Optimal Magnetic Sensor Vests for Cardiac Source Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dataset
2.2. Goal Functions
2.3. Optimization
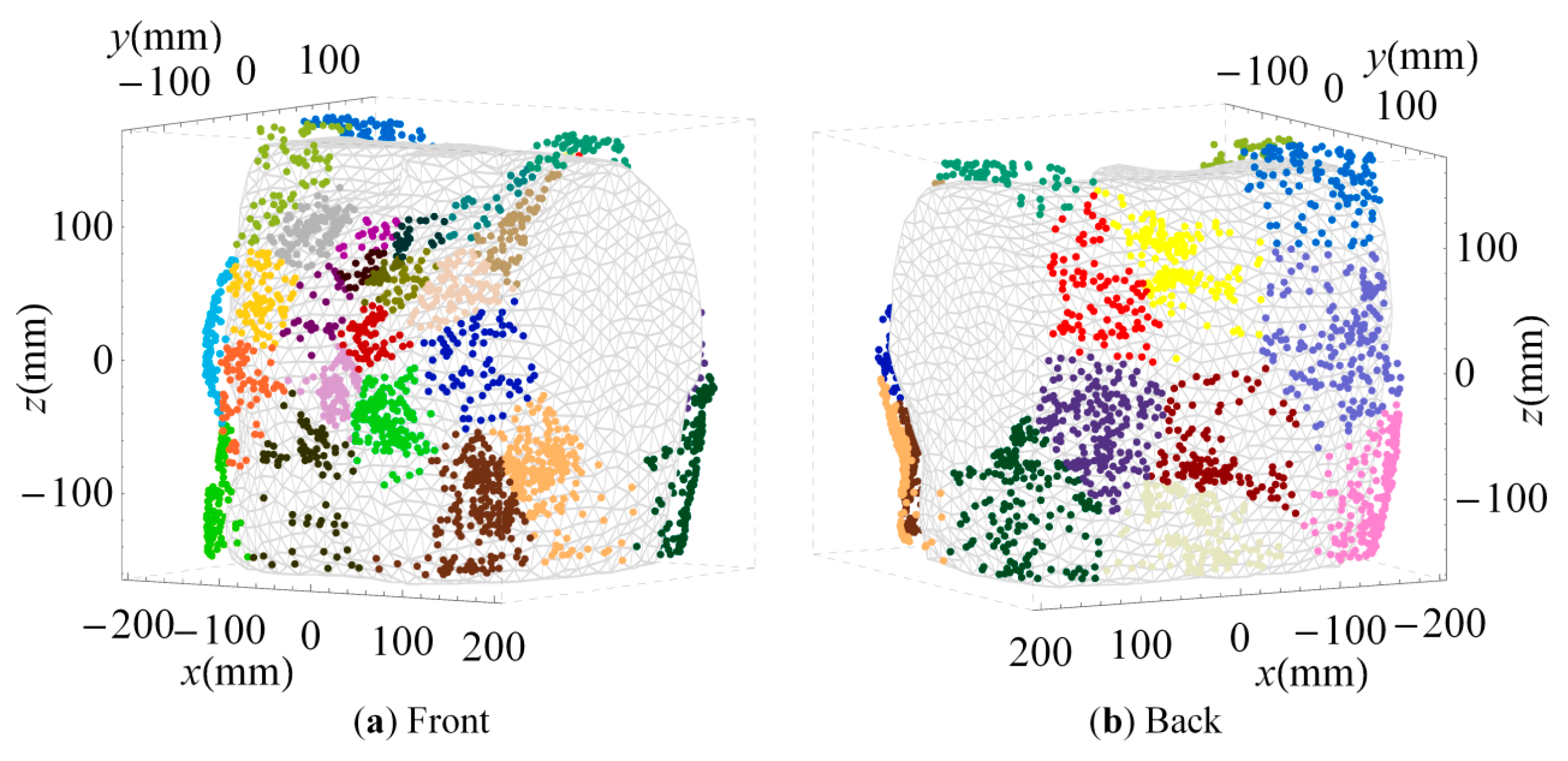
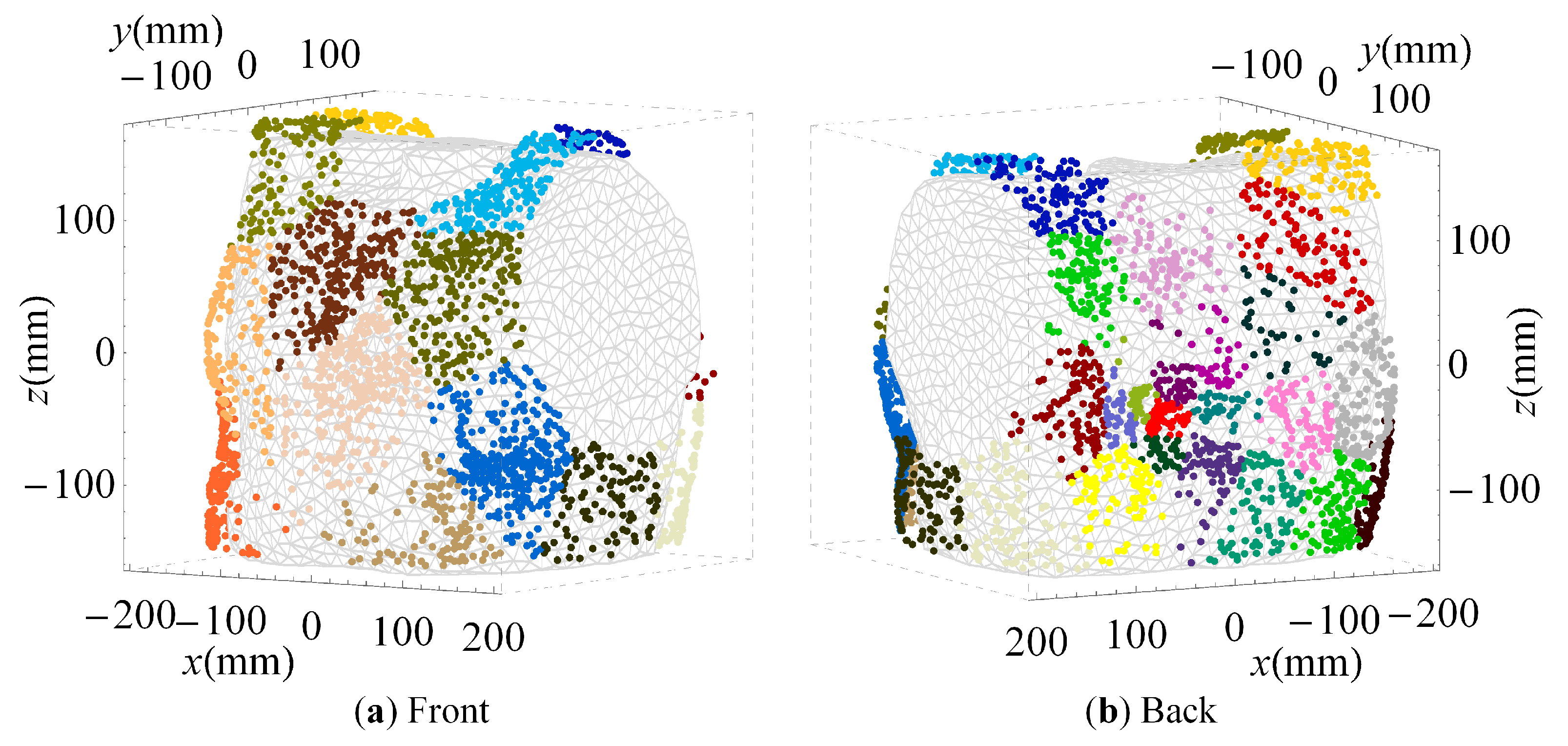
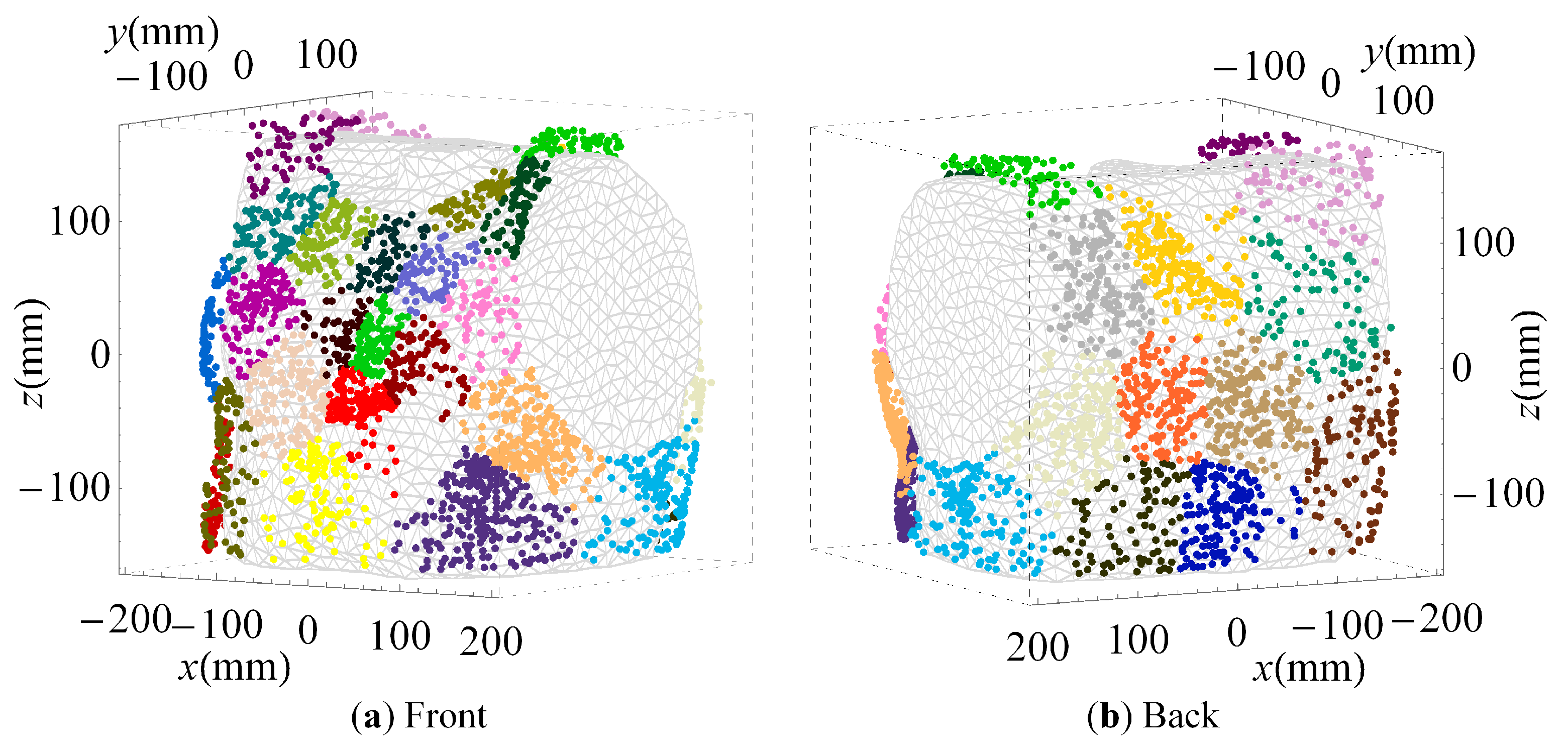
2.4. Cluster Analysis
3. Results
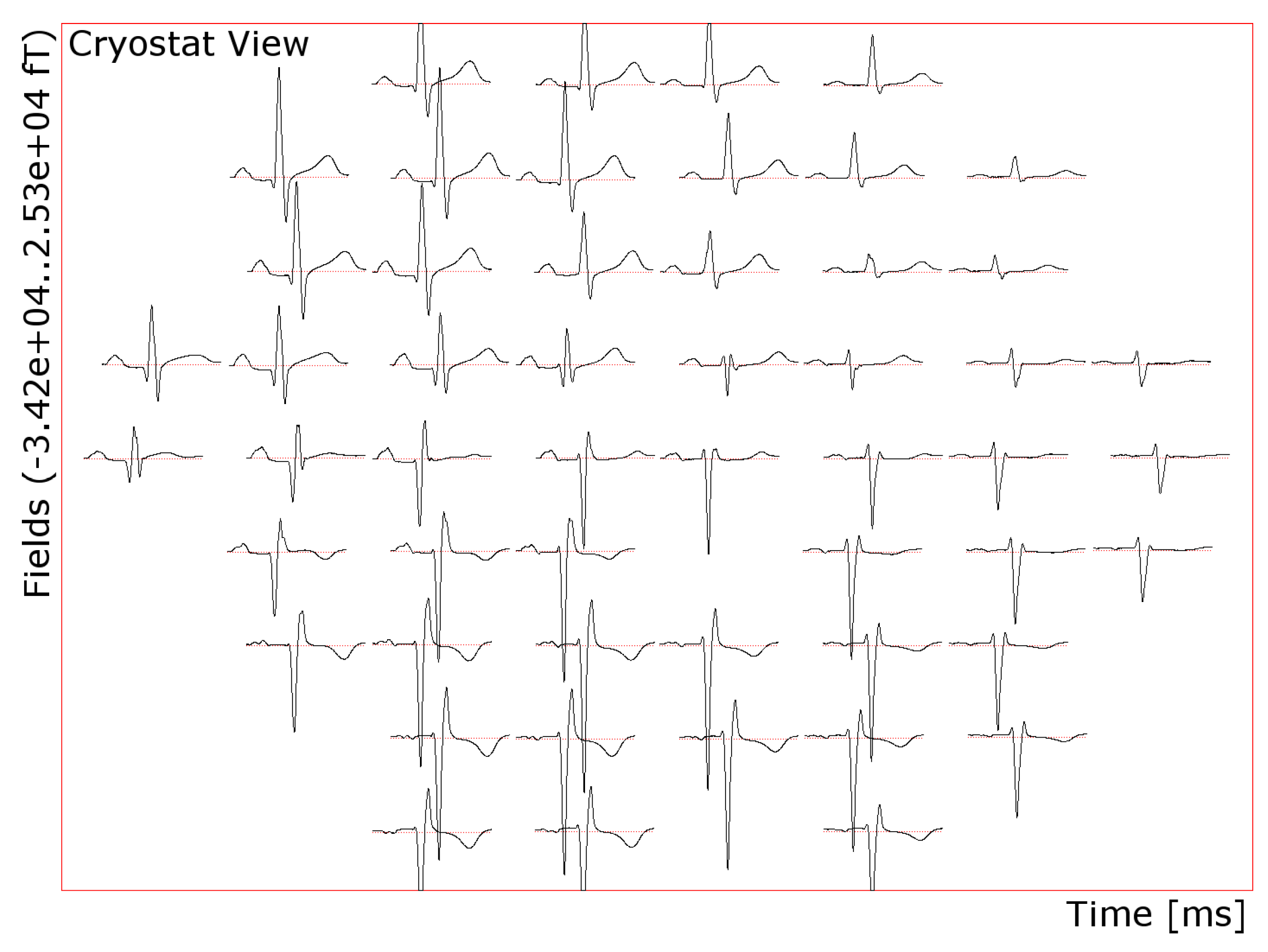
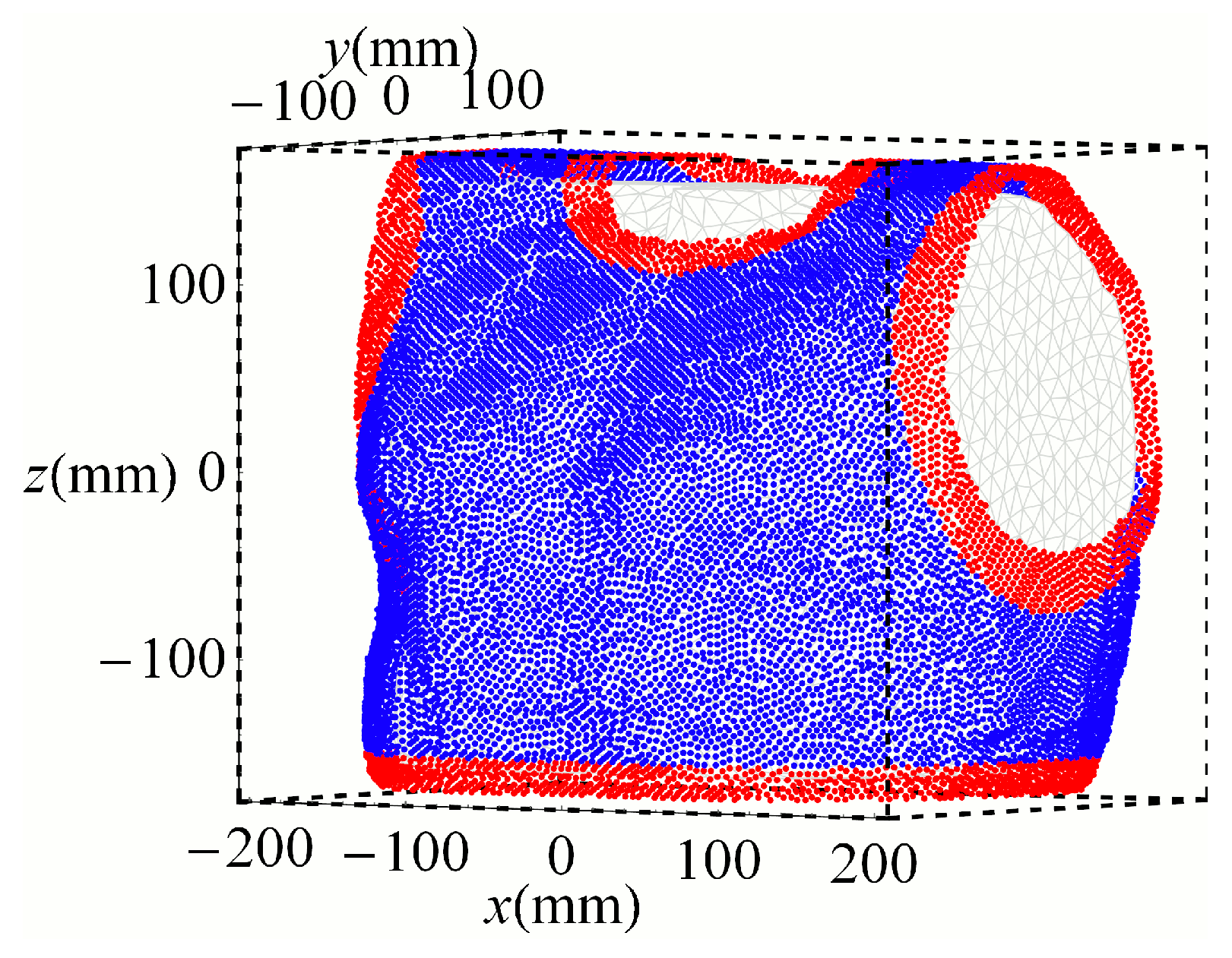
3.1. Optimized Arrangements
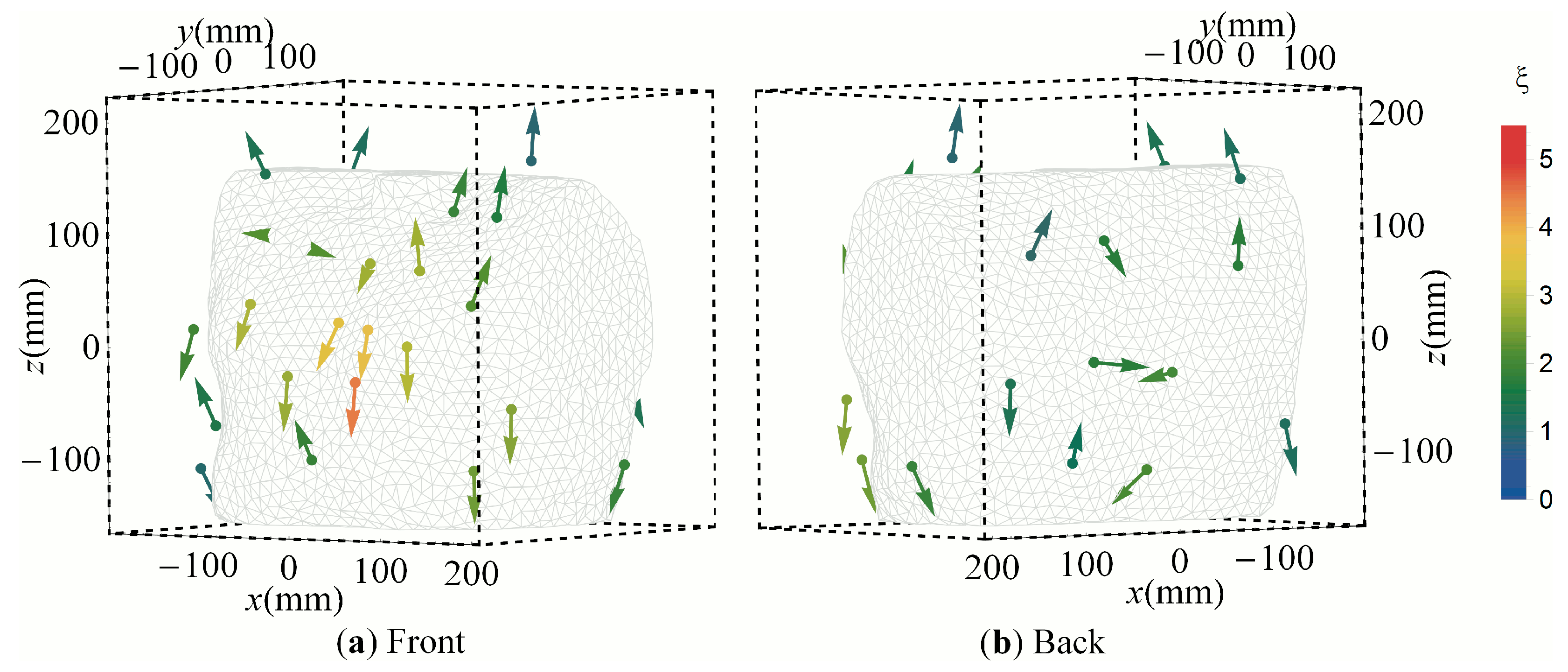
3.2. Clustered Positions
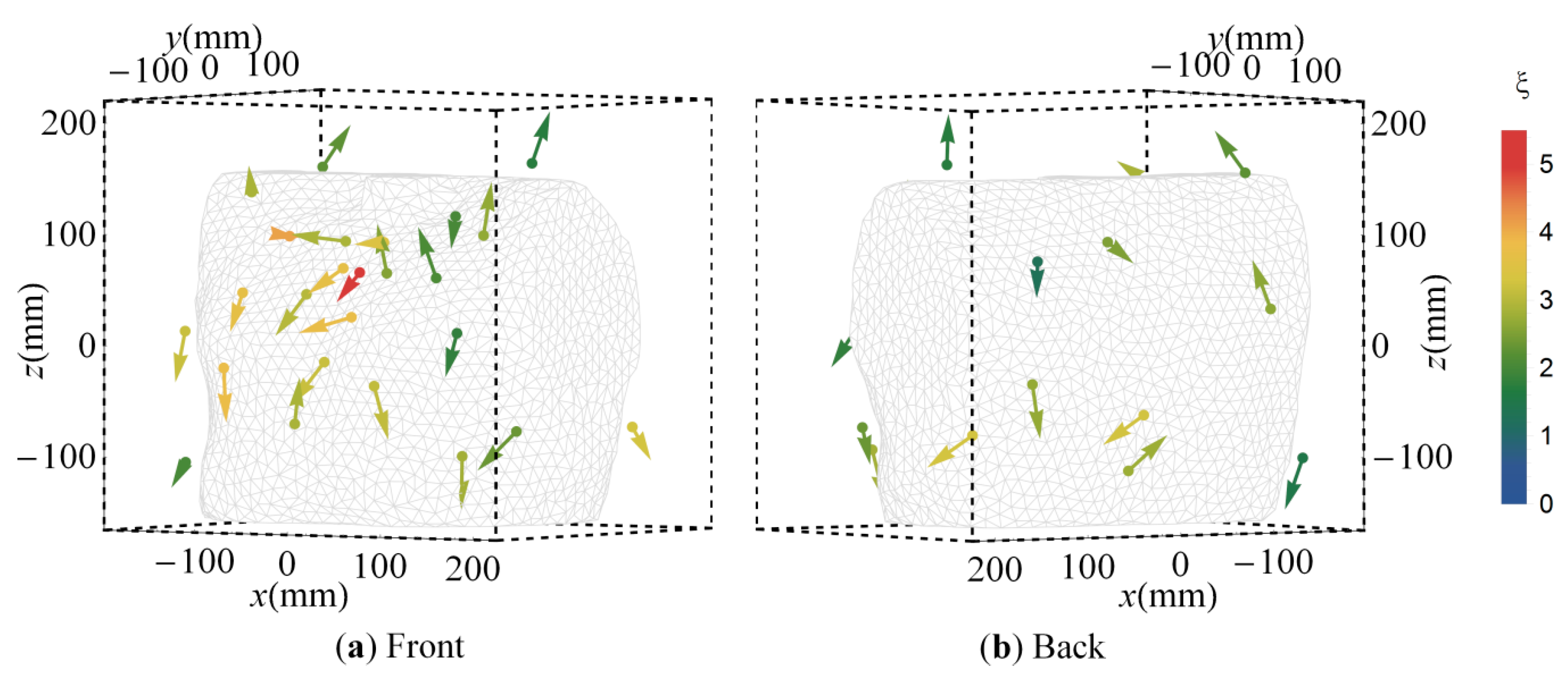
