Current Developments on Optical Feedback Interferometry as an All-Optical Sensor for Biomedical Applications
Abstract
:1. Introduction
2. Optical Path Changes
2.1. OFI for the Monitoring of Transient Path Changes
2.1.1. Introduction
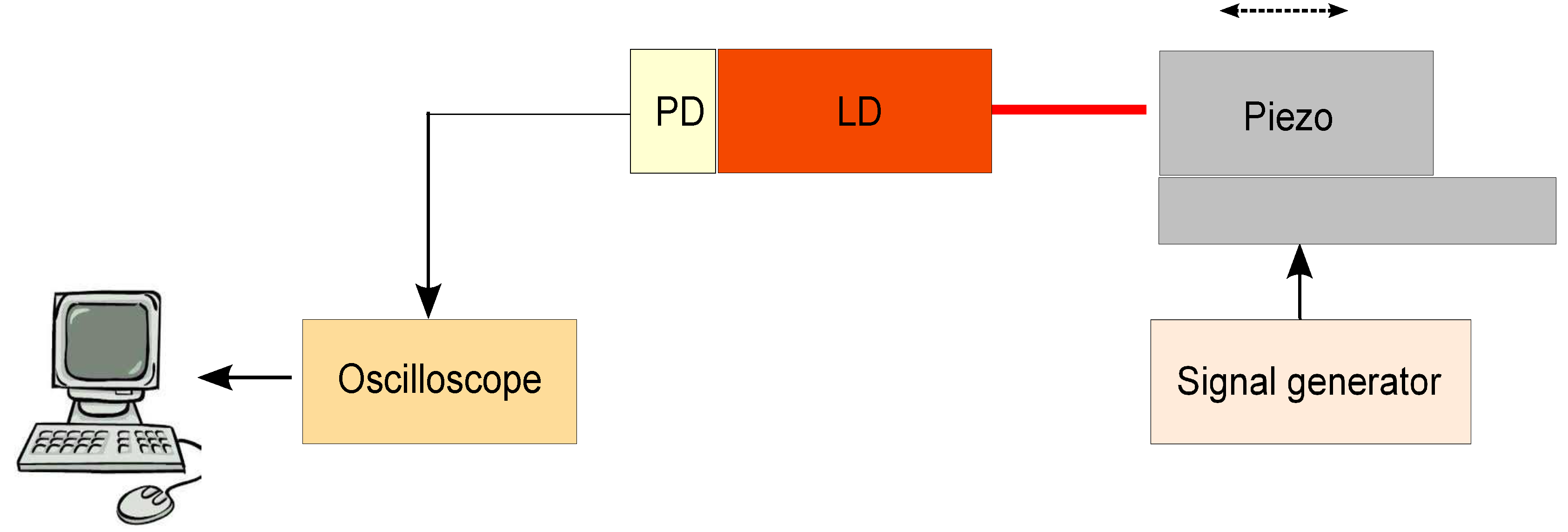
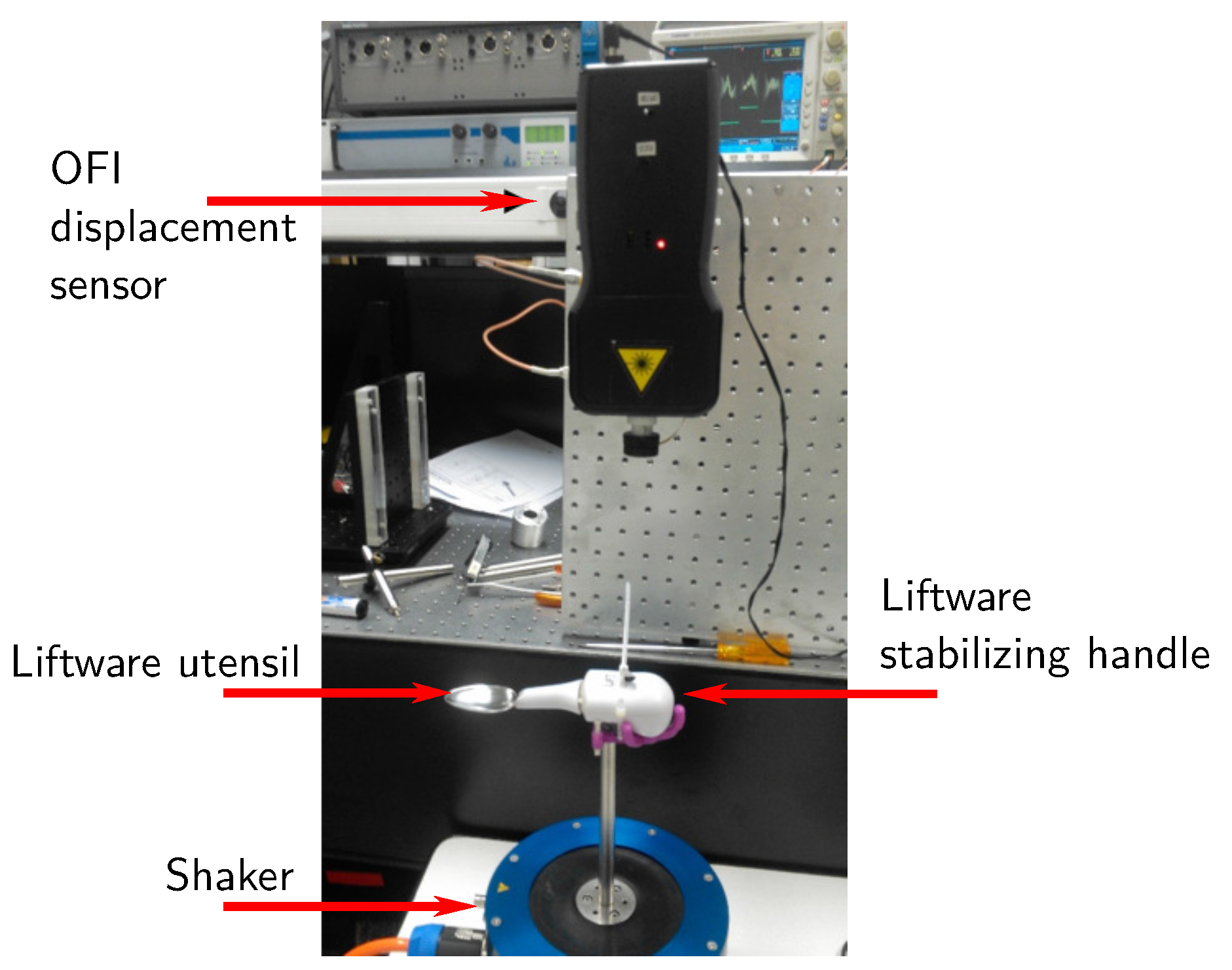
2.1.2. Experimental Setup
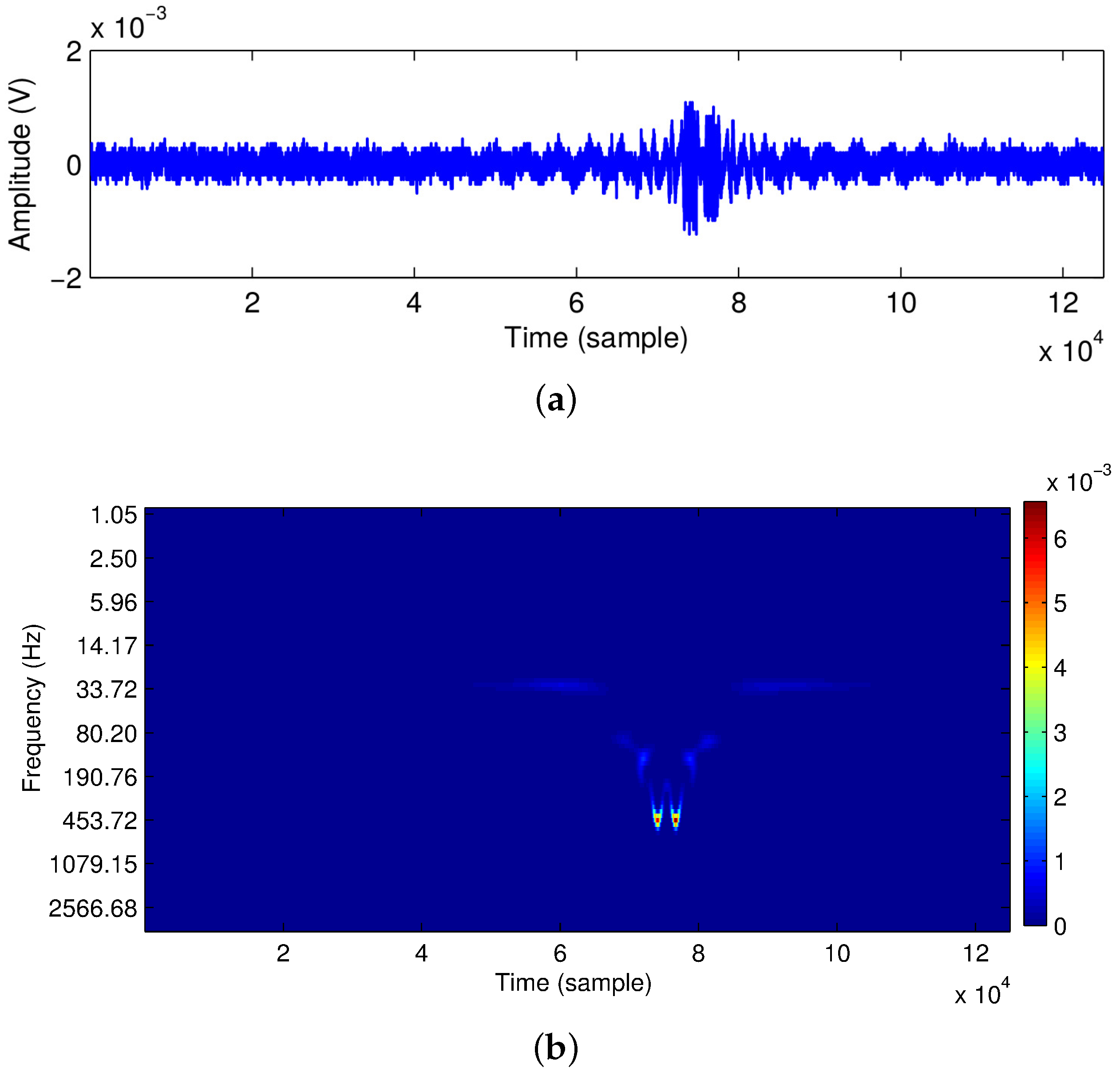
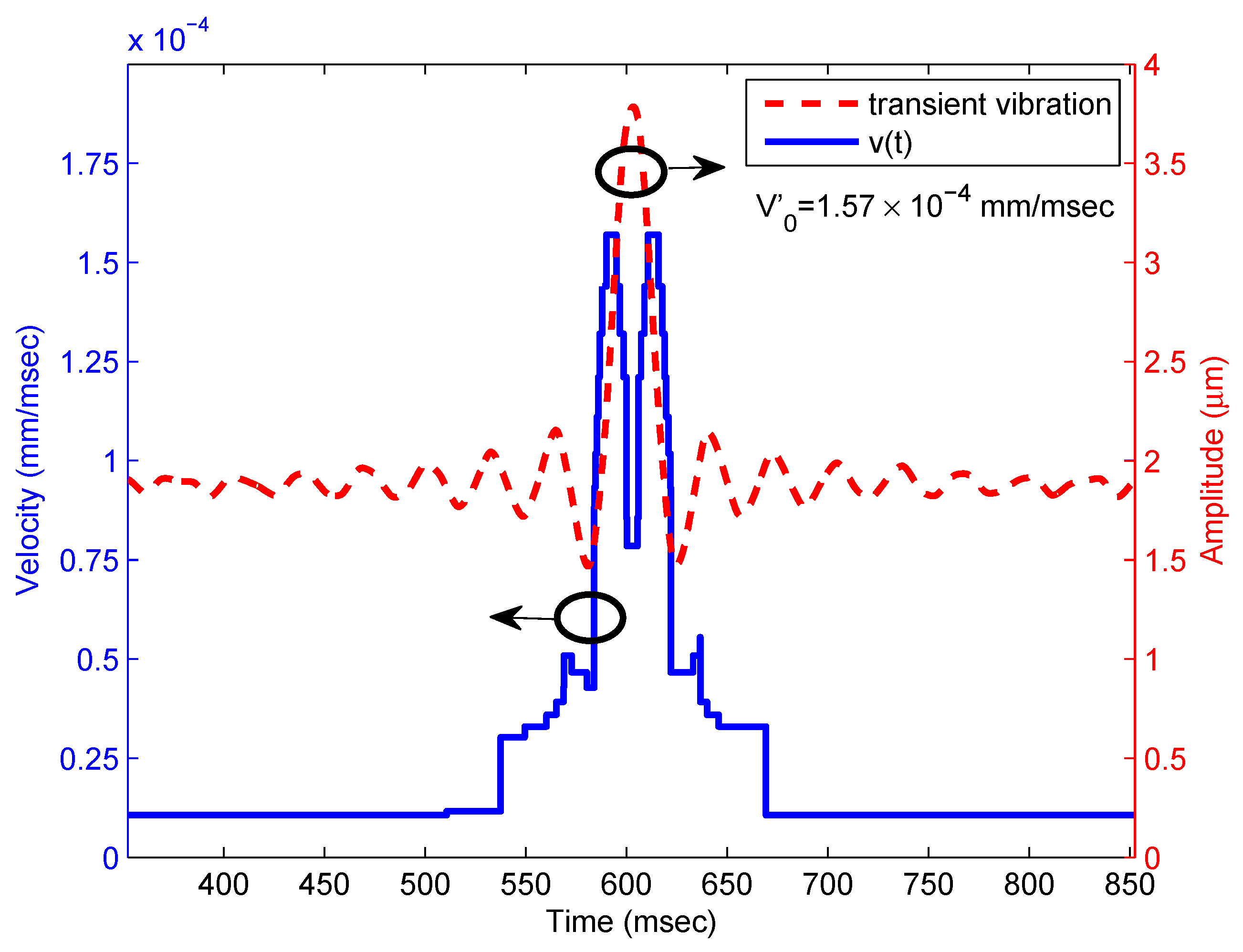
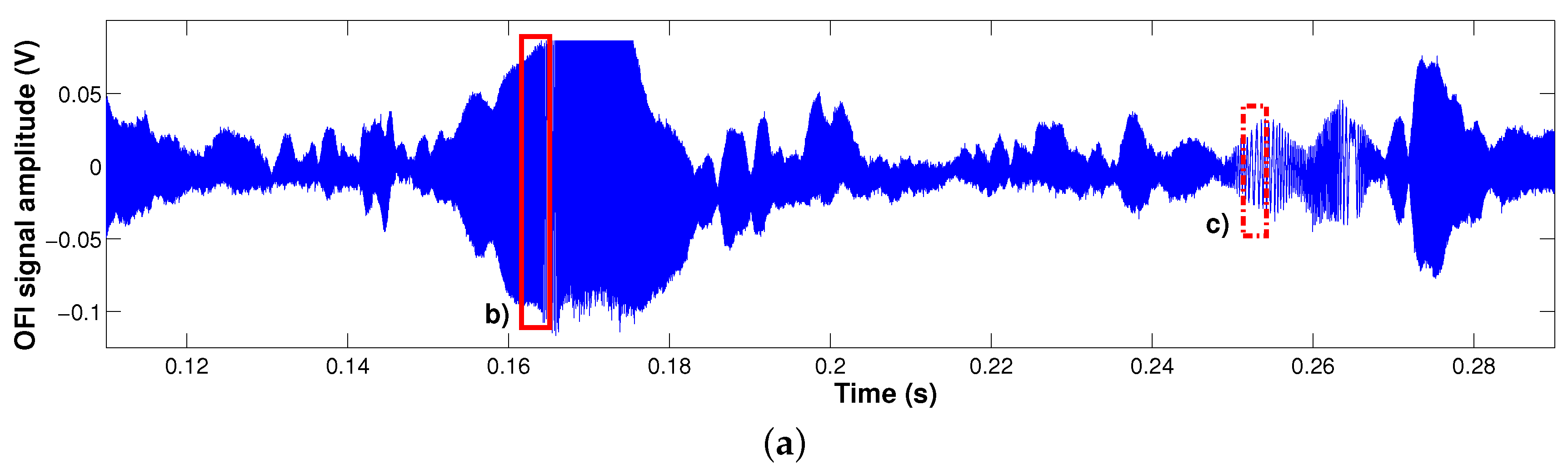
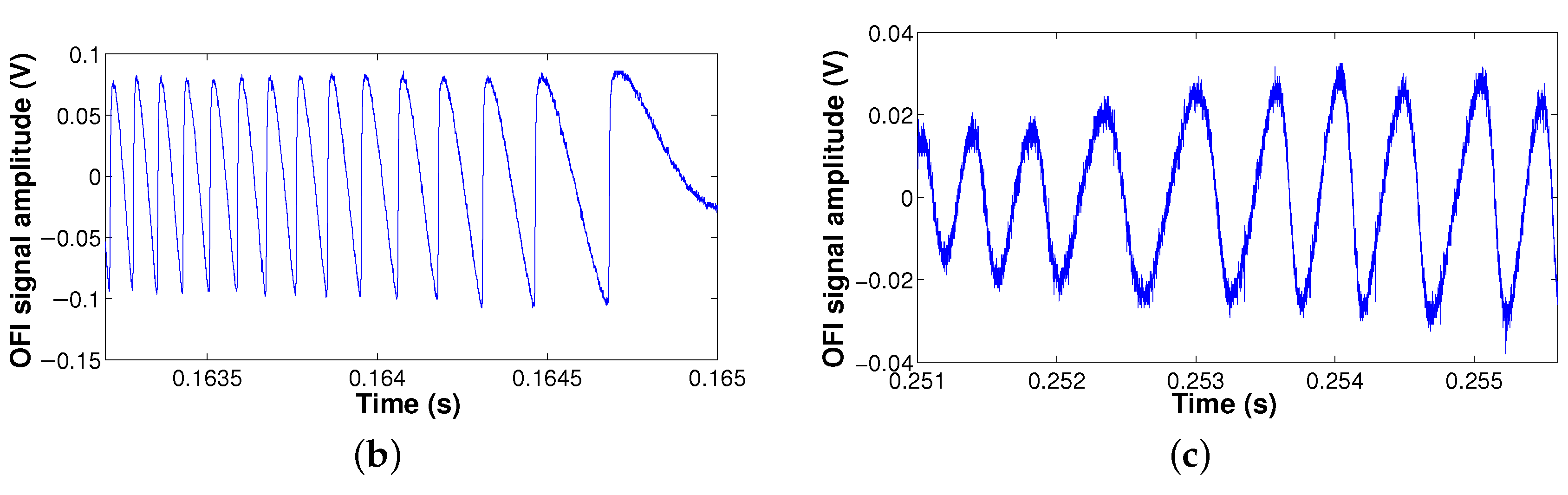
2.1.3. Results
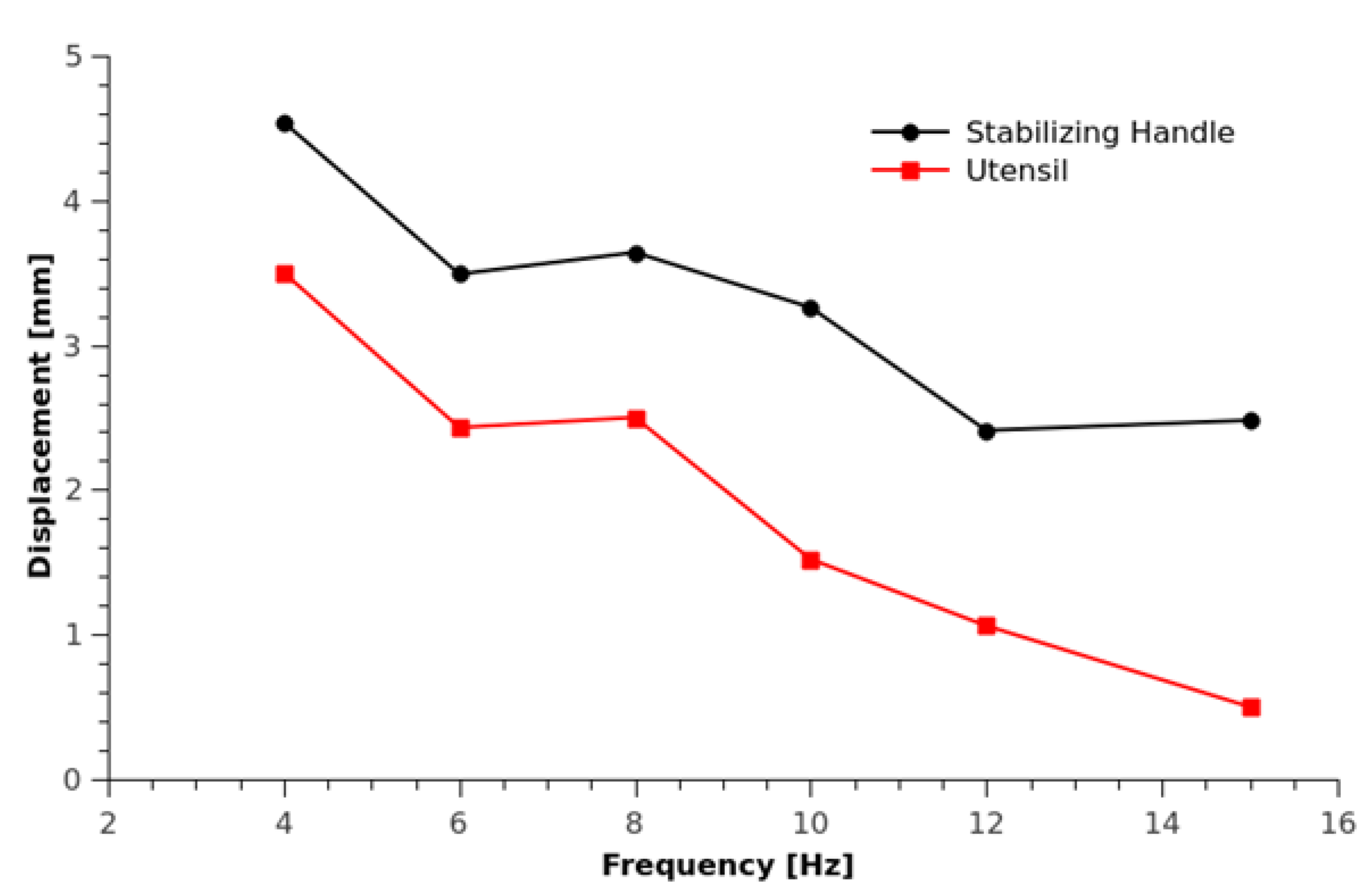
2.2. OFI Sensor for Large Displacements
- Using two piezo-actuators to move a lens for a speckle-tracking technique [43],
- Using a voltage-controlled liquid lens and a double-headed laser diode sensor with different laser beam spot sizes have also been proposed to avoid speckle [44]
- Keeping the operating point of the OFI interferometer fixed at the half-fringe [45]
- Using OFI signal envelope tracking and dynamic fringe detection [46]
3. OFI for the Monitoring of Flows
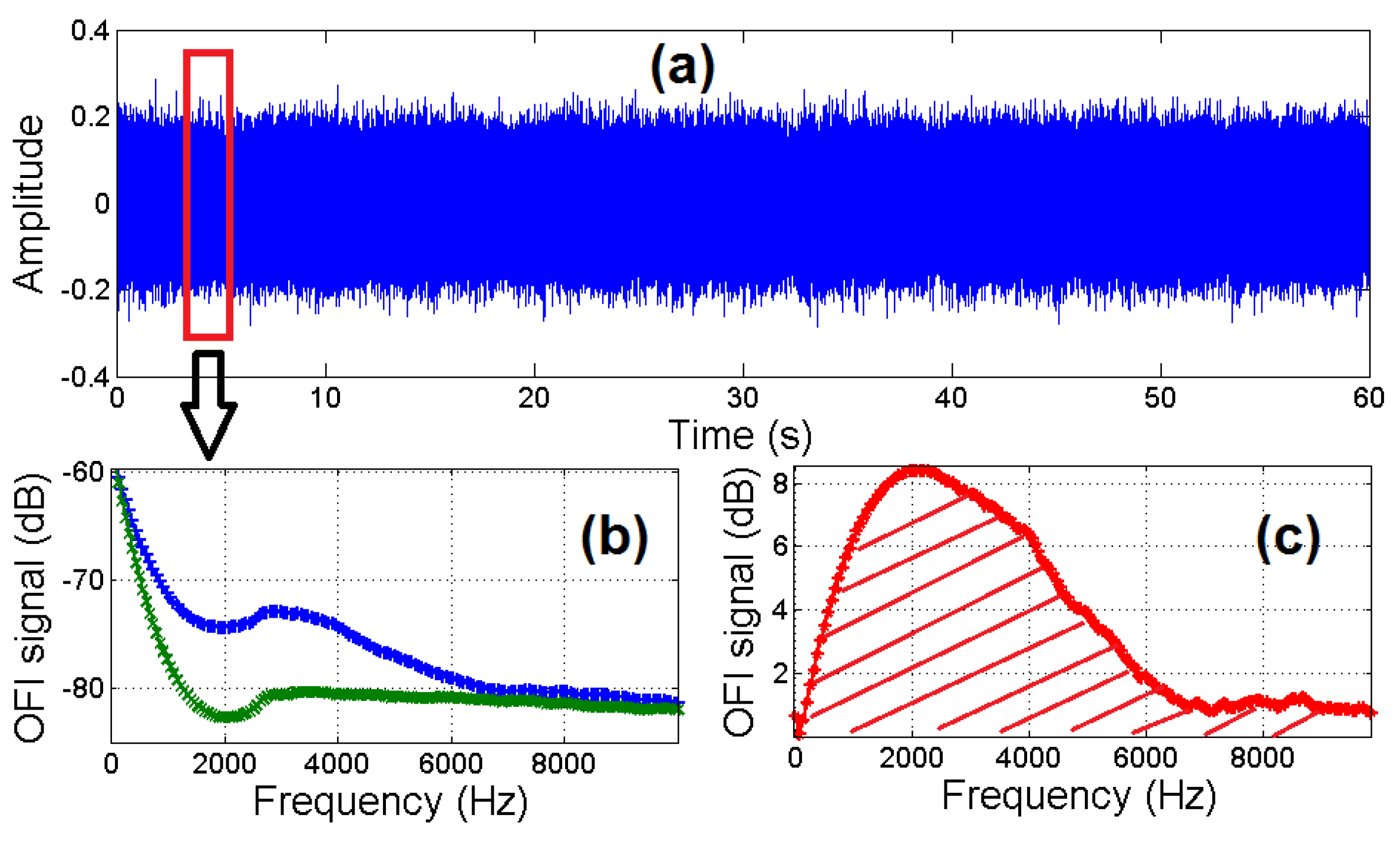
3.1. Processing of OFI Flow Signals under Different Scattering Regimes
3.1.1. Introduction to Signal Processing for Flow Monitoring
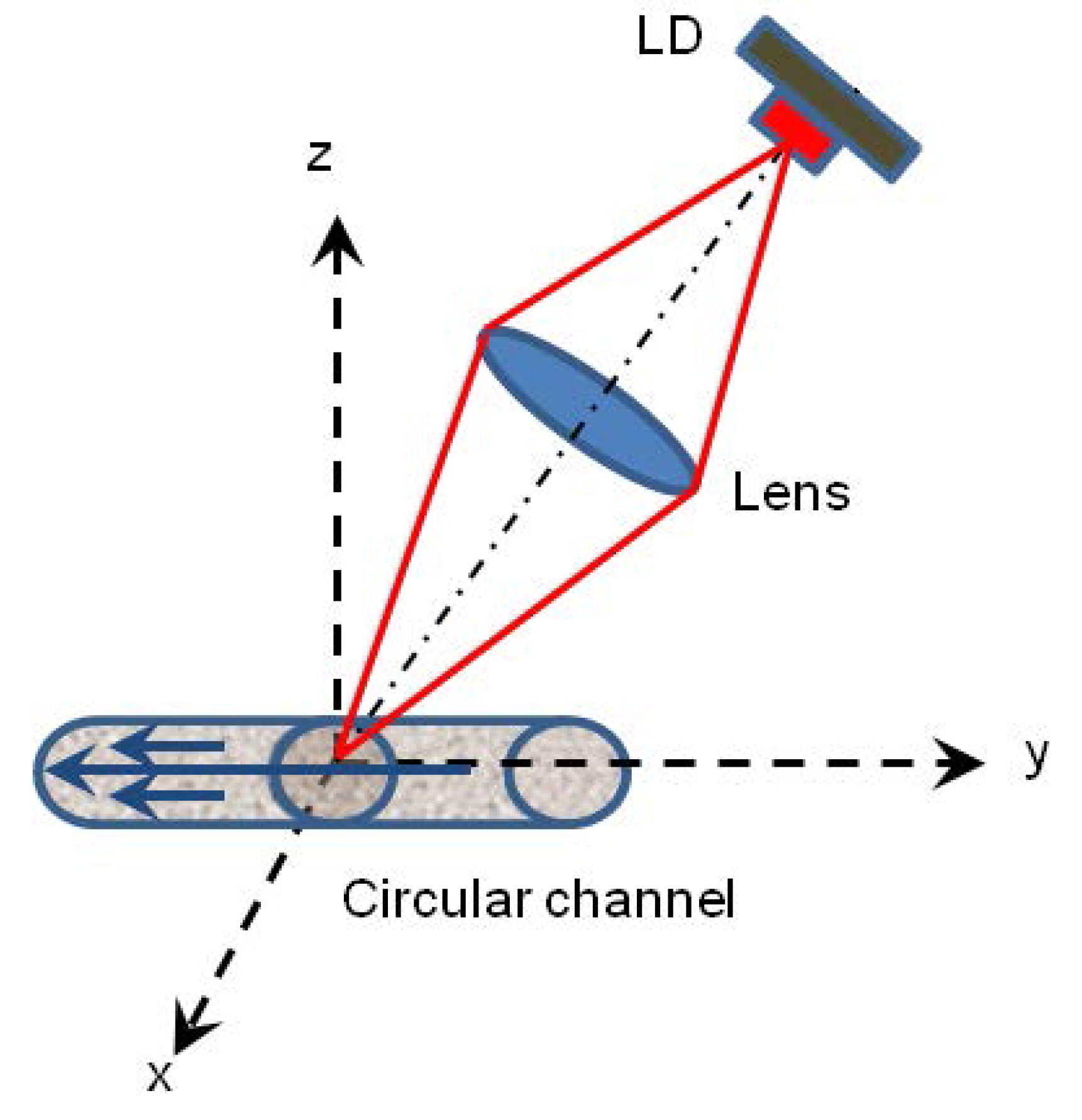
3.1.2. Experiment
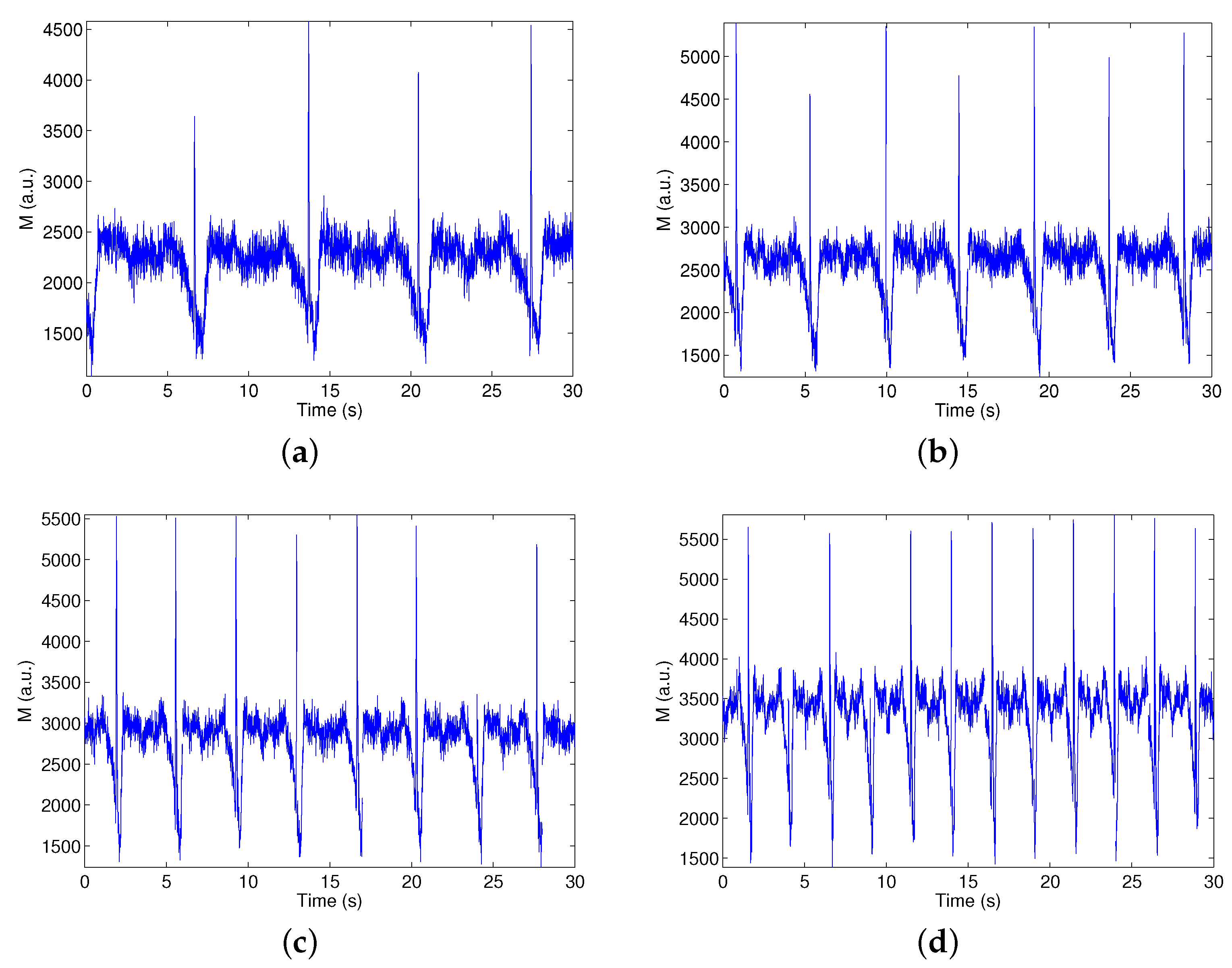
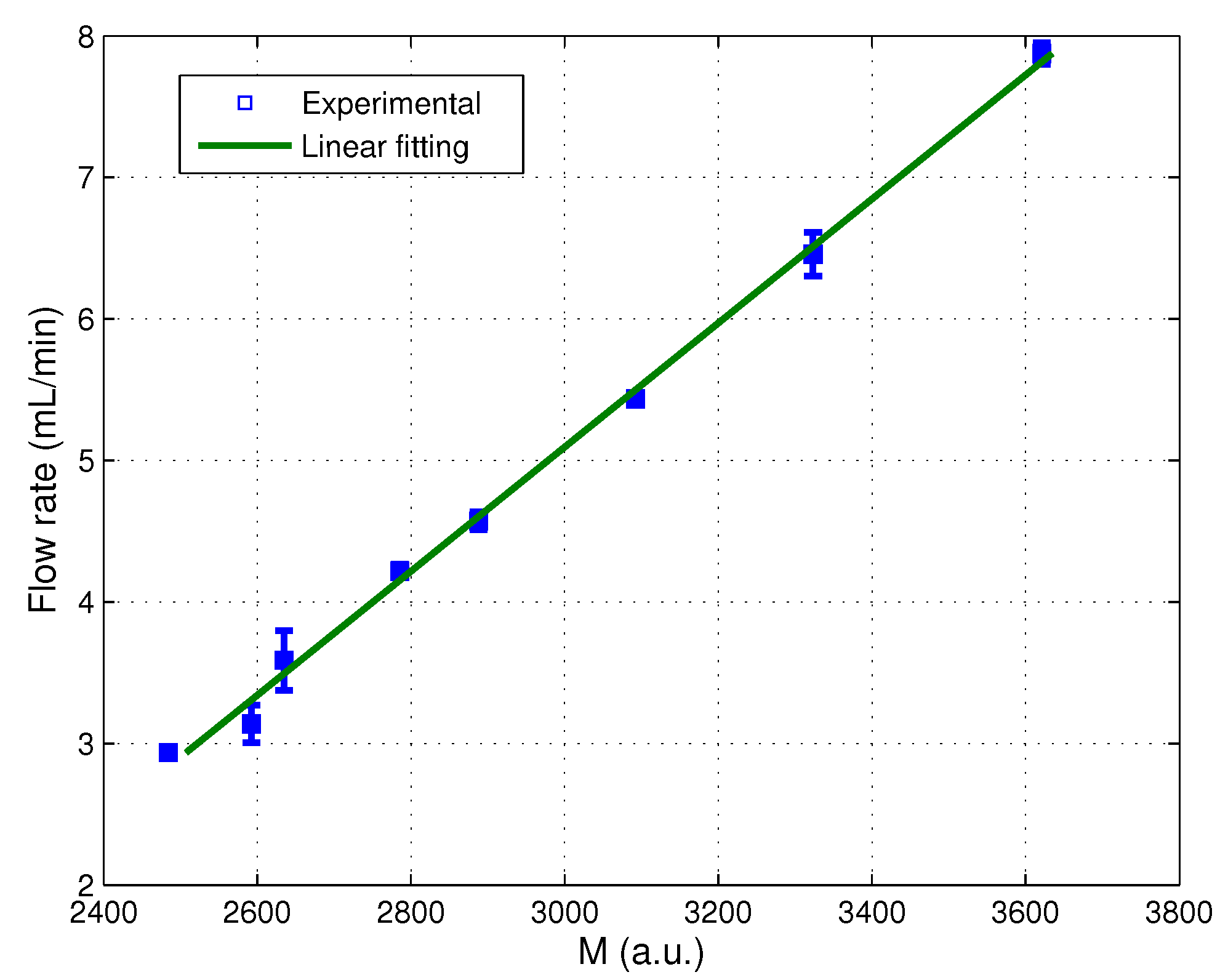
3.2. Real-Time Monitoring of Non-Steady Flows
3.2.1. Methodology
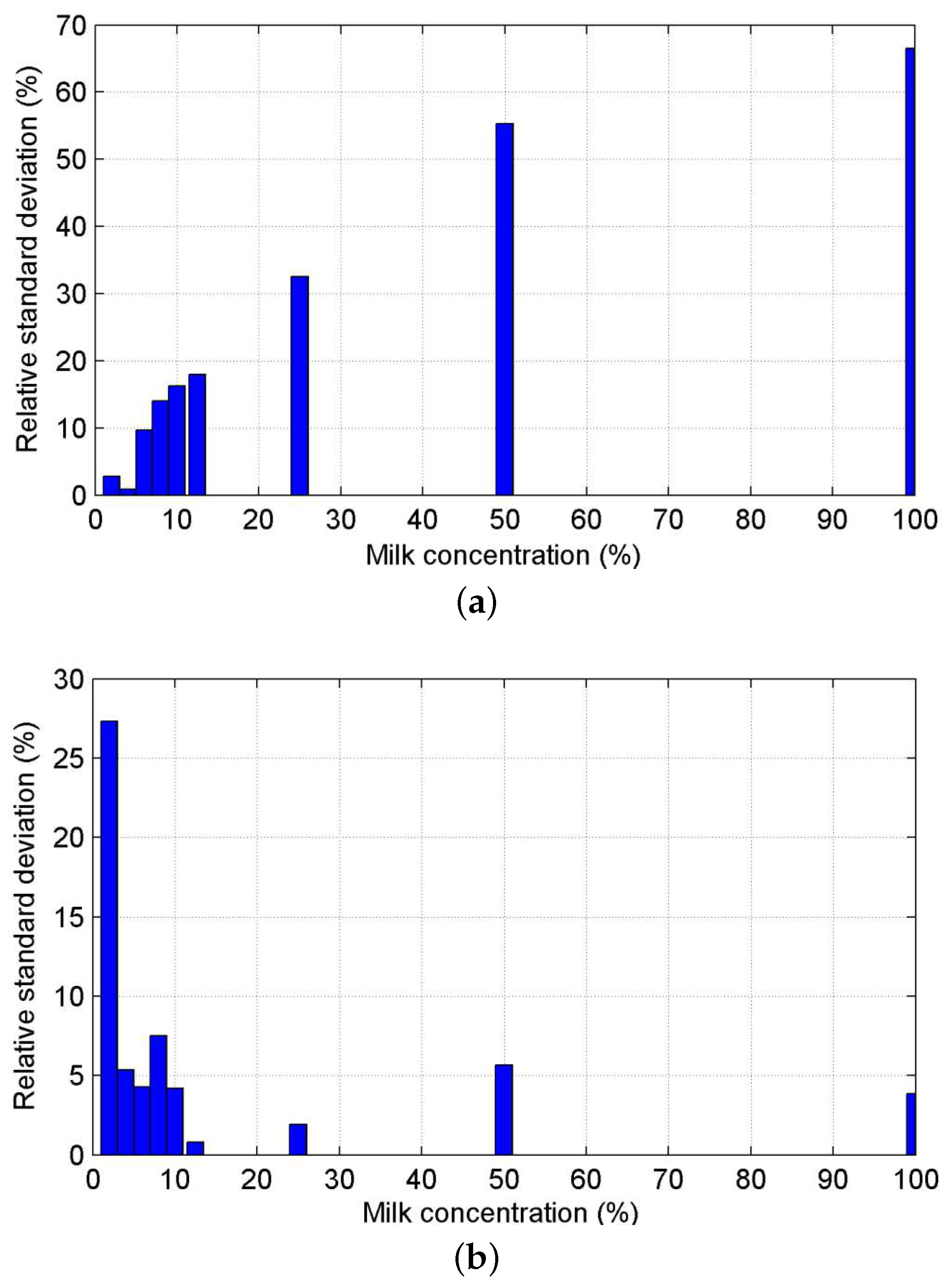
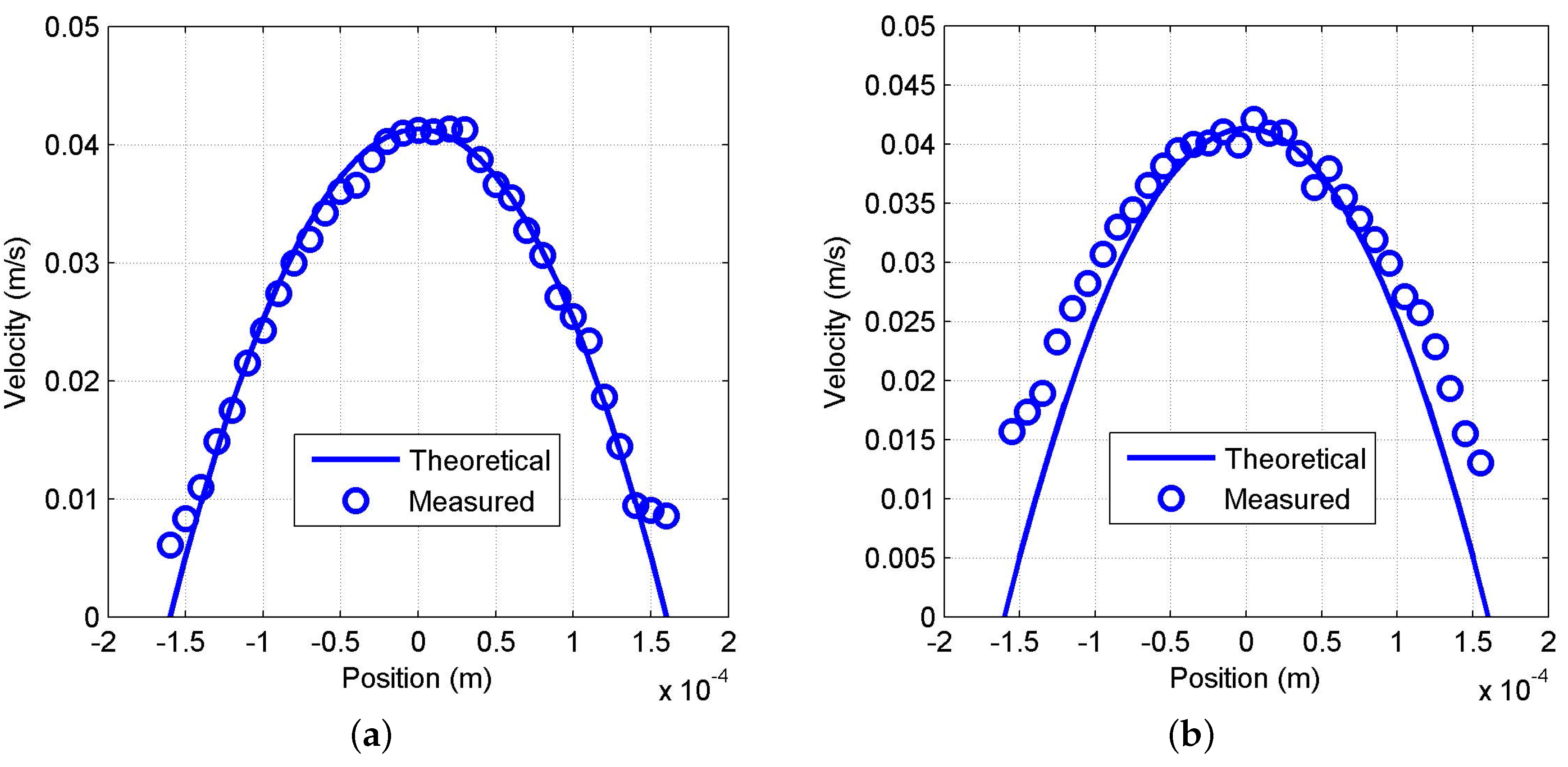
