Fabrication of a Micro-Needle Array Electrode by Thermal Drawing for Bio-Signals Monitoring
Abstract
:1. Introduction
2. Experimental
2.1. MAE Fabrication
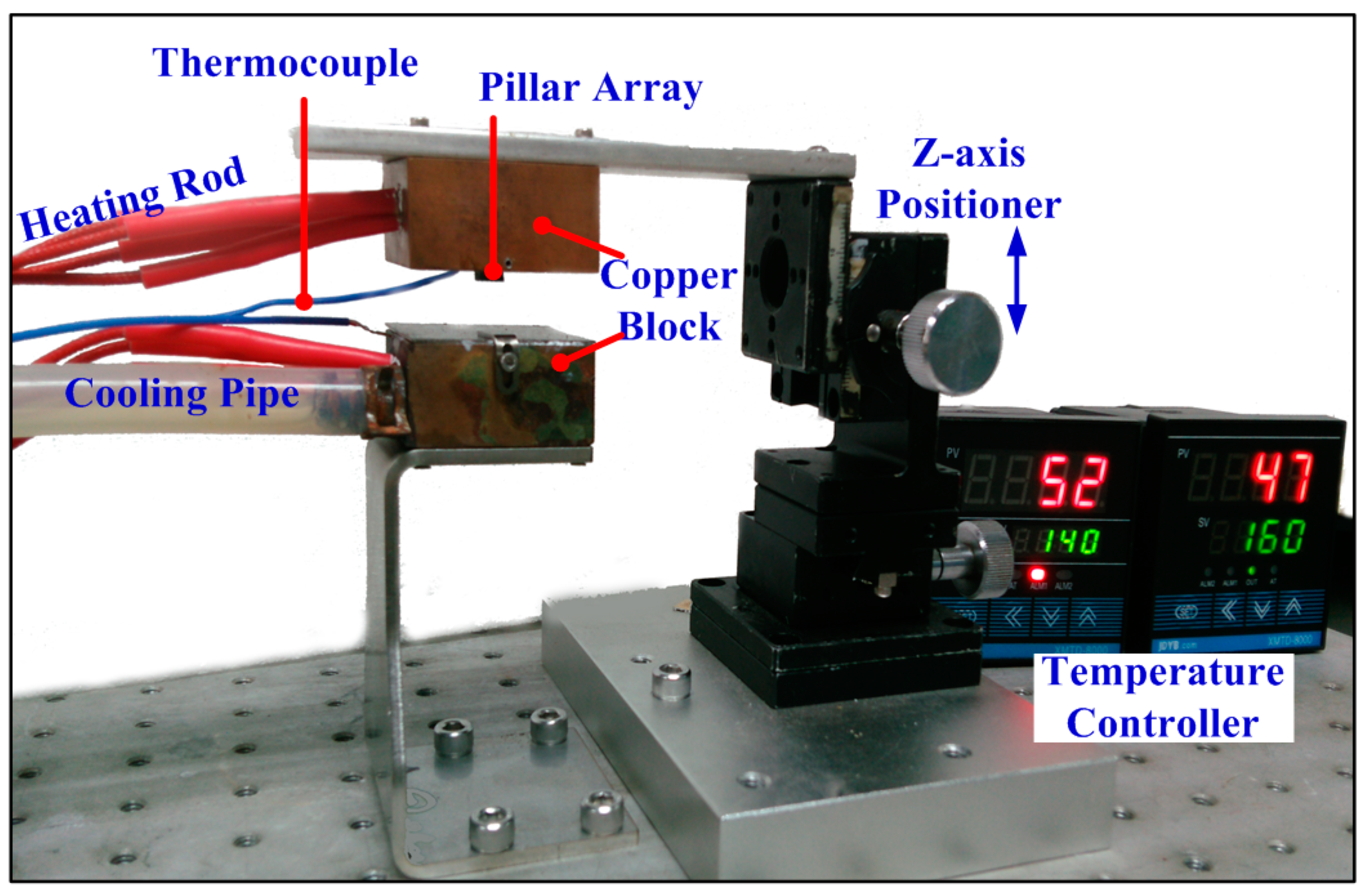
2.1.1. Experimental Setup
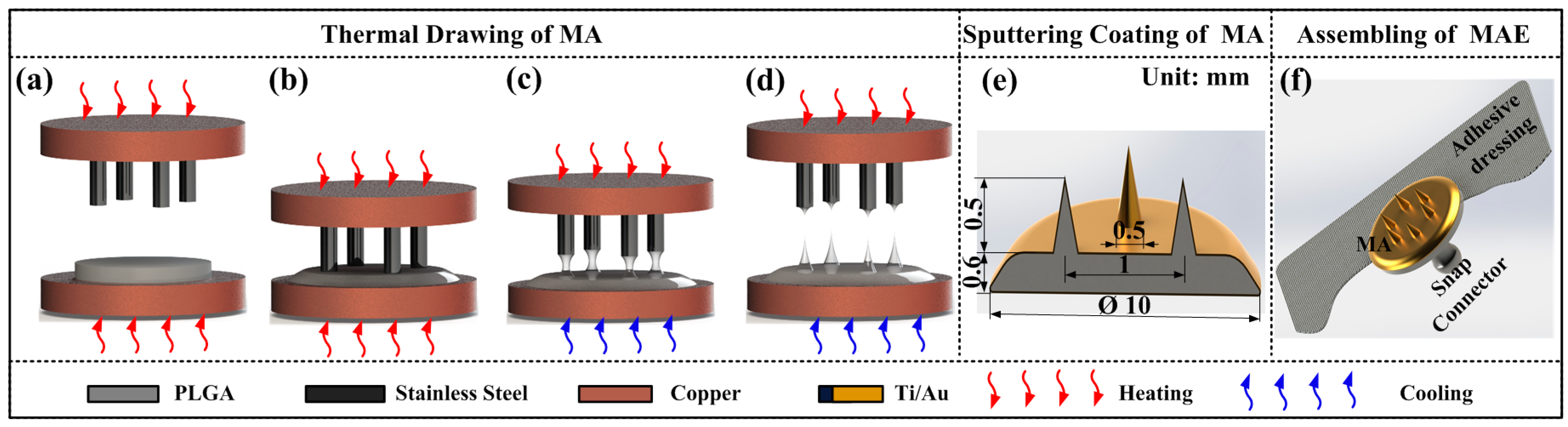
2.1.2. Fabrication Process