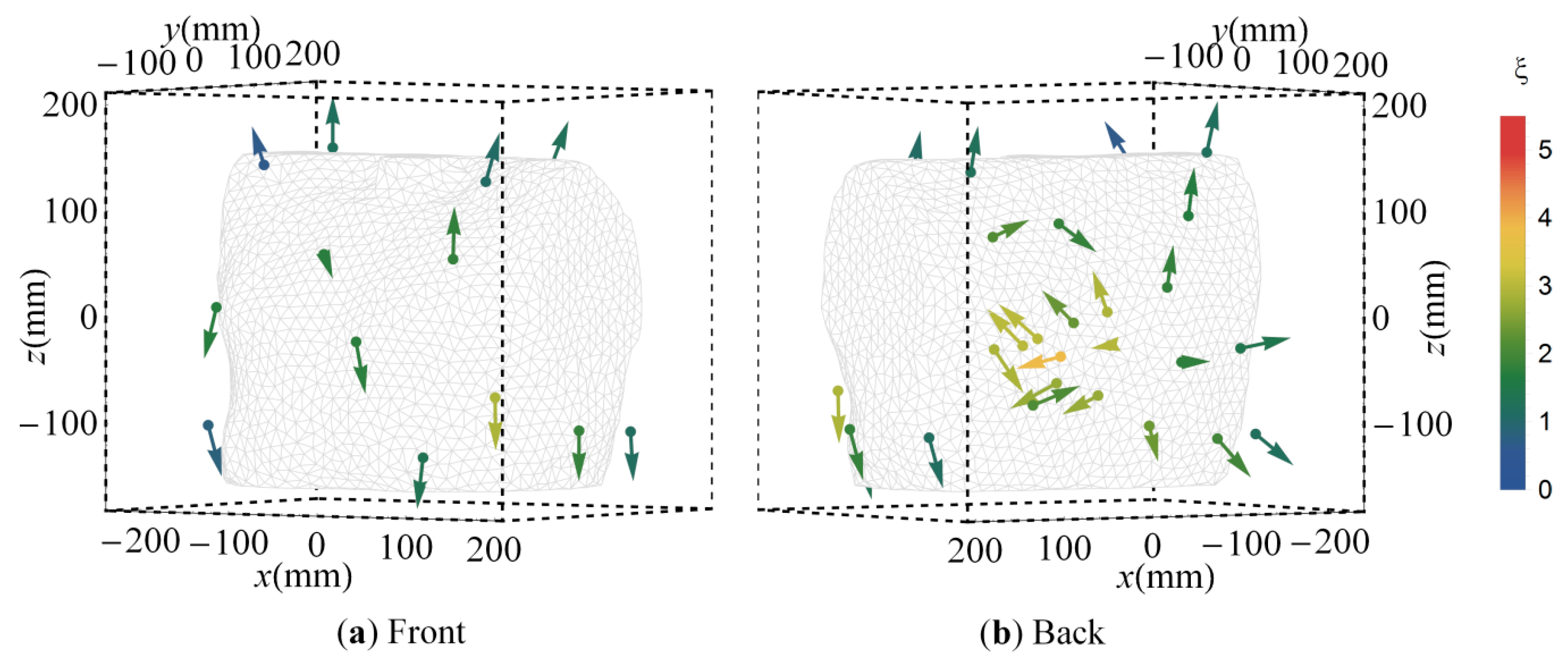
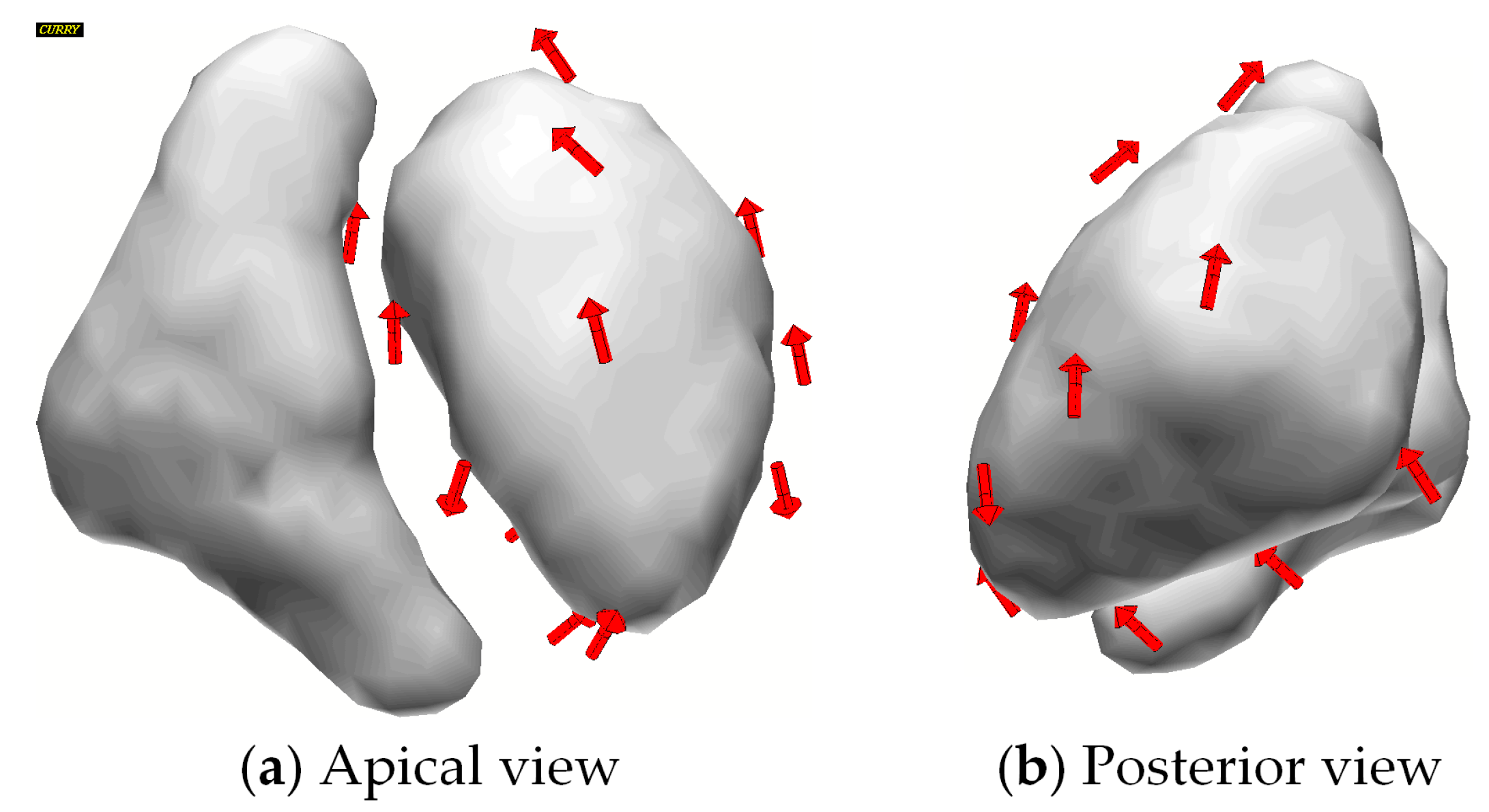
3.3. Cluster Orientations
4. Discussion
4.1. MCG Vest Optimization
4.2. Sensor Positions
4.3. Sensor Orientations
4.4. Limitations and Future Work
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AV | Atrioventricular |
| BSPM | Body surface potential map |
| CN | Condition number |
| ECG | Electrocardiography |
| MCG | Magnetocardiography |
| MR | Magnetoresistive |
| OPM | Optically pumped magnetometer |
| PAM | Partitioning around medoids |