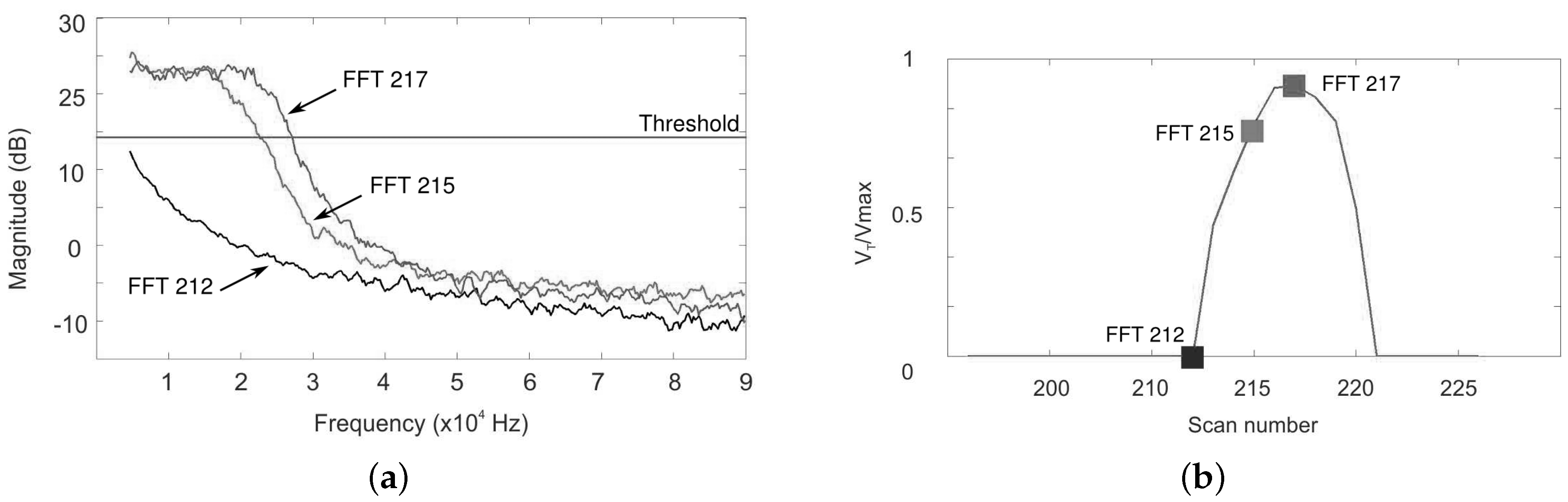
3.2.2. Characterization of the Sensor and Evaluation of the Signal Processing
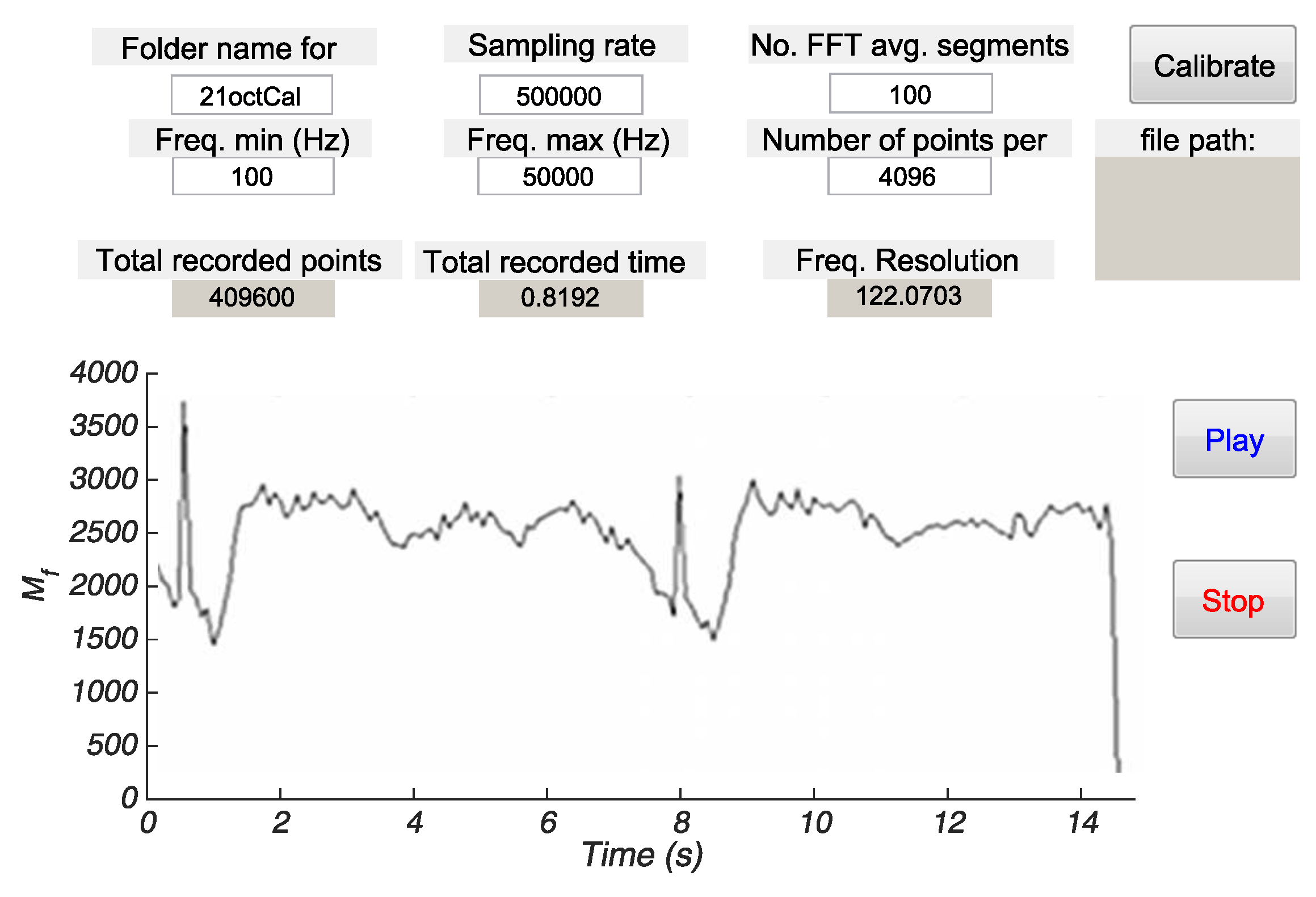
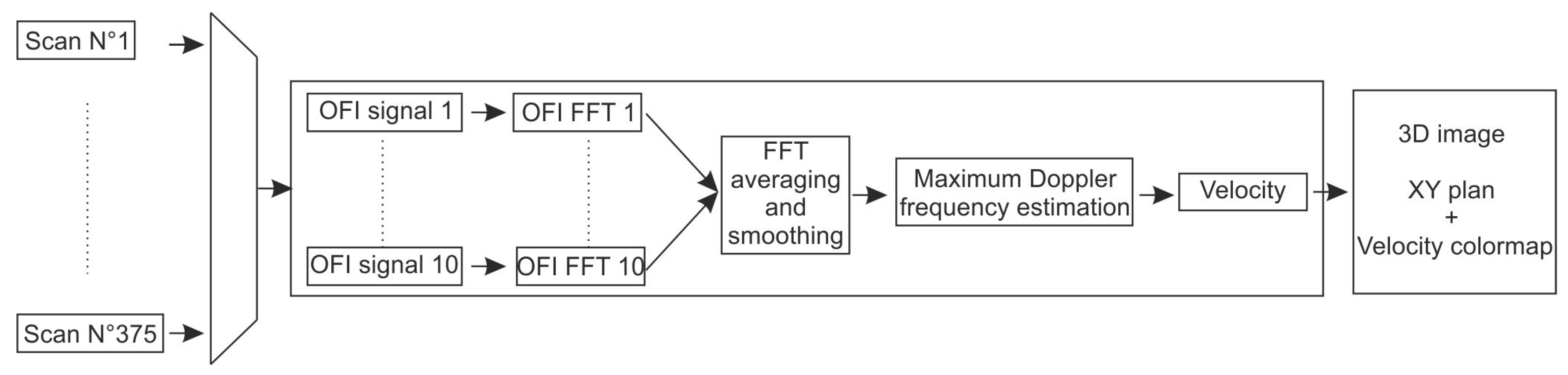
3.2.3. Real-Time Implementation
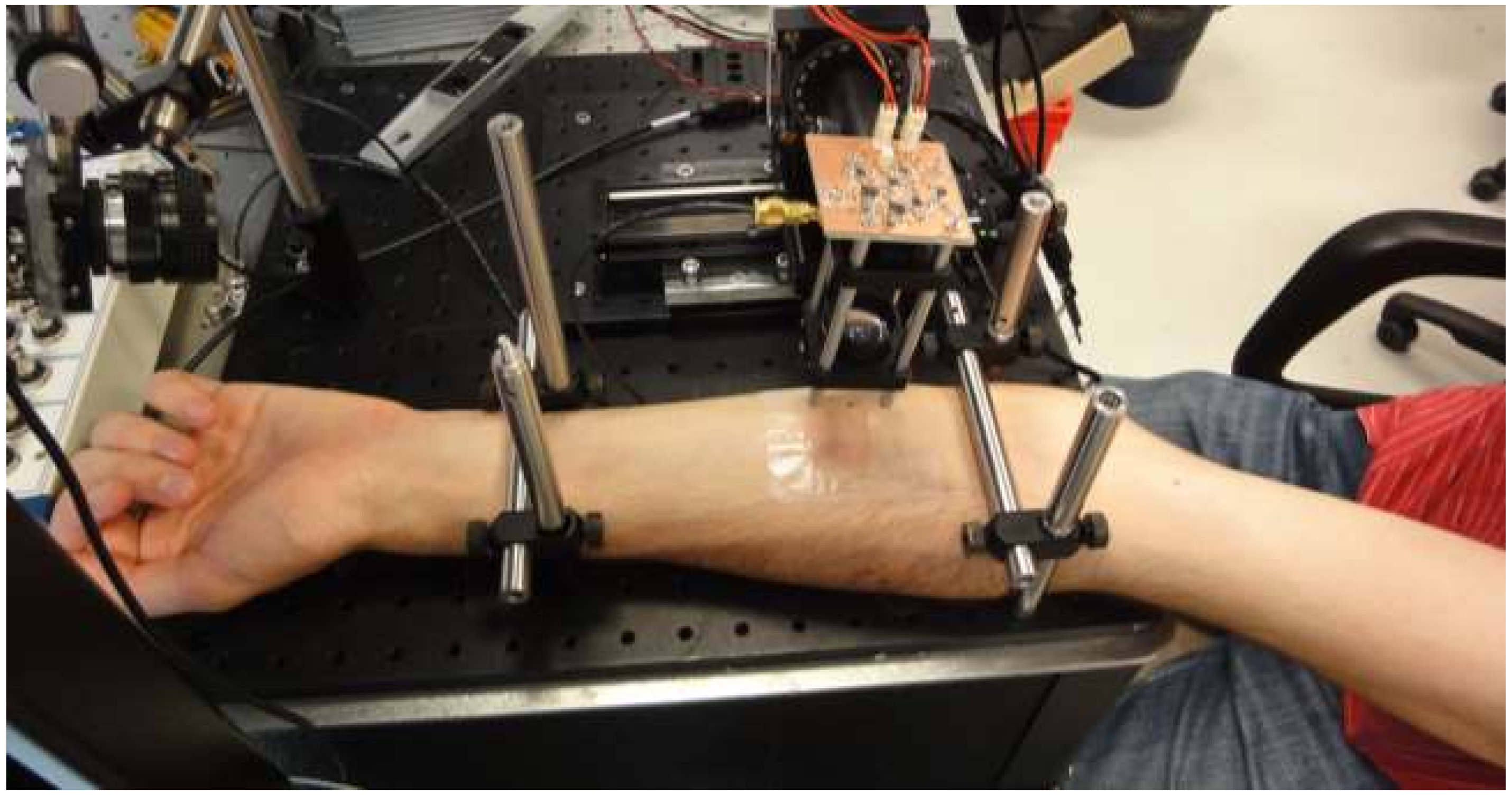
3.3. Human Skin Blood Flow Measurements
3.3.1. Skin Preparation
3.3.2. Experimental Setup
3.3.3. Signal Processing
3.3.4. Preliminary Results
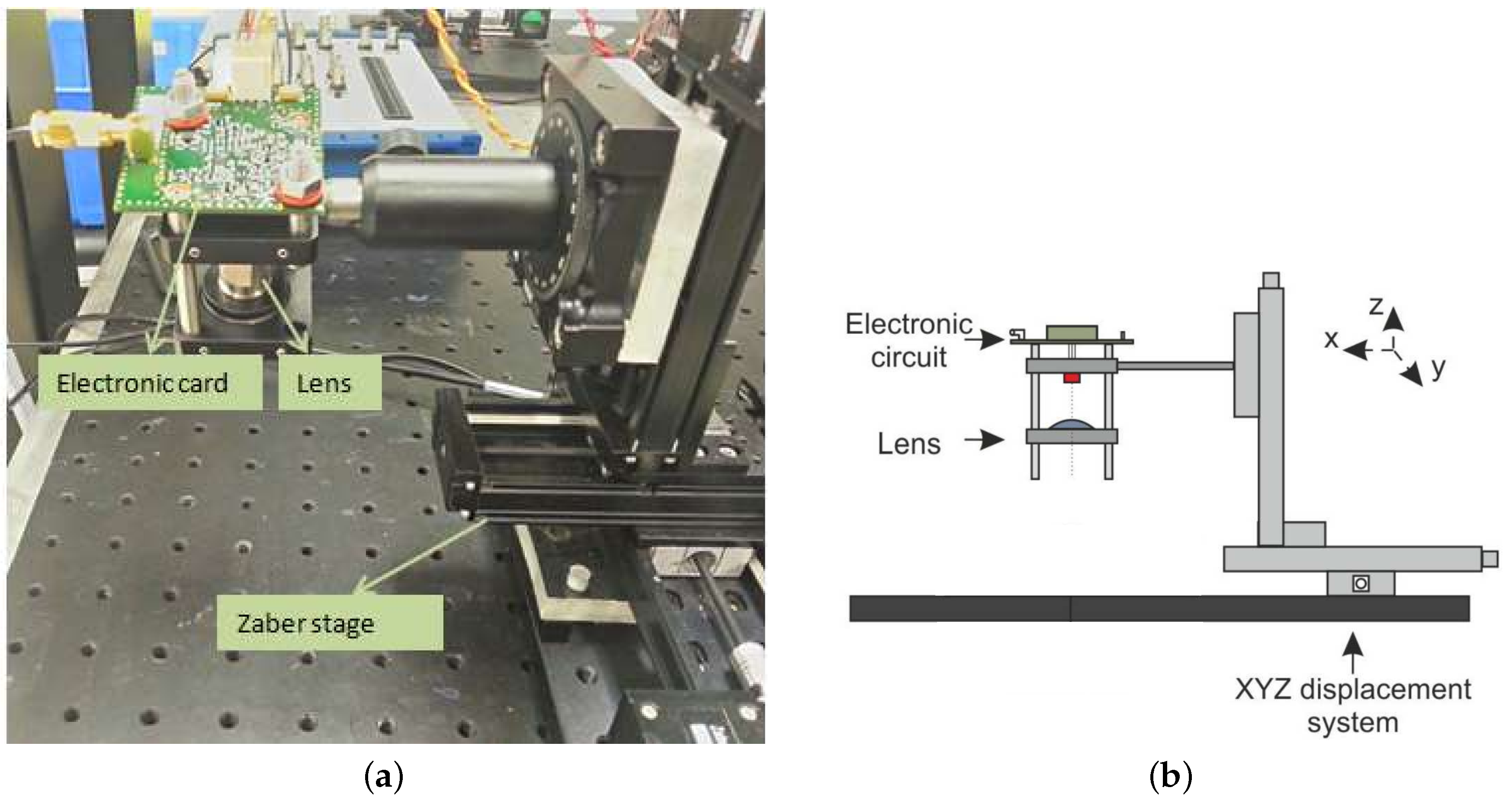
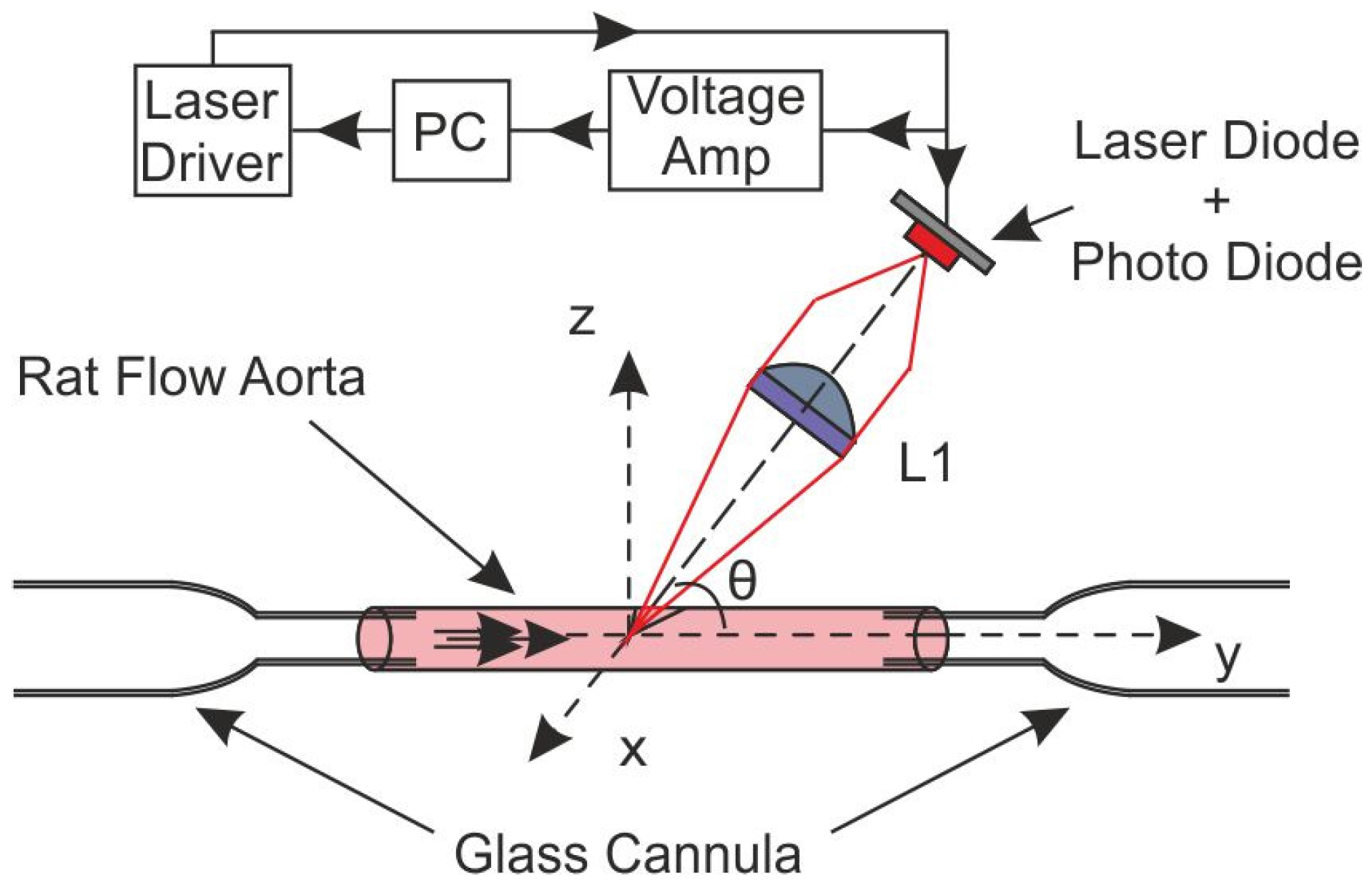
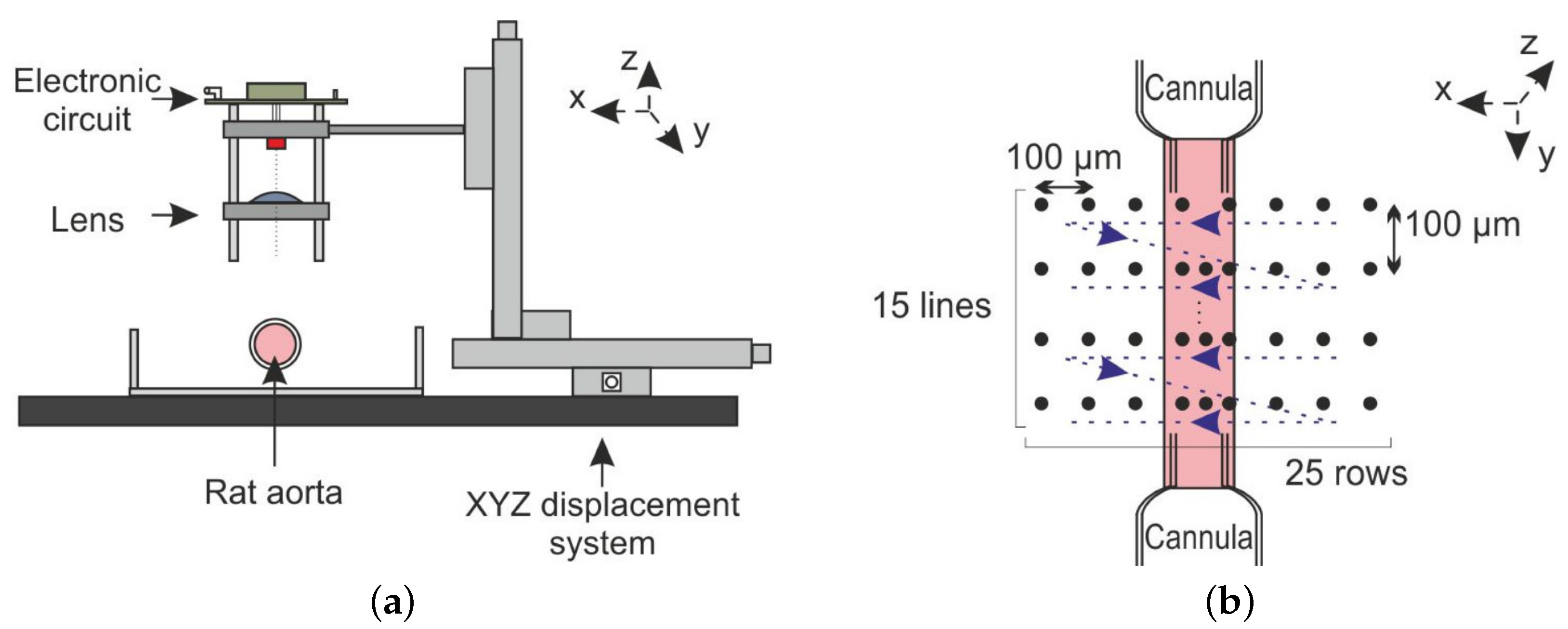
3.4. OFI Pressure Myograph Sensor
3.4.1. Aorta Cannulation
3.4.2. OFI Sensor and Signal Processing
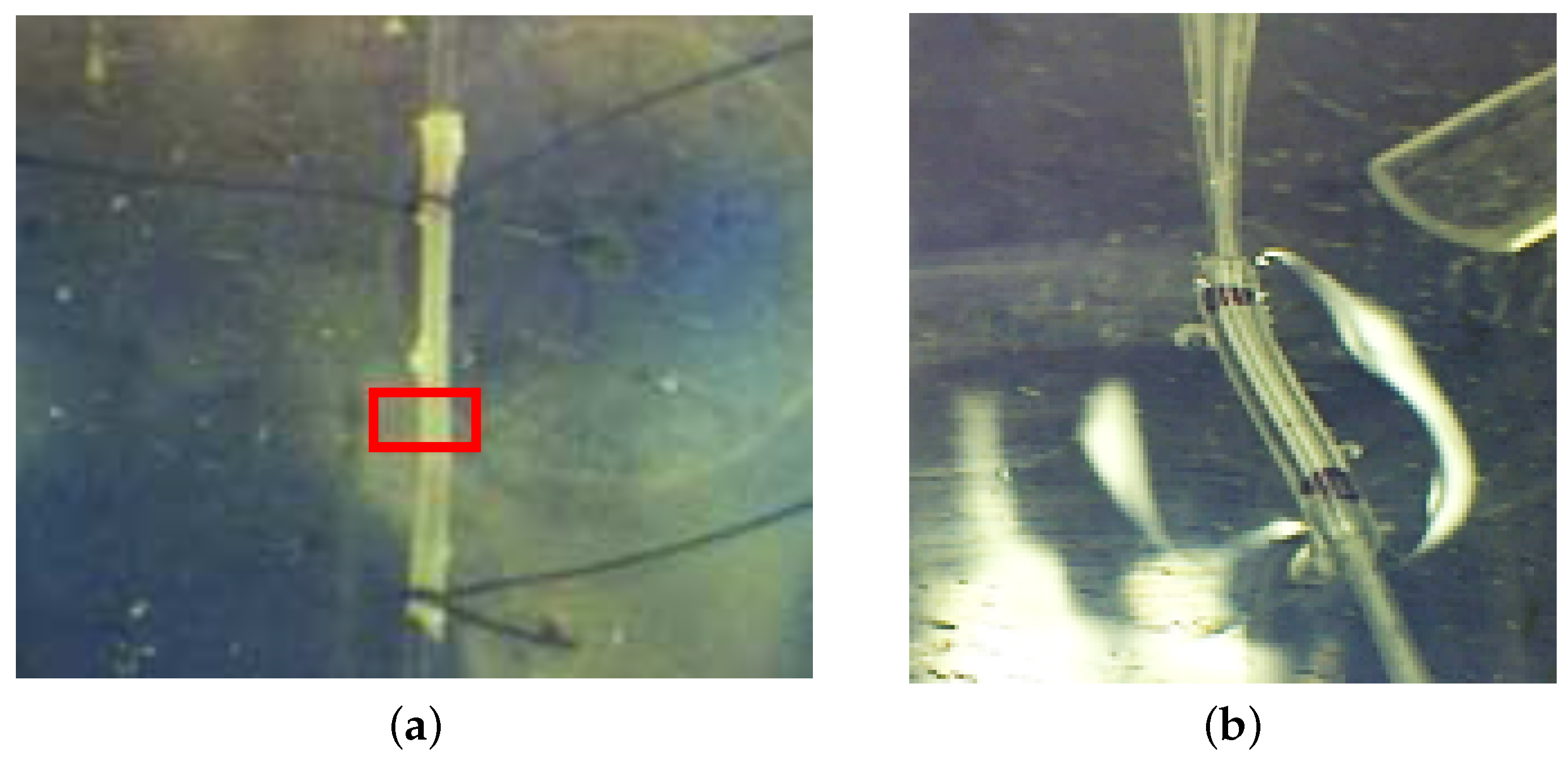
3.4.3. Photography-Based Diameter Assessment
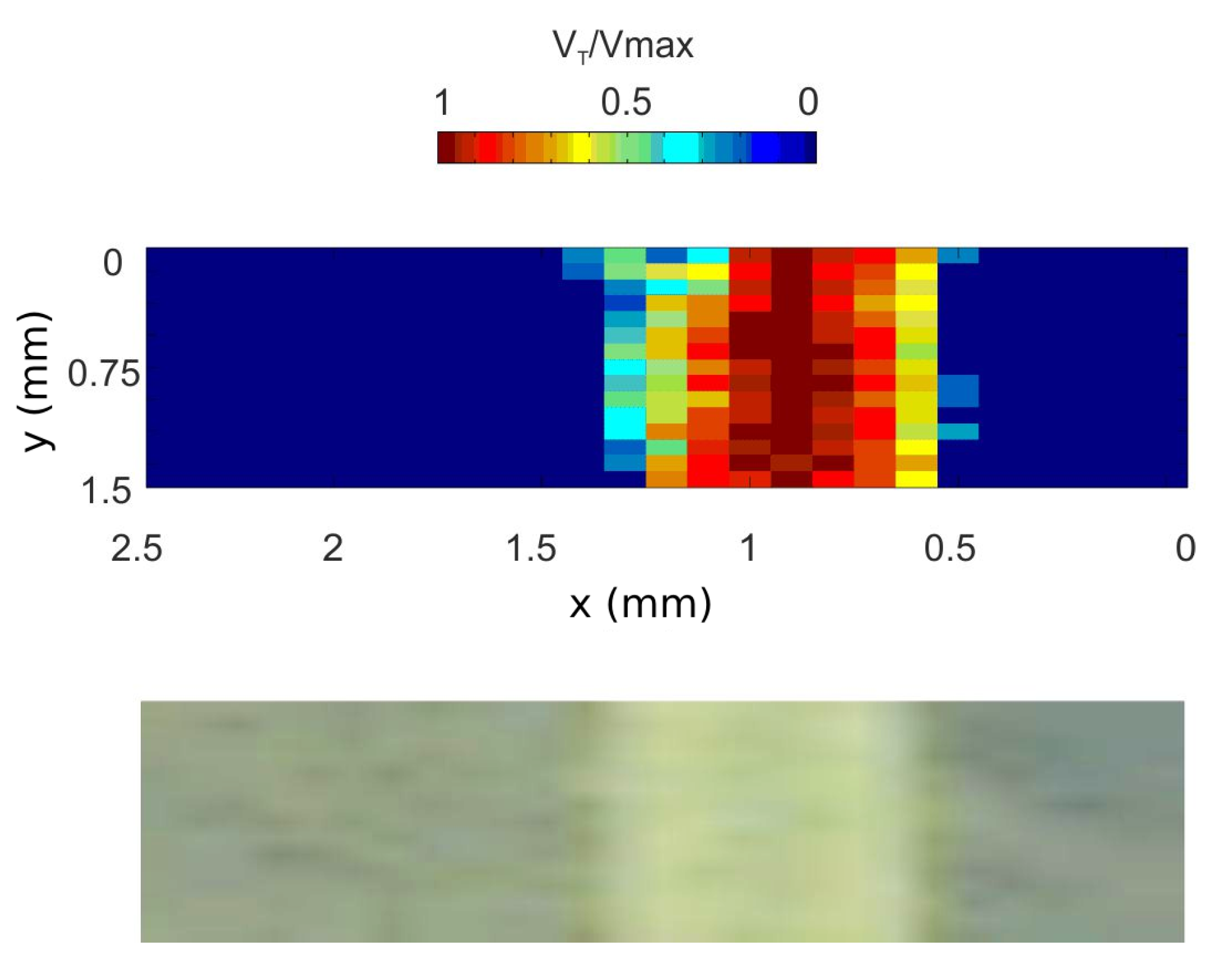
3.4.4. OFI Diameter Assessment
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kane, D.M.; Shore, K.A. Unlocking Dynamical Diversity; John Wiley and Sons, Ltd.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Taimre, T.; Nikolić, M.; Bertling, K.; Lim, Y.L.; Bosch, T.; Rakić, A.D. Laser feedback interferometry: A tutorial on the self-mixing effect for coherent sensing. Adv. Opt. Photonics 2015, 7, 570–631. [Google Scholar] [CrossRef]
- Donati, S. Developing self-mixing interferometry for instrumentation and measurements. Laser Photonics Rev. 2011, 6, 393–417. [Google Scholar] [CrossRef]
- Lang, R.; Kobayashi, K. External optical feedback effects on semiconductor injection laser properties. IEEE J. Quantum Electron. 1980, 16, 347–355. [Google Scholar] [CrossRef]