Materials Preparation
Thermal Drawing of MA
MA Coating
MAE Assembling
2.2. Insertion, EII Test, and Numerical Simulation
2.2.1. EII Test during the Insertion Process
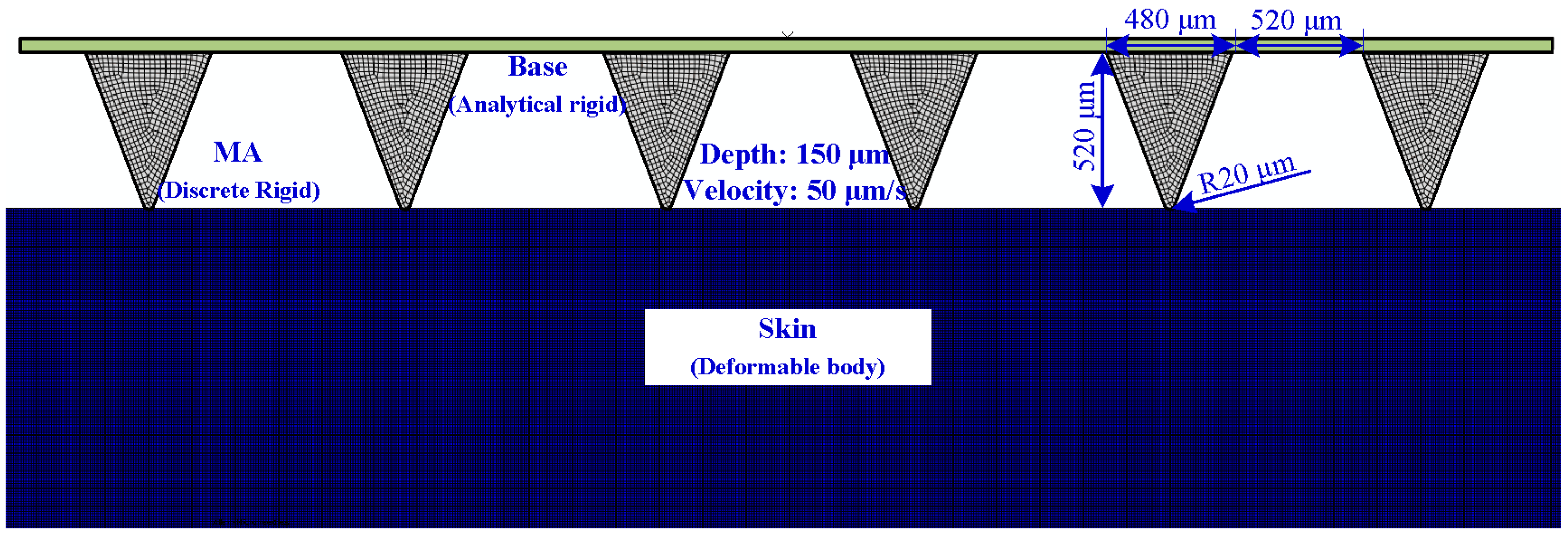
2.2.2. Numerical Simulation of Insertion and Pull Process
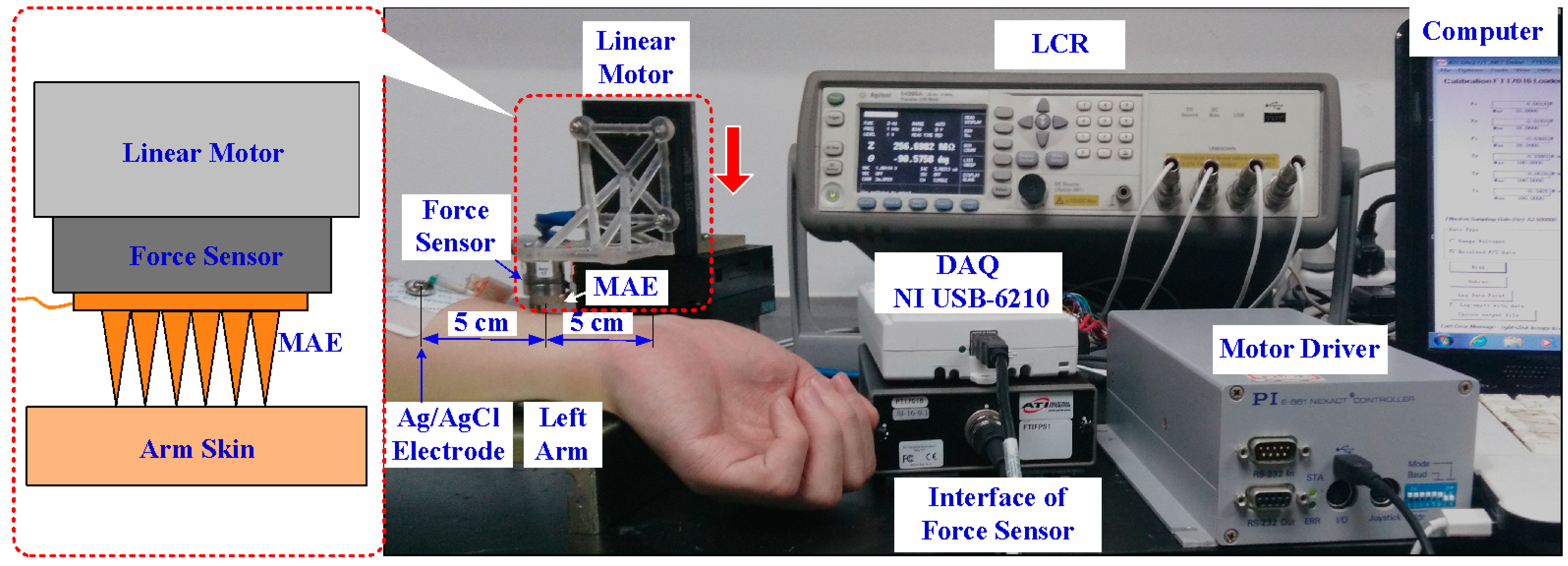