| PSO | Particle swarm optimization |
| SNR | Signal-to-noise ratio |
| SQUID | Superconducting quantum interference device |
Appendix A
References
- Leder, U.; Haueisen, J.; Huck, M.; Nowak, H. Non-invasive imaging of arrhytmogenic left-ventricular myocardium after infarction. Lancet 1998, 352. [Google Scholar] [CrossRef]
- Stroink, G.; Hailer, B.; van Leeuwen, P. Cardiomagnetism. In Magnetism in Medicine: A Handbook, 2nd ed.; Andrä, W., Nowak, H., Eds.; Wiley: Weinheim, Germany, 2006. [Google Scholar]
- Dössel, O. Inverse Problem of Electro- and Magnetocardiography: Review and Recent Progress. Available online: http://www.ijbem.org/volume2/number2/doessel/paper_ijbem.htm (accessed on 7 May 2016).
- Freitas, P.P.; Ferreira, R.; Cardoso, S.; Cardoso, F. Magnetoresistive sensors. J. Phys. Condens. Matter 2007, 19. [Google Scholar] [CrossRef]
- Moodera, J.S.; Kinder, L.R.; Wong, T.M.; Meservey, R. Large magnetoresistance at room temperature in ferromagnetic thin film tunnel junctions. Phys. Rev. Let. 1995, 74, 3273–3276. [Google Scholar] [CrossRef] [PubMed]
- Paz, E.; Serrano-Guisan, S.; Ferreira, R.; Freitas, P.P. Room temperature direct detection of low frequency magnetic fields in the 100 pT/Hz1/2 range using large arrays of magnetic tunnel junctions. J. App. Phys. 2014, 115, 17E501. [Google Scholar] [CrossRef]
- Cardoso, S.; Gameiro, L.; Leitao, D.C.; Cardoso, F.; Ferreira, R.; Paz, E.; Freitas, P.P. Magnetic tunnel junction sensors with pTesla sensitivity for biomedical imaging. In Proceedings of the SPIE Smart Sensors, Actuators, and MEMS VI, Grenoble, France, 24–26 April 2013.
