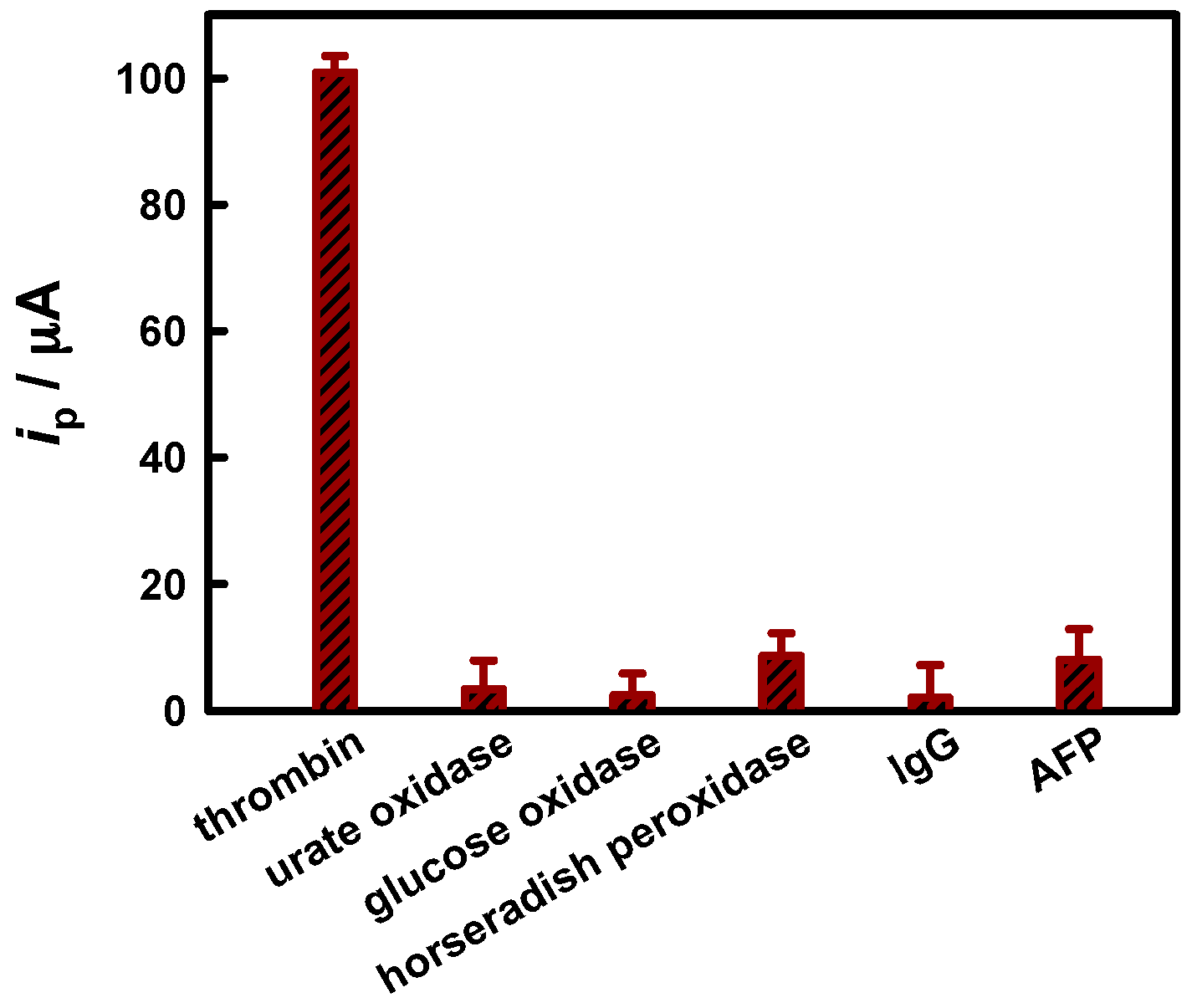
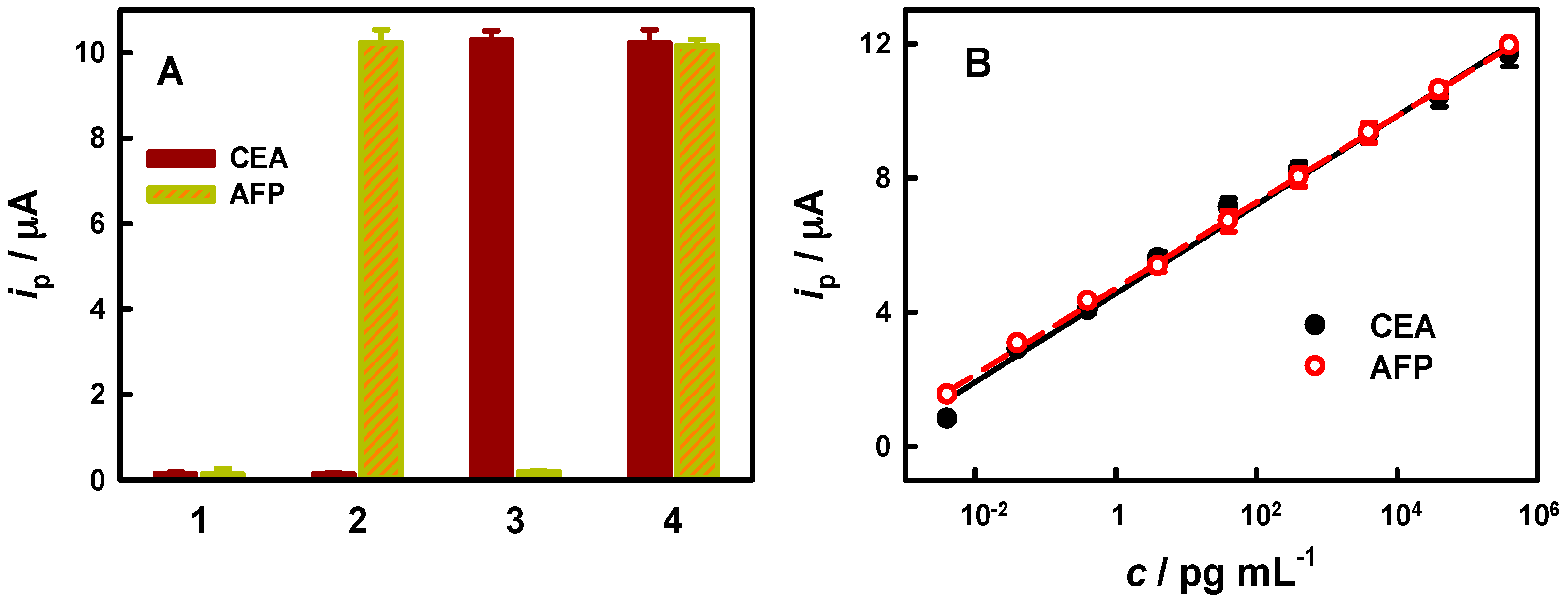
| Analyte | Label | Analytical Technique | LDR/ng·mL−1 | LOD/ng·mL−1 | Ref. |
|---|---|---|---|---|---|
| IgG | FITC | CRET | 0.03–0.6 | 4.35 × 10−3 | [33] |
| CdS QDs | Photoelectrochemical | 5 × 10−4–5 | 5 × 10−4 | [15] | |
| Glucose | Chronoamperometry | 0.005–1 | 0.002 | [34] | |
| CdTe QDs | Fluorometry/SWV | 0.1–500/5 × 10−3−100 | 0.03/0.005 | [35] | |
| AuNPs | ASV for Au(III) | 0.5–100 | 0.5 | [36] | |
| AuNPs and ALP | ASV for catalytically-deposited Ag | 0.01–250 | 4.8 × 10−3 6.1 × 10−3 | [37] | |
| CdS QDs | Differential pulse ASV | 5 × 10−6–500 | 4.5 × 10−6 | This work | |
| AFP | CNSs-HRP | SWV | 0.05–6 | 0.02 | [38] |
| CdTe-GOx | Photoelectrochemistry | 5 × 10−4–1 × 104 | 1.3 × 10−4 | [39] | |
| Au-MNCs | Dynamic light scattering | 0.01–50 | 0.01 | [40] | |
| PLNPs | FRET | 0.8–45 | 0.41 | [41] | |
| Label-free | Electrochemiluminescence | 1 × 10−4–10, 10–320 | 1 × 10−4 | [42] | |
| Label-free | Differential pulse ASV | 0.5–50 | 0.1 | [43] | |
| CdS QDs | Differential pulse ASV | 5 × 10−6–500 | 4.9 × 10−6 | This work | |
| CEA | Label-free | Differential pulse ASV | 0.5–80 | 0.05 | [43] |
| AuNPs | Differential pulse ASV | 1 × 10−5–100 | 3.0 × 10−6 | [44] | |
| Cy3 | Fluorescence | 0.3–100 | 0.09 | [45] | |