2.3. Bio-Signals Recording
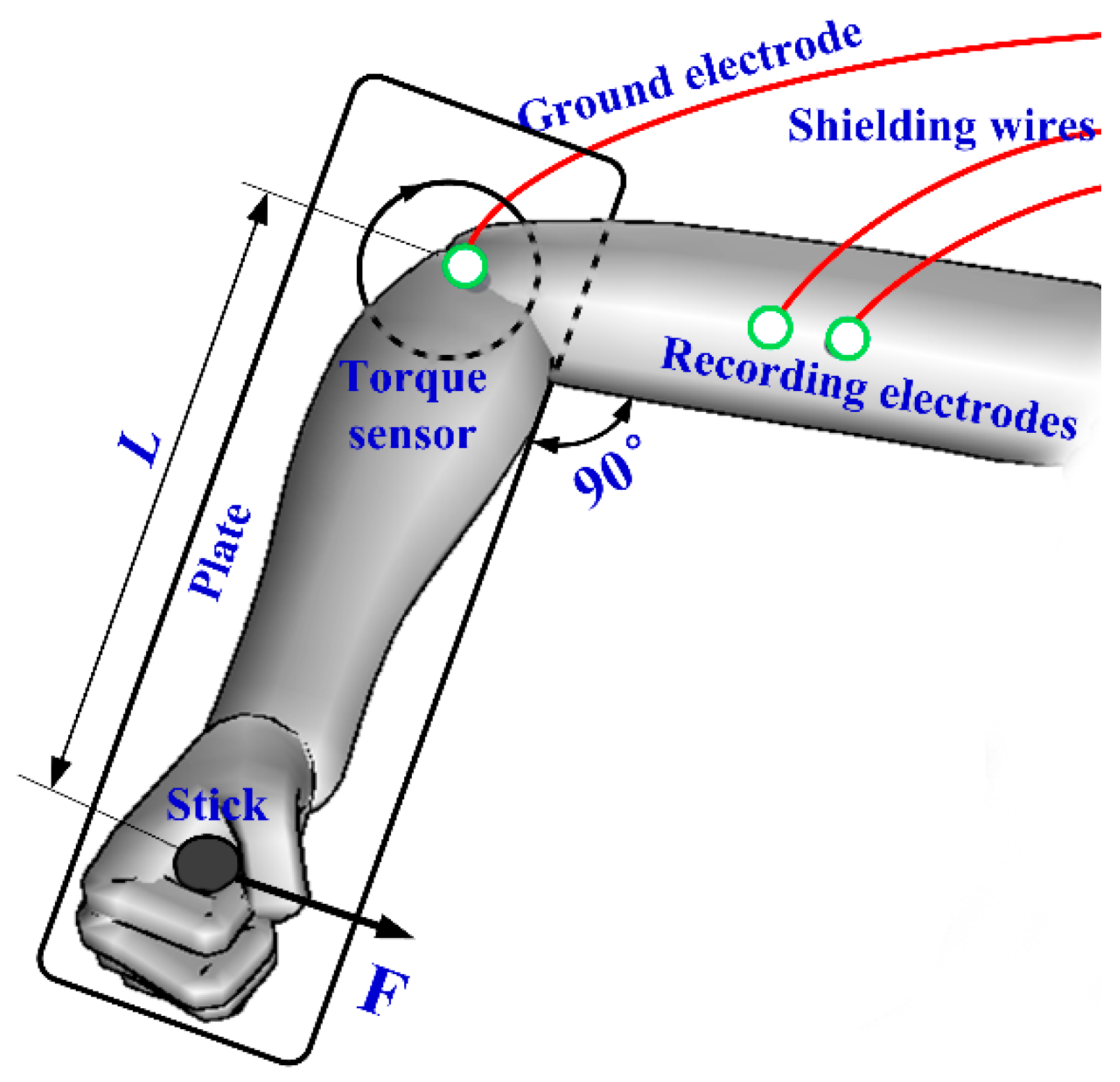
2.3.1. EMG Test
2.3.2. ECG Test
2.3.3. EEG Test
3. Results and Discussion
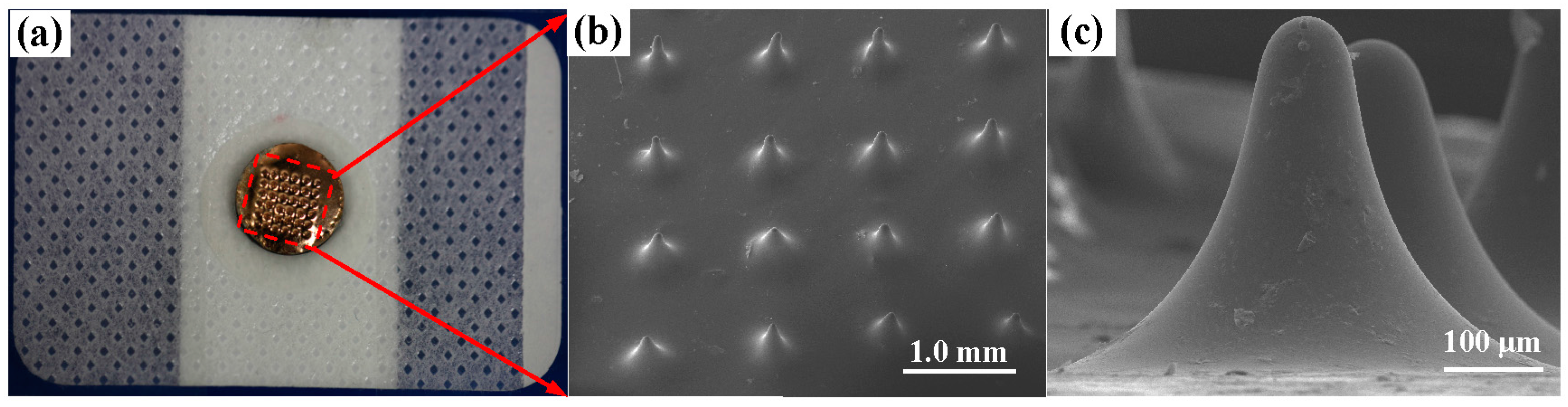
3.1. Characterization of the MAE
3.2. EII Test and Insertion Process
3.2.1. EII Recording during MAE Compression