- Edelstein, A.S.; Fischer, G.A.; Pedersen, M.; Nowak, E.R.; Cheng, S.F.; Nordman, C.A. Progress toward a thousandfold reduction in 1/f noise in magnetic sensors using an AC microelectromechanical system flux concentrator. Appl. Phys. Lett. 2006, 99, 08B317. [Google Scholar] [CrossRef]
- Bison, G.; Castagna, N.; Hofer, A.; Knowles, P.; Schenker, J.L.; Kasprzak, M.; Saudan, H.; Weis, A. A room temperature 19-channel magnetic field mapping device for cardiac signals. Appl. Phys. Lett. 2009, 95. [Google Scholar] [CrossRef]
- Budker, D.; Romalis, M. Optical magnetometry. Nat. Phys. 2007, 3, 227–234. [Google Scholar] [CrossRef]
- Sander, T.H.; Preusser, J.; Mhaskar, R.; Kitching, J.; Trahms, L.; Knappe, S. Magnetoencephalography with a chip-scale atomic magnetometer. Biomed. Opt. Express 2012, 3, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Schultze, V.; IJsselsteijn, R.; Scholtes, T.; Woetzel, S.; Meyer, H. Characteristics and performance of an intensity-modulated optically pumped magnetometer in comparison to the classical MX magnetometer. Opt. Express 2012, 20, 14201–14212. [Google Scholar] [CrossRef] [PubMed]
- Weis, A. Optically pumped alkali magnetometers for biomedical applications. Europhys. News 2012, 43, 20–23. [Google Scholar] [CrossRef]
- Yin, X.L.; Liu, Y.F.; Ewing, D.; Ruder, C.K.; De Rego, P.J.; Edelstein, A.S.; Liou, S.H. Tuning magnetic nanostructures and flux concentrators for magnetoresistive sensors. In Proceedings of the SPIE 9551 Spintronics VIII, San Diego, CA, USA, 9–13 August 2015.
- Ozbay, A.; Nowak, E.R.; Edelstein, A.S.; Fischer, G.A.; Nordman, C.A.; Cheng, S.F. Magnetic-field dependence of the noise in a magnetoresistive sensor having MEMS flux concentrators. IEEE Trans. Magn. 2006, 42, 3306–3308. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, J.; White, R.M.; Wang, S.X. Reduction of magnetic 1/f noise in miniature anisotropic magnetoresistive sensors. Appl. Phys. Lett. 2015, 106. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Viehland, D. A differential magnetoelectric heterostructure: Internal noise reduction and external noise cancellation. J. Appl. Phys. 2015, 118. [Google Scholar] [CrossRef]
- Valadeiro, J.P.; Amaral, J.; Leitão, D.C.; Ferreira, R.; Freitas Cardoso, S.; Freitas, P.J.P. Strategies for pTesla field detection using magnetoresistive sensors with a soft pinned sensing layer. IEEE Trans. Magn. 2015, 51. [Google Scholar] [CrossRef]
- Edelstein, A.; Fischer, G.A.; Burnette, J.E.; Egelhoff, W.E., Jr.; Cheng, S.F. Achieving 1/f noise reduction with the MEMS flux concentrator. In Proceedings of the 2009 IEEE Sensors, Christchurch, New Zealand, 25–28 October 2009.
- Liou, S.H.; Sellmyer, D.; Russek, S.E.; Heindl, R.; Da Silva, F.C.S.; Moreland, J.; Pappas, D.P. Magnetic noise in a low-power picotesla magnetoresistive sensor. In Proceedings of the 2009 IEEE Sensors, Christchurch, New Zealand, 25–28 October 2009.
- Fischer, G.A.; Edelstein, A.S. Magnetic modeling of a MEMS flux concentrator. In Proceedings of the 2010 IEEE Sensors, Kona, HI, USA, 1–4 November 2010.
- Hu, J.; Pan, M.; Tian, W.; Chen, D.; Luo, F. Integrating magnetoresistive sensors with microelectromechanical systems for noise reduction. Appl. Phys. Lett. 2012, 101. [Google Scholar] [CrossRef]
- Cardoso, S.; Leitao, D.C.; Gameiro, L.; Cardoso, F.; Ferreira, R.; Paz, E.; Freitas, P.P. Magnetic tunnel junction sensors with pTesla sensitivity. Microsyst. Technol. 2014, 20, 793–802. [Google Scholar] [CrossRef]
- Tian, W.; Hu, J.; Pan, M.; Chen, D.; Zhao, J. Magnetic flux vertical motion modulation for 1/f noise suppression in magnetoresistance field sensors using MEMS device. IEEE Trans. Magn. 2016, 52. [Google Scholar] [CrossRef]
- Multi Dimension. TMR9001 Linear Sensor, Datasheet. Available online: http://www.dowaytech.com/en/1866.html (accessed on 7 May 2016).
- Yin, X.; Liou, S.H. Novel magnetic nanostructured multilayer for high sensitive magnetoresistive sensor. In Proceedings of the 2012 IEEE Sensors, Taipei, Taiwan, 28–31 October 2012.