- Donati, S. Electro-Optical Instrumentation; Pearson Education, Inc.: Upper Saddle River, NJ, USA, 2004. [Google Scholar]
- Bosch, T.; Servagent, N.; Donati, S. Optical feedback interferometry for sensing application. Opt. Eng. 2001, 40, 20–27. [Google Scholar] [CrossRef]
- Mezzapesa, F.P.; Columbo, L.; Brambilla, M.; Dabbicco, M.; Ancona, A.; Sibillano, T.; Lucia, F.D.; Lugarà, P.M.; Scamarcio, G. Simultaneous measurement of multiple target displacements by self-mixing interferometry in a single laser diode. Opt. Express 2011, 19, 16160–16173. [Google Scholar] [CrossRef] [PubMed]
- Donati, S.; Rossi, D.; Norgia, M. Single channel self-mixing interferometer measures simultaneously displacement and tilt and yaw angles of a reflective target. IEEE J. Quantum Electron. 2015, 51, 1–8. [Google Scholar] [CrossRef]
- Donati, S.; Giuliani, G.; Merlo, S. Laser diode feedback interferometer for measurement of displacements without ambiguity. IEEE J. Quantum Electron. 1995, 31, 113–119. [Google Scholar] [CrossRef]
- Wang, M.; Lai, G. Displacement measurement based on Fourier transform method with external laser cavity modulation. Rev. Sci. Instrum. 2001, 72, 3440–3445. [Google Scholar] [CrossRef]
- Bernal, O.; Zabit, U.; Bosch, T. Study of laser feedback phase under self-mixing leading to improved phase unwrapping for vibration sensing. IEEE Sens. J. 2013, 13, 4962–4971. [Google Scholar] [CrossRef]