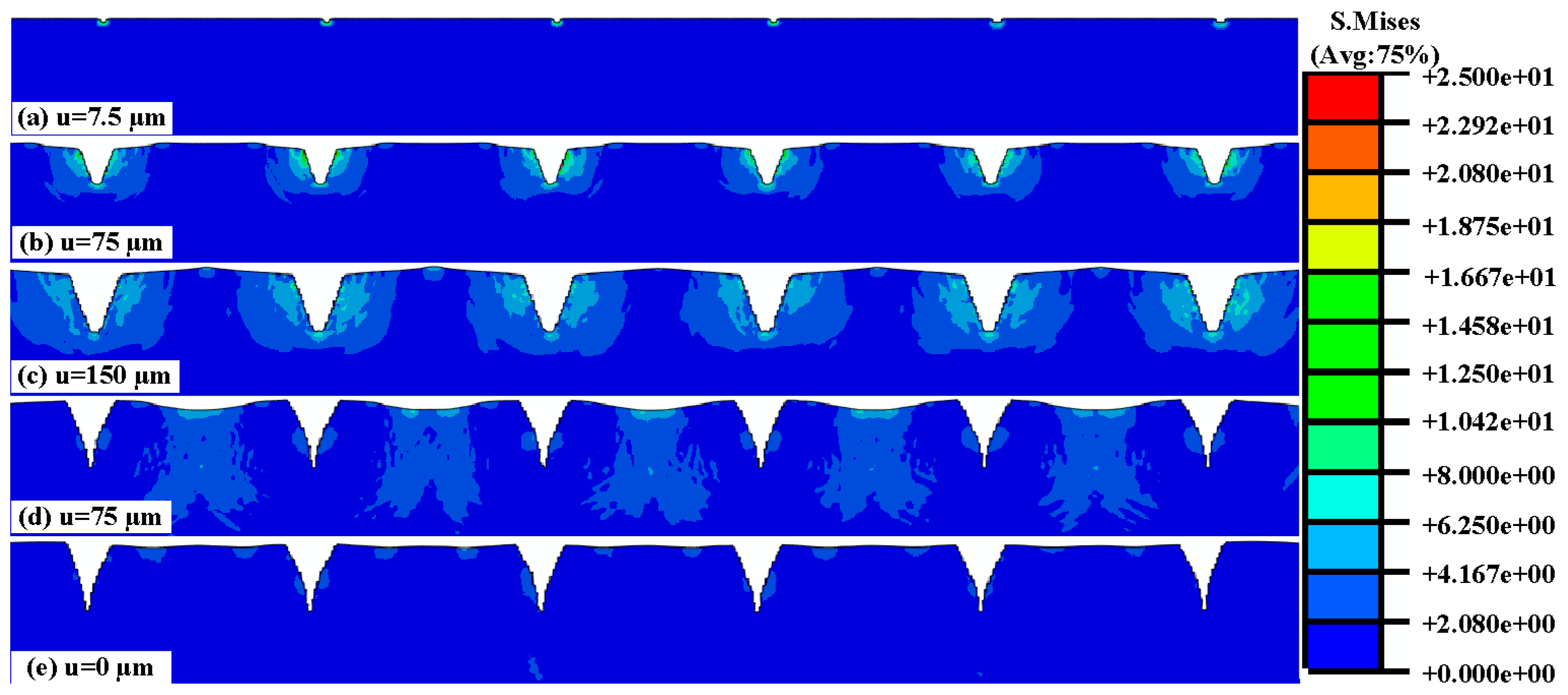
3.2.2. Numerical Simulation of the Insertion and Pull Process
3.3. Bio-Signals Measurement
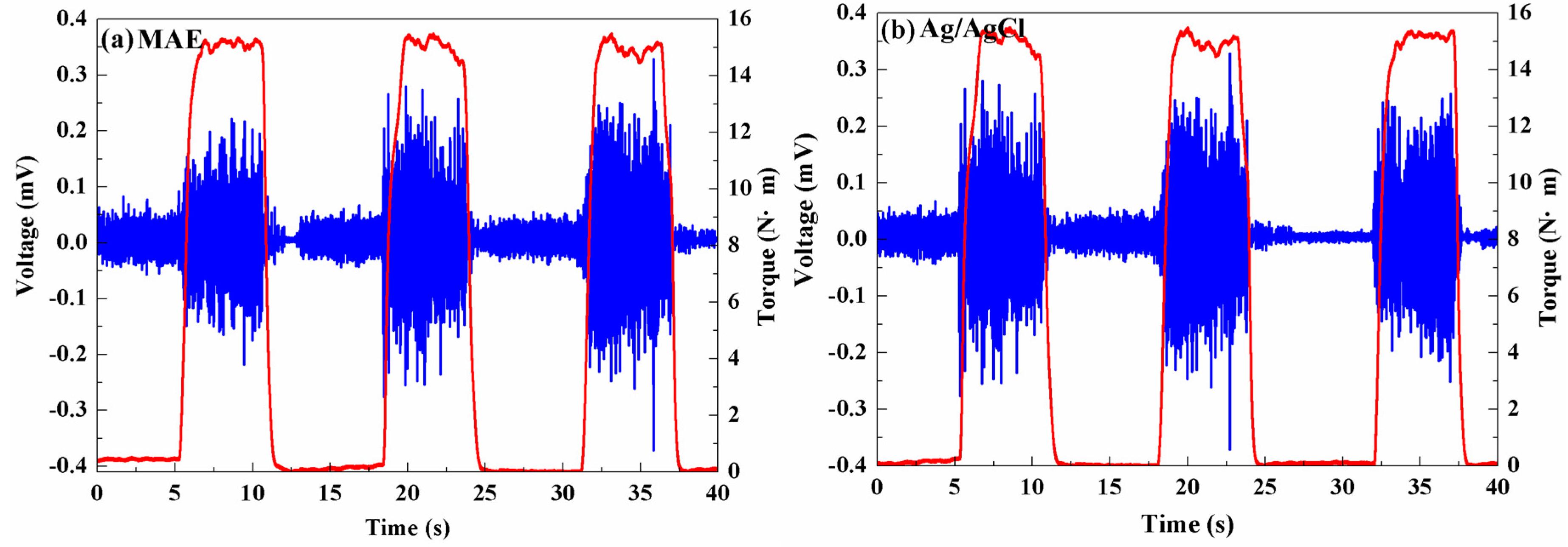
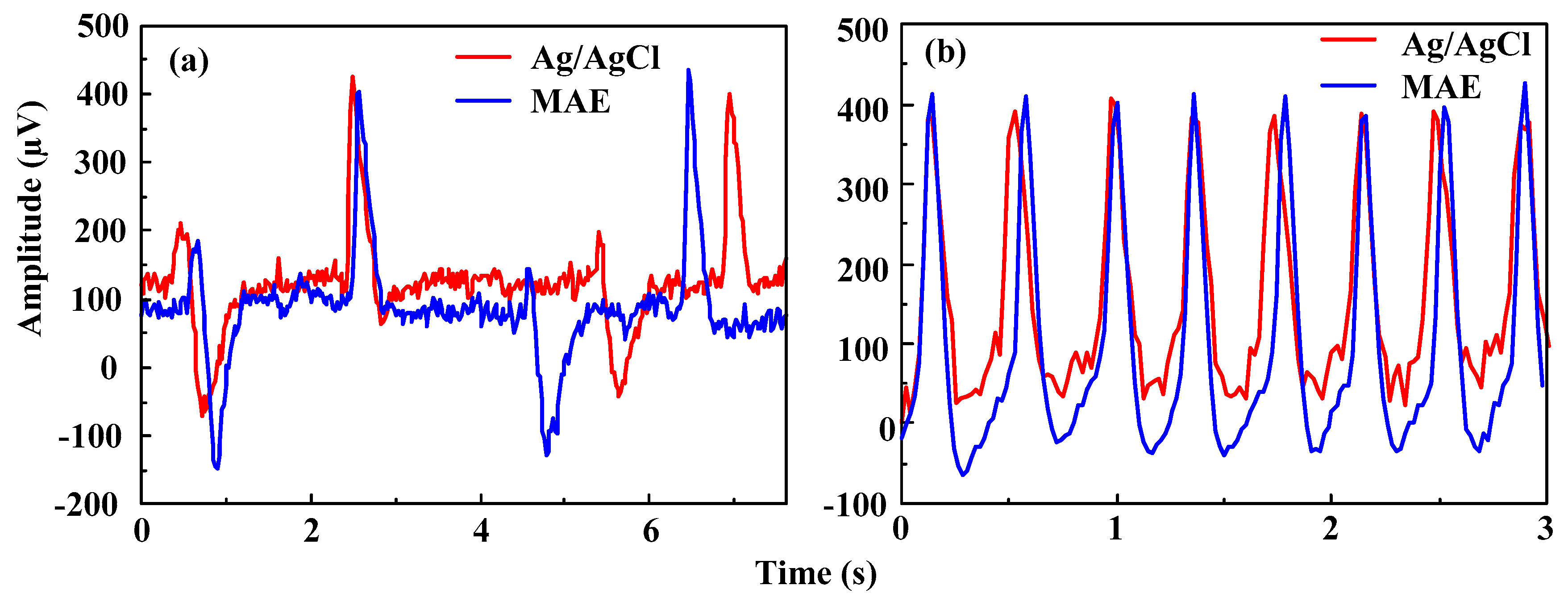
3.3.1. EMG Measurement
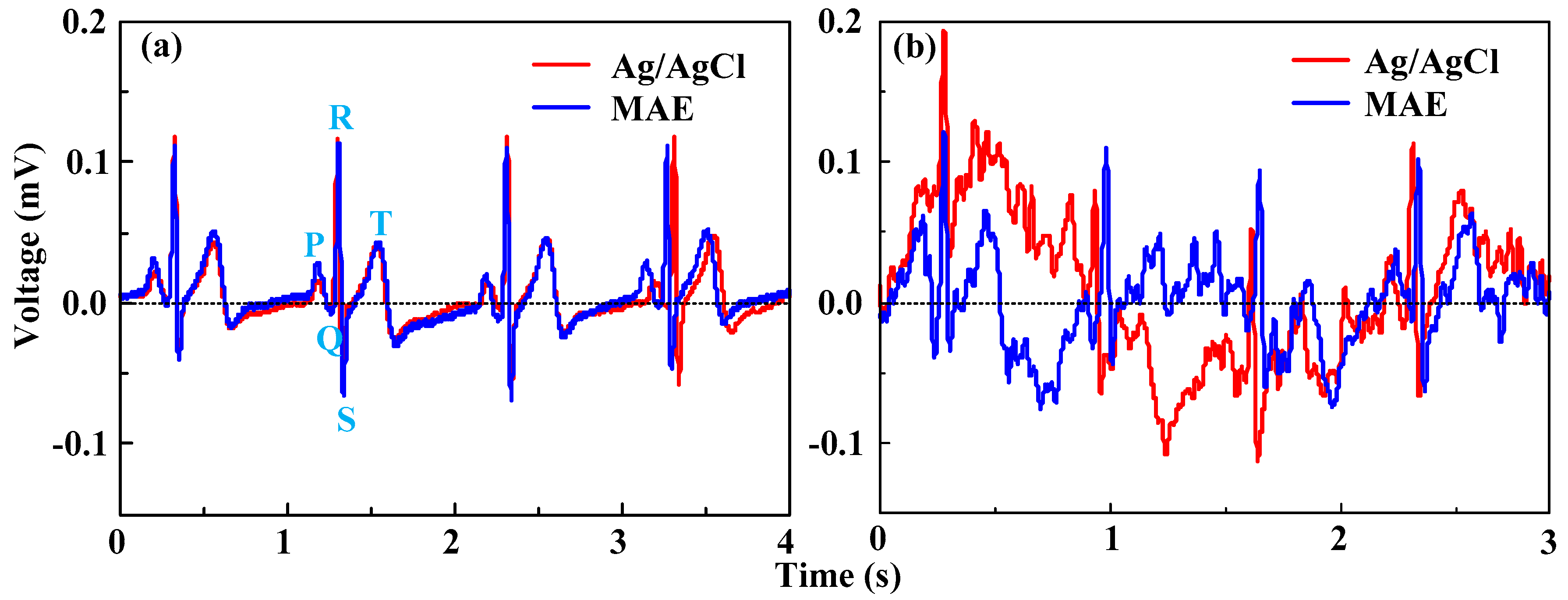
3.3.2. ECG Measurement
4. Conclusions
- (1)
- The MAE, biocompatible for humans, can be fabricated by a thermal drawing method and magnetron sputtering in mass production. The micro-needle tips can “tear” and insert the human skin due to the stress concentration near the tips. When the compression force on the MAE is larger than 1 N, EII of the MAE is about 85 KΩ which is lower than that measured by Ag/AgCl electrodes (120 KΩ). EII slightly decreases with the insertion force at a given driving current frequency. The MAE can record EII at a relatively low insertion force.