- Knappe, S.; Sander, T.; Trahms, L. Optically-pumped magnetometers for MEG. In Magnetoencephalography; Supek, S., Aine, C.J., Eds.; Springer: New York, NY, USA, 2014; pp. 993–1000. [Google Scholar]
- Griffith, W.C.; Knappe, S.; Kitching, J. Femtotesla atomic magnetometry in a microfabricated vapor cell. Opt. Express 2010, 18, 27167–27172. [Google Scholar] [CrossRef] [PubMed]
- Knappe, S.; Sander, T.H.; Kosch, O.; Wiekhorst, F.; Kitching, J.; Trahms, L. Cross-validation of microfabricated atomic magnetometers with superconducting quantum interference devices for biomagnetic applications. Appl. Phys. Lett. 2010, 97. [Google Scholar] [CrossRef]
- Wyllie, R.; Kauer, M.; Wakai, R.T.; Walker, T.G. Optical magnetometer array for fetal magnetocardiography. Opt. Lett. 2012, 37, 2247–2249. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.K.; Wakai, R.T. A compact, high performance atomic magnetometer for biomedical applications. Phys. Med. Biol. 2013, 58, 8153–8161. [Google Scholar] [CrossRef] [PubMed]
- Lembke, G.; Erne, S.N.; Nowak, H.; Menhorn, B.; Pasquarelli, A. Optical multichannel room temperature magnetic field imaging system for clinical application. Biomed. Opt. Express 2014, 5, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Alem, O.; Sander, T.H.; Mhaskar, R.; LeBlanc, J.; Eswaran, H.; Steinhoff, U.; Okada, Y.; Kitching, J.; Trahms, L.; Knappe, S. Fetal magnetocardiography measurements with an array of microfabricated optically pumped magnetometers. Phys. Med. Biol. 2015, 60, 4797–4811. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.B.; Maloof, A.C.; Romalis, M.V. Ultrahigh sensitivity magnetic field and magnetization measurements with an atomic magnetometer. Appl. Phys. Lett. 2010, 97. [Google Scholar] [CrossRef]
- Kamada, K.; Taue, S.; Kobayashi, T. Optimization of bandwidth and signal responses of optically pumped atomic magnetometers for biomagnetic applications. Jpn. J. Appl. Phys. 2011, 50. [Google Scholar] [CrossRef]
- Kamada, K.; Yosuke Ito, Y.; Ichihara, S.; Mizutani, N.; Kobayashi, T. Noise reduction and signal-to-noise ratio improvement of atomic magnetometers with optical gradiometer configurations. Opt. Express 2015, 23, 6976–6987. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzo, L.; Haueisen, J. Numerical comparison of sensor arrays for magnetostatic linear inverse problems based on a projection method. COMPEL 2007, 26, 356–367. [Google Scholar]
- Tsukada, K.; Haruta, Y.; Adachi, A.; Ogata, H.; Komuro, T.; Ito, T.; Takada, Y.; Kandori, A.; Noda, Y.; Terada, Y.; et al. Multichannel SQUID system detecting tangential components of the cardiac magnetic field. Rev. Sci. Instrum. 1995, 66, 5085–5091. [Google Scholar] [CrossRef]
- Arturi, C.M.; Di Rienzo, L.; Haueisen, J. Information content in single-component versus three component cardio-magnetic fields. IEEE Trans. Magn. 2004, 40, 631–634. [Google Scholar] [CrossRef]
- Di Rienzo, L.; Haueisen, J.; Arturi, C.M. Three component magnetic field data: Impact on minimum norm solutions in a biomedical application. COMPEL 2005, 24, 869–881. [Google Scholar] [CrossRef]
- Schnabel, A.; Burghoff, M.; Hartwig, S.; Petsche, F.; Steinhoff, U.; Drung, D.; Koch, H. A sensor configuration for a 304 SQUID vector magnetometer. Neurol. Clin. Neurophys. 2004, 1. PMID 16012698. [Google Scholar]
- Kang, C.S.; Lee, S.; Kim, K.; Kwon, H.; Lee, Y.-H.; Yu, K.K.; Lim, H.K.; Park, Y.K. Performance investigation of a three-dimensional SQUID magnetocardiography system by using a computer simulation. J. Korean Phys. Soc. 2008, 53, 3444–3449. [Google Scholar] [CrossRef]
- Lux, R.L.; Smith, C.R.; Wyatt, R.F.; Abildskov, J.A. Limited lead selection for estimation of body-surface potential maps in electrocardiography. IEEE Trans. Biomed. Eng. 1978, 25, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Lux, R.L.; Evans, A.K.; Burgess, M.J.; Abildskov, J.A. Redundancy reduction for improved display and analysis of body-surface potential maps Ⅰ. Spatial compression. Circ. Res. 1981, 49, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.C.; Spach, M.S.; Herman-Giddens, S. Selection of the number and position of measuring locations for electrocardiography. IEEE Trans. Biomed. Eng. 1971, 18, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Finlay, D.D.; Nugent, C.D.; Donnelly, M.P.; Lux, R.L.; McCullagh, P.J.; Black, N.D. Selection of optimal recording sited for limited lead body surface potential mapping: A sequential selection based approach. BMC Med. Inform. Decis. Mak. 2006, 6. [Google Scholar] [CrossRef] [PubMed]
- Kornreich, F.; Rautaharju, P.; Warren, J.; Montague, T.J.; Horacek, B.M. Identification of best electrocardiographic leads for diagnosing myocardial infarction by statistical analysis of body surface potential maps. Am. J. Cardiol. 1985, 56, 852–856. [Google Scholar] [CrossRef]
- Dössel, O.; Schneider, F.; Müller, M. Optimization of electrode positions for multichannel electrocardiography with respect to electrical imaging of the heart. In Proceedings of the 20th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Hong Kong, China, 29 October–1 November 1998; pp. 71–74.
- Donnelly, M.P.; Finlay, D.D.; Nugent, C.D.; Black, N.D. Lead selection: Old and new methods for locating the most electrocardiogram information. J. Electrocardiol. 2008, 41, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Jazbinšek, V.; Burghoff, M.; Kosch, O.; Steinhoff, U.; Hren, R.; Trahms, L.; Trontelj, Z. Selection of optimal recording sites in electrocardiography and magnetocardiography. Biomed. Eng. Biomed. Te. 2004, 48, 174–177. [Google Scholar]
- Jazbinšek, V.; Hren, R.; Trontelj, Z. Influence of limited lead selection on source localization in magnetocardiography and electrocardiography. Int. Congr. Ser. 2007, 1300, 492–495. [Google Scholar] [CrossRef]
- Nalbach, M.; Dössel, O. Comparison of sensor arrangements of MCG and ECG with respect to information content. Physica. C Supercond. 2002, 372–376, 254–258. [Google Scholar] [CrossRef]
- Lau, S.; Eichardt, R.; Di Rienzo, L.; Haueisen, J. Tabu search optimization of magnetic sensor systems for magnetocardiography. IEEE Trans. Magn. 2008, 44, 1442–1445. [Google Scholar] [CrossRef]
- Golub, G.H.; Loan, C.F.V. Matrix Computations; John Hopkins University Press: Baltimore, MD, USA, 1996. [Google Scholar]
- Skeel, R.D. Scaling for numerical stability in Gaussian elimination. J. ACM 1979, 26, 494–526. [Google Scholar] [CrossRef]
- Demmel, J.; Higham, N. Improved error bounds for underdetermined system solvers. SIAM J. Matrix Anal. Appl. 1993, 14, 1–14. [Google Scholar] [CrossRef]
- Eichardt, R.; Baumgarten, D.; Petković, B.; Wiekhorst, F.; Trahms, L.; Haueisen, J. Adapting source grid parameters to improve the condition of the magnetostatic linear inverse problem of estimating nanoparticle distributions. Med. Biol. Eng. Comput. 2012, 50, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Engelbrecht, A.P. Fundamentals of Swarm Intelligence; Wiley: Chichester, UK, 2005. [Google Scholar]
- SimBio: A generic environment for bio-numerical simulations. Available online: https://www.mrt.uni-jena.de/simbio (accessed on 15 March 2016).
- Kaufman, L.; Rousseeuw, P.J. Clustering by means of medoids. In Statistical Data Analysis Based on the L1 Norm and Related Methods; Dodge, Y., Ed.; North Holland/Elsevier: Amsterdam, The Netherlands, 1987; pp. 405–416. [Google Scholar]
- Fisher, N.I.; Lewis, T.; Embleton, B.J.J. Statistical Analysis of Spherical Data; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Tilg, B.; Wach, P.; Lafer, G.; Nenonen, J.; Katila, T. Simultaneous use of multiple MCG sensor arrays—A study on the localization accuracy. In Proceedings of the 18th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 1996, Bridging Disciplines for Biomedicine. Amsterdam, The Netherlands, 31 October–3 November 1996; pp. 1445–1446.