- Arriaga, A.L.; Bony, F.; Bosch, T. Speckle-insensitive fringe detection method based on Hilbert transform for self-mixing interferometry. Appl. Opt. 2014, 53, 6954–6962. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Azcona, F.J.; Yañez, C.; Royo, S. Extraction of vibration parameters from optical feedback interferometry signals using wavelets. Appl. Opt. 2015, 54, 10106–10113. [Google Scholar] [CrossRef] [PubMed]
- Atashkhooei, R.; Urresty, J.C.; Royo, S.; Riba, J.R.; Romeral, L. Runout tracking in electric motors using self-mixing interferometry. IEEE/ASME Trans. Mechatron. 2014, 19, 184–190. [Google Scholar] [CrossRef]
- Dabbicco, M.; Intermite, A.; Scamarcio, G. Laser-self-mixing fiber sensor for integral strain measurement. J. Lightwave Technol. 2011, 29, 335–340. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, Y.; Xi, J.; Guo, Q. Simultaneous measurement of vibration and parameters of a semiconductor laser using self-mixing interferometry. Appl. Opt. 2014, 53, 4256–4263. [Google Scholar] [CrossRef] [PubMed]
- Bertling, K.; Perchoux, J.; Taimre, T.; Malkin, R.; Robert, D.; Rakić, A.D.; Bosch, T. Imaging of acoustic fields using optical feedback interferometry. Opt. Express 2014, 22, 30346–30356. [Google Scholar] [CrossRef] [PubMed]
- Pruijmboom, A.; Booij, S.; Schemmann, M.; Werner, K.; Hoeven, P.; van Limpt, H.; Intemann, S.; Jordan, R.; Fritzsch, T.; Oppermann, H.; et al. VCSEL-based miniature laser-self-mixing interferometer with integrated optical and electronic components. In Proceedings of the SPIE 7221, Photonics Packaging, Integration, and Interconnects IX, San Jose, CA, USA, 26–28 January 2009; pp. 72210S1–72210S12.