- (2)
- The MAE can clearly sense and record the surface EMG signals as well as Ag/AgCl electrodes. The geometry of the MAE can be customized by a thermal drawing method based on a monitored muscle to improve the selectivity of EMG and eliminate the crosstalk. The ECG features measured by both the MAE and Ag/AgCl electrodes are all distinguishable in the static state. ECG signals in the dynamic state measured by both Ag/AgCl electrodes and the MAE are seriously disturbed by motion artifacts, but ECG signals of the MAE is more distinguishable than the signal collected by Ag/AgCl electrodes. Both the MAE and Ag/AgCl electrodes can record EEG signals. The MAE could directly capture bio-signals without skin preparation and application of electrolytic gel.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Spinelli, E.; Haberman, M. Insulating electrodes: A review on biopotential front ends for dielectric skin-electrode interfaces. Physiol. Meas. 2010, 31. [Google Scholar] [CrossRef] [PubMed]
- Forvi, E.; Bedoni, M.; Carabalona, R.; Soncini, M.; Mazzoleni, P.; Rizzo, F.; O’Mahony, C.; Morasso, C.; Cassarà, D.G.; Gramatica, F. Preliminary technological assessment of microneedles-based dry electrodes for biopotential monitoring in clinical examinations. Sens. Actuators A Phys. 2012, 180, 177–186. [Google Scholar] [CrossRef]
- Zhou, W.; Song, R.; Pan, X.; Peng, Y.; Qi, X.; Peng, J.; Hui, K.S.; Hui, K.N. Fabrication and impedance measurement of novel metal dry bioelectrode. Sens. Actuators A Phys. 2013, 201, 127–133. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Liu, M.; Cui, L.; Yu, Y. Microneedle electrode array for electrical impedance myography to characterize neurogenic myopathy. Ann. Biomed. Eng. 2015, 44, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, C.; Pini, F.; Blake, A.; Webster, C.; O’Brien, J.; McCarthy, K.G. Microneedle-based electrodes with integrated through-silicon via for biopotential recording. Sens. Actuators A Phys. 2012, 186, 130–136. [Google Scholar] [CrossRef]
- Salvo, P.; Raedt, R.; Carrette, E.; Schaubroeck, D.; Vanfleteren, J.; Cardon, L. A 3D printed dry electrode for ECG EEG recording. Sens. Actuators A Phys. 2012, 174, 96–102. [Google Scholar] [CrossRef]
- Yu, L.M.; Tay, F.E.H.; Guo, D.G.; Xu, L.; Yap, K.L. A microfabricated electrode with hollow microneedles for ECG measurement. Sens. Actuators A Phys. 2009, 151, 17–22. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Bhartia, B.; Mukhopadhyay, K.; Sharma, A. Long term biopotential recording by body conformable photolithography fabricated low cost polymeric microneedle arrays. Sens. Actuators A Phys. 2015, 236, 164–172. [Google Scholar] [CrossRef]
- Hsu, L.S.; Tung, S.W.; Kuo, C.H.; Yang, Y.J. Developing barbed microtip-based electrode arrays for biopotential measurement. Sensors 2014, 14, 12370–12386. [Google Scholar] [CrossRef] [PubMed]
- Griss, P.; Enoksson, P.; Tolvanen-Laakso, H.K.; Merilainen, P.; Ollmar, S.; Stemme, G. Micromachined Electrodes for Biopotential Measurements. J. Microelectromech. Syst. 2001, 10, 10–16. [Google Scholar] [CrossRef]
- Ng, W.C.; Seet, H.L.; Lee, K.S.; Ning, N.; Tai, W.X.; Sutedja, M.; Fuh, J.Y.H.; Li, X.P. Micro-spike EEG electrode and the vacuum-casting technology for mass production. J. Mater. Process. Technol. 2009, 209, 4434–4438. [Google Scholar] [CrossRef]
- Chinteng, L.; Lunde, L.; Yuhang, L.; Lin, B.S.; Chang, J.Y. Novel dry polymer foam electrodes for long-term eeg measurement. IEEE Trans. Biomed. Eng. 2011, 58, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Griss, P.; Tolvanen-Laakso, H.K.; Merilainen, P.; Stemme, G. Characterization of micromachined spiked biopotential electrodes. IEEE Trans. Biomed. Eng. 2002, 49, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.X.; Zhao, W.; Wang, W.; Li, Z.H. A Flexible Microneedle Electrode Array With Solid Silicon Needles. J. Microelectromech. Syst. 2012, 21, 1084–1089. [Google Scholar] [CrossRef]
- Wilke, N.; Hibert, C.; O’Brien, J.; Morrissey, A. Silicon microneedle electrode array with temperature monitoring for electroporation. Sens. Actuators A. Phys. 2005, 123–124, 319–325. [Google Scholar] [CrossRef]
- Xiang, Z.; Yen, S.-C.; Xue, N.; Sun, T.; Tsang, W.M.; Zhang, S.; Liao, L.-D.; Thakor, N.V.; Lee, C. Ultra-thin flexible polyimide neural probe embedded in a dissolvable maltose-coated microneedle. J. Micromech. Microeng. 2014, 24, 065015. [Google Scholar] [CrossRef]