- Sato, M.; Terada, Y.; Mitsui, T.; Miyashita, T.; Kandori, A.; Tsukada, K. Visualization of atrial excitation by magnetocardiogram. Int. J. Cardiovasc. Imaging 2002, 28, 305–312. [Google Scholar] [CrossRef]
- Schneider, F.; Dössel, O.; Müller, M. Filtering characteristics of the human body and reconstruction limits in the inverse problem of electrocardiography. Comput. Cardiol. 1998, 25, 698–692. [Google Scholar]
- Kim, K.; Lee, Y.H.; Kwon, H.; Kim, J.M.; Kim, I.S.; Park, Y.K. Optimal sensor distribution for measuring the tangential field components in MCG. Neurol. Clin. Neurophys. 2004, 1. PMID 16012625. [Google Scholar]
- Jazbinšek, V.; Kosch, O.; Meindl, P.; Steinhoff, U.; Trontelj, Z.; Trahms, L. Multichannel vector MFM and BSPM of chest and back. In Proceedings of the 12th International Conference on Biomagnetism Biomag, Espoo, Finland, 13–17 August 2000; pp. 583–586.
- Diekmann, V.; Becker, W.; Grözinger, B.; Jürgens, R.; Kornhuber, C. A comparison of normal and tangential magnetic field component measurements in biomagnetic investigations. Clin. Phys. Physiol. Meas. 1991, 12, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Kandori, A.; Tsukada, K.; Haruta, Y.; Noda, Y.; Terada, Y.; Mitsui, T.; Sekihara, K. Reconstruction of two-dimensional current distribution from tangential MCG measurement. Phys. Med. Biol. 1996, 41, 1705–1716. [Google Scholar] [CrossRef]
- Kim, K.; Lee, Y.; Kwon, H.; Kim, J.M.; Kim, I.S.; Park, Y.K.; Lww, K.W. Design of a SQUID sensor array measuring the tangential field components in magnetocardiogram. Prog. Supercond. 2004, 45, 56–63. [Google Scholar]
- Lee, Y.; Kim, J.; Kim, K.; Kwon, H.; Kim, I.; Park, Y.; Ko, Y.; Chung, N. Tangential cardiomagnetic field measurement system based on double relaxation oscillation SQUID planar gradiometers. IEEE Trans. Appl. Supercond. 2005, 15, 648–651. [Google Scholar] [CrossRef]
- Hochwald, B.; Nehorai, A. Magnetoencephalography with diversely oriented and multicomponent sensors. IEEE Trans. Biomed. Eng. 1997, 44, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.J.; Pritchett, L.C.; Sealy, W.C.; Kassel, J.; Wallace, A.G. The pre-excitation syndromes. Prog. Cardiovasc. Dis. 1978, 20, 285–327. [Google Scholar] [CrossRef]
- Purcell, C.J.; Stroink, G.; Horacek, B.M. Effect of torso boundaries on electric potential and magnetic field of a dipole. IEEE Trans. Biomed. Eng. 1988, 35, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Hren, R.; Zhang, X.; Stroink, G. Comparison between electrocardiographic and magnetocardiographic inverse solutions using the boundary element method. Med. Biol. Eng. Comput. 1996, 34, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Mulyadi, I.H.; Fiedler, P.; Eichardt, R.; Zelle, D.; Haueisen, J.; Supriyanto, E. Optimal electrode placement for smart clothing: Compromises between electrophysiological and practical aspects. IEEE J. Biomed. Health Inform. 2016, in press. [Google Scholar]
- Leder, U.; Schrey, F.; Haueisen, J.; Dörrer, L.; Schreiber, J.; Liehr, M.; Schwarz, G.; Solbrig, O.; Figulla, H.R.; Seidel, P. Reproducibility of HTS-SQUID magnetocardiography in an unshielded clinical environment. Int. J. Cardiol. 2001, 79, 237–243. [Google Scholar] [CrossRef]
- Schneider, U.; Haueisen, J.; Loeff, M.; Bondarenko, N.; Schleussner, E. Prenatal diagnosis of a long QT syndrome by fetal magnetocardiography in an unshielded bedside environment. Prenat. Diagn. 2005, 25, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Zhang, G.F.; Wang, Y.L.; Liu, M.; Li, H.; Qiu, Y.; Zeng, J.; Kong, X.Y.; Xie, X.M. Multichannel fetal magnetocardiography using SQUID bootstrap circuit. Chin. Phys. B 2013, 22. [Google Scholar] [CrossRef]
- Comani, S.; Mantini, D.; Alleva, G.; Di Luzio, S.; Romani, G.L. Optimal filter design for shielded and unshielded ambient noise reduction in fetal magnetocardiography. Phys. Med. Biol. 2005, 50, 5509–5521. [Google Scholar] [CrossRef] [PubMed]
- Maurits van der Graaf, A.W.; Bhagirath, P.; Ramanna, H.; van Driel, V.J.H.M.; de Hooge, J.; de Groot, N.M.S.; Götte, M.J.W. Noninvasive imaging of cardiac excitation: Current status and future perspective. Ann. Noninvasive Electrocardiol. 2014, 19, 105–113. [Google Scholar] [CrossRef] [PubMed]
| CN | Skeel | ρ | |
|---|---|---|---|
| Value of randomly initialized vest setups | 209 ± 88 | 159 ± 50 | 5.48 ± 0.64 |
| Value of optimized vest setups | 19.05 ± 1.15 | 26.21 ± 1.21 | 2.51 ± 0.05 |
| Number of sensors at edge of vest | 47.2% | 46.9% | 40.3% |
| Median distance to cluster medoid | 30.8 mm | 27.9 mm | 29.6 mm |
| Maximal distance to cluster medoid | 108.1 mm | 110.8 mm | 131.3 mm |
| Shape of orientation distribution per cluster | 1.76 ± 1.80 | 1.45 ± 0.84 | 1.91 ± 2.31 |
| Strength of principal orientation per cluster | 2.08 ± 0.84 | 2.84 ± 0.84 | 1.99 ± 0.77 |
| Evaluated with: | ||||
|---|---|---|---|---|
| CN | Skeel | ρ | ||
| Cluster-based representative setup derived using: | CN | 49.49 | 56.79 | 3.41 |
| Skeel | 133.97 | 99.39 | 3.29 | |
| ρ | 106.90 | 85.01 | 3.49 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, S.; Petković, B.; Haueisen, J. Optimal Magnetic Sensor Vests for Cardiac Source Imaging. Sensors 2016, 16, 754. https://doi.org/10.3390/s16060754
Lau S, Petković B, Haueisen J. Optimal Magnetic Sensor Vests for Cardiac Source Imaging. Sensors. 2016; 16(6):754. https://doi.org/10.3390/s16060754
Chicago/Turabian StyleLau, Stephan, Bojana Petković, and Jens Haueisen. 2016. "Optimal Magnetic Sensor Vests for Cardiac Source Imaging" Sensors 16, no. 6: 754. https://doi.org/10.3390/s16060754
APA StyleLau, S., Petković, B., & Haueisen, J. (2016). Optimal Magnetic Sensor Vests for Cardiac Source Imaging. Sensors, 16(6), 754. https://doi.org/10.3390/s16060754