- Liess, M.; Weijers, G.; Heinks, C.; van der Horst, A.; Rommers, A.; Duijve, R.; Mimnagh, G. A miniaturized multidirectional optical motion sensor and input device based on laser self-mixing. Meas. Sci. Technol. 2002, 13, 2001. [Google Scholar] [CrossRef]
- Rakic, A.D.; Taimre, T.; Bertling, K.; Lim, Y.L.; Wilson, S.J.; Nikolic, M.; Valavanis, A.; Indjin, D.; Linfield, E.H.; Davies, A.G.; et al. THz QCL self-mixing interferometry for biomedical applications. In Proceedings of the SPIE 9199, Terahertz Emitters, Receivers, and Applications V, San Diego, CA, USA, 17–18 August 2014.
- Mohr, T.; Breuer, S.; Blomer, D.; Simonetta, M.; Patel, S.; Schlosser, M.; Deninger, A.; Birkl, G.; Giuliani, G.; Elsaber, W. Terahertz homodyne self-mixing transmission spectroscopy. Appl. Phys. Lett. 2015, 106, 061111. [Google Scholar] [CrossRef]
- Taimre, T.; Bertling, K.; Lim, Y.L.; Dean, P.; Indjin, D.; Rakić, A.D. Methodology for materials analysis using swept-frequency feedback interferometry with terahertz frequency quantum cascade lasers. Opt. Express 2014, 22, 18633–18647. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Bertling, K.; Dean, P.; Keeley, J.; Burnett, A.D.; Lim, Y.L.; Khanna, S.P.; Valavanis, A.; Linfield, E.H.; Davies, A.G.; et al. Laser feedback interferometry as a tool for analysis of granular materials at terahertz frequencies: Towards imaging and identification of plastic