- Kim, M.; Kim, T.; Kim, D.S.; Chung, W.K. Curved microneedle array-based semg electrode for robust long-term measurements and high selectivity. Sensors 2015, 15, 16265–16280. [Google Scholar] [CrossRef] [PubMed]
- Sabir, M.I.; Xu, X.; Li, L. A review on biodegradable polymeric materials for bone tissue engineering applications. J. Mater. Sci. 2009, 44, 5713–5724. [Google Scholar] [CrossRef]
- Xu, S.; Dai, M.; Xu, C.; Chen, C.; Tang, M.; Shi, X.; Dong, X. Performance evaluation of five types of Ag/AgCl bio-electrodes for cerebral electrical impedance tomography. Ann. Biomed. Eng. 2011, 39, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.J.; Xu, F. Mechanical properties of skin: A review. Adv. Mech. 2008, 38, 393–426. [Google Scholar]
- Xu, F.; Lu, T.J.; Seffen, K.A. Biothermomechanics of skin tissues. J. Mech. Phys. Solids. 2008, 56, 1852–1884. [Google Scholar] [CrossRef]
- Wildnauer, R.H.; Bothwell, J.W.; Douglass, A.B. Stratum corneum biomechanical propepties I. Influence of relative humidity on normal and extracted human stratum corneum. J. Investig. Dermatol. 1971, 56, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, F.M.; Brokken, D.; Oomens, C.W.; Bader, D.L.; Baaijens, F.P. The relative contributions of different skin layers to the mechanical behavior of human skin in vivo using suction experiments. Med. Eng. Phys. 2006, 28, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Gattiker, G.E.; Kaler, K.V.I.S.; Mintchev, M.P. Electronic Mosquito: Designing a semi-invasive Microsystem for blood sampling, analysis and drug delivery applications. Microsyst. Technol. 2005, 12, 44–51. [Google Scholar] [CrossRef]
- Elkhyat, A.; Carol, C.M.; Gharbi, T.; Humbert, P. Influence of the hydrophobic and hydrophilic characteristics of sliding and slider surfaces on friction coefficient: In vivo human skin friction comparison. Skin. Res. Technol. 2004, 10, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Pol. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.; Veiseh, M.; Zhang, M. Surface modification of silicon and gold-patterned silicon surfaces for improved biocompatibility and cell patterning selectivity. Biosens. Bioelectron. 2005, 20, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Zingler, S.; Erber, R.; Lux, C.J.; Seeberger, R.; Bister, D.; Ludwig, B. Biocompatibility of gold and stainless steel chains used for forced eruption of impacted teeth—An in vitro investigation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Larraneta, E.; Moore, J.; Vicente-Perez, E.M.; Gonzalez-Vazquez, P.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. A proposed model membrane and test method for microneedle insertion studies. Int. J. Pharm. 2014, 472, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L.; Zipes, D.P.; Libby, P.; Bonow, R.O. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine, 5th ed.; Braunwald, E., Ed.; Philadelphia, W.B. Saunders Co.: St. Louis, MO, USA, 1997. [Google Scholar]
- YU, L.M.; Tay, F.E.H.; Guo, D.G.; Xu, L.; Nyan, M.N.; Chong, F.W.; Yap, K.L.; Xu, B. A MEMS-based Bioelectrode for ECG measurement. In Proceedings of the IEEE Sensors 2008 Conference, Lecce, Italy, 26–29 October 2008; pp. 1068–1071.
- Merletti, R. The electrode-skin interface and optimal detection of bioelectric signals. Physiol. Meas. 2010, 31, 10. [Google Scholar] [CrossRef]

| Stratum Corneum | Epidermis | Dermis | |
|---|---|---|---|
| C10 (MPa) | 10 | 0.11 | 0.16 |
| Failure stress (MPa) | 25 | -- | 7.3 |
| Density (kg/m3) | 1300 | 1200 | 1200 |
| Thickness (mm) | 0.07 | 0.05 | 0.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, L.; Jiang, Q.; Chen, K.; Chen, Z.; Pan, C.; Jiang, L. Fabrication of a Micro-Needle Array Electrode by Thermal Drawing for Bio-Signals Monitoring. Sensors 2016, 16, 908. https://doi.org/10.3390/s16060908
Ren L, Jiang Q, Chen K, Chen Z, Pan C, Jiang L. Fabrication of a Micro-Needle Array Electrode by Thermal Drawing for Bio-Signals Monitoring. Sensors. 2016; 16(6):908. https://doi.org/10.3390/s16060908
Chicago/Turabian StyleRen, Lei, Qing Jiang, Keyun Chen, Zhipeng Chen, Chengfeng Pan, and Lelun Jiang. 2016. "Fabrication of a Micro-Needle Array Electrode by Thermal Drawing for Bio-Signals Monitoring" Sensors 16, no. 6: 908. https://doi.org/10.3390/s16060908
APA StyleRen, L., Jiang, Q., Chen, K., Chen, Z., Pan, C., & Jiang, L. (2016). Fabrication of a Micro-Needle Array Electrode by Thermal Drawing for Bio-Signals Monitoring. Sensors, 16(6), 908. https://doi.org/10.3390/s16060908