| ALP | Chemiluminescence | 1–120 | 0.6 | [46] | |
| Pt–Ag alloy | Electrogenerated chemiluminescence | 1 × 10−5–10 | 3.0 × 10−6 | [47] | |
| Label-free | Differential pulse ASV | 0.5–45 | 0.2 | [48] | |
| CdS QDs | Differential pulse ASV | 5 × 10−6–500 | 3.0 × 10−6 | This work | |
| Thrombin | QDs | SWV | 0.02–0.5 | 0.02 | [1] |
| Fe3O4@CdSe | Electrochemiluminescence | 1 × 10−3–5.0 nM | 0.12 pM | [49] | |
| AuNPs | Colorimetric detection | 0.115–3.7 pM | 14 fM | [50] | |
| AuNPs | Absorption spectra for catalytically deposited Au | 2–167 nM | 2 nM | [51] | |
| Label-free | EIS | 0.12–30 nM | 0.06 nM | [52] | |
| AuNPs | SPR | 0.1–75 nM | 0.1 nM | [53] | |
| CdS QDs | Differential pulse ASV | 1 × 10−6–10 nM | 0.9 fM | This work |
| Added/nmol·L−1 | Measured/nmol·L−1 | RSD/% | Recovery/% |
|---|---|---|---|
| 1.00 | 0.97 | 6.4 | 97 |
| 2.00 | 1.96 | 7.5 | 98 |
| 3.00 | 2.95 | 5.2 | 98 |
| 4.00 | 4.07 | 8.0 | 101 |
| 5.00 | 4.81 | 6.9 | 96 |
| Serum Sample | Reference Method a/ng·mL−1 | CEA | AFP | ||||
|---|---|---|---|---|---|---|---|
| CEA | AFP | Our Protocol b/ng·mL−1 | RD/% | Our Protocol b/ng·mL−1 | RD/% | ||
| 1 | Normal | 2.28 | 11.3 | 2.19 | −3.9 | 11.1 | −1.8 |
| 2 | Normal | 1.72 | 1.40 | 1.67 | −2.9 | 1.33 | −5.0 |
| 3 | Normal | 1.34 | 1.31 | 1.42 | 6.0 | 1.39 | 6.1 |