explosives. Sensors 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Dean, P.; Lim, Y.L.; Valavanis, A.; Kliese, R.; Nikolić, M.; Khanna, S.P.; Lachab, M.; Indjin, D.; Ikonić, Z.; Harrison, P.; et al. Terahertz imaging through self-mixing in a quantum cascade laser. Opt. Lett. 2011, 36, 2587–2589. [Google Scholar] [CrossRef] [PubMed]
- Lui, H.S.; Taimre, T.; Bertling, K.; Lim, Y.L.; Dean, P.; Khanna, S.P.; Lachab, M.; Valavanis, A.; Indjin, D.; Linfield, E.H.; et al. Terahertz inverse synthetic aperture radar imaging using self-mixing interferometry with a quantum cascade laser. Opt. Lett. 2014, 39, 2629–2632. [Google Scholar] [CrossRef] [PubMed]
- Donati, S.; Norgia, M. Self-Mixing Interferometry for Biomedical Signals Sensing. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 104–111. [Google Scholar] [CrossRef]
- De Mul, F.F.M.; van Spijker, J.; van der Plas, D.; Greve, J.; Aarnoudse, J.G.; Smits, T.M. Mini laser-Doppler (blood) flow monitor with diode laser source and detection integrated in the probe. Appl. Opt. 1984, 23, 2970–2973. [Google Scholar] [CrossRef] [PubMed]
- Hast, J.; Myllylä, R.; Sorvoja, H.; Miettinen, J. Arterial pulse shape measurement using self-mixing effect in a diode laser. Quantum Electron. 2002, 32, 975. [Google Scholar] [CrossRef]
- Capelli, G.; Bollati, C.; Giuliani, G. Non-contact monitoring of heart beat using optical laser diode vibrocardiography. In Proceedings of the 2011 International Workshop on BioPhotonics, Parma, Italy, 8–10 June 2011; pp. 1–3.
- Arasanz, A.; Azcona, F.; Royo, S.; Jha, A.; Pladellorens, J. A new method for the acquisition of arterial pulse wave using self-mixing interferometry. Opt. Laser Technol. 2014, 63, 98–104. [Google Scholar] [CrossRef]
- Rovati, L.; Cattini, S.; Palanisamy, N. Measurement of the fluid-velocity profile using a self-mixing superluminescent diode. Meas. Sci. Technol. 2011, 22, 025402. [Google Scholar] [CrossRef]
- Capelli, G.; Giuliani, G. Laser velocimeter for the measurement of eye movements. In Proceedings of the 2011 International Workshop on BioPhotonics, Parma, Italy, 8–10 June 2011; pp. 1–3.
- Donati, S.; Annovazzi-Lodi, V.; Bottazzi, L.; Zambarbieri, D. Pickup of head movement in vestibular reflex experiments with an optical fiber gyroscope. IEEE J. Sel. Top. Quantum Electron. 1996, 2, 890–893. [Google Scholar] [CrossRef]
- Wang, M.; Lai, G. Self-mixing microscopic interferometer for the measurement of microprofile. Opt. Commun. 2004, 238, 237–244. [Google Scholar] [CrossRef]
- Gaynor, J.E.; Lim, Y.L.; Bertling, K.; Rakic, A.D. Towards a scanning laser confocal microscope using the self-mixing effect. In Proceedings of the 2012 Conference on Optoelectronic and Microelectronic Materials Devices (COMMAD), Melbourne, Australia, 12–14 December 2012; pp. 81–82.
- Lee, S.; White, P. Fault diagnosis of rotating machinery using Wigner higher order moment spectra. Mech. Syst. Sig. Process. 1997, 11, 637–650. [Google Scholar] [CrossRef]