| 4 | Pregnant | 2.53 | 14.3 | 2.62 | 3.6 | 13.9 | −2.8 |
| 5 | Lung cancer | 5.58 | 80.3 | 5.37 | −3.8 | 79.6 | −0.9 |
| 6 | Rectal cancer | 34.5 | 370 | 33.1 | −4.0 | 369 | −0.3 |
| 7 | Liver cancer | 5.02 | 30.6 | 5.21 | 3.8 | 32.1 | 4.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.; Wang, L.; Xie, Q. Sensitive Bioanalysis Based on in-Situ Droplet Anodic Stripping Voltammetric Detection of CdS Quantum Dots Label after Enhanced Cathodic Preconcentration. Sensors 2016, 16, 1342. https://doi.org/10.3390/s16091342
Qin X, Wang L, Xie Q. Sensitive Bioanalysis Based on in-Situ Droplet Anodic Stripping Voltammetric Detection of CdS Quantum Dots Label after Enhanced Cathodic Preconcentration. Sensors. 2016; 16(9):1342. https://doi.org/10.3390/s16091342
Chicago/Turabian StyleQin, Xiaoli, Linchun Wang, and Qingji Xie. 2016. "Sensitive Bioanalysis Based on in-Situ Droplet Anodic Stripping Voltammetric Detection of CdS Quantum Dots Label after Enhanced Cathodic Preconcentration" Sensors 16, no. 9: 1342. https://doi.org/10.3390/s16091342
APA StyleQin, X., Wang, L., & Xie, Q. (2016). Sensitive Bioanalysis Based on in-Situ Droplet Anodic Stripping Voltammetric Detection of CdS Quantum Dots Label after Enhanced Cathodic Preconcentration. Sensors, 16(9), 1342. https://doi.org/10.3390/s16091342