- Scalise, L.; Morbiducci, U. Non-contact cardiac monitoring from carotid artery using optical vibrocardiography. Med. Eng. Phys. 2008, 30, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Barun, S. Mechanical Signature Analysis-Theory and Applications; New York Academic Press: New York, NY, USA, 1986. [Google Scholar]
- McFadeen, P. Examination of a technique for the early detection of failure in gears by signal processing of the time domain average of the meshing vibration. Mech. Syst. Sig. Process. 1987, 1, 173–183. [Google Scholar] [CrossRef]
- Jha, A.; Royo, S.; Azcona, F.; Yañez, C. Extracting vibrational parameters from the time-frequency map of a self mixing signal: An approach based on wavelet analysis. In Proceedings of the IEEE SENSORS, Valencia, Spain, 2–5 November 2014; pp. 1881–1884.
- Vetterli, M.; Kovacevic, J. Wavelets and Subband Coding; Prentice-Hall: Upper Saddle River, NJ, USA, 1995. [Google Scholar]
- Kliese, R.; Rakić, A.D. Spectral broadening caused by dynamic speckle in self-mixing velocimetry sensors. Opt. Express 2012, 20, 18757–18771. [Google Scholar] [CrossRef] [PubMed]
- Norgia, M.; Donati, S.; D’Alessandro, D. Interferometric measurements of displacement on a diffusing target by a speckle tracking technique. IEEE J. Quantum Electron. 2001, 37, 800–806. [Google Scholar] [CrossRef]
- Atashkhooei, R.; Royo, S.; Azcona, F.; Zabit, U. Analysis and control of speckle effects in self-mixing interferometry. In Proceedings of the 2011 IEEE Sensors, Limerick, Ireland, 28–31 October 2011; pp. 1390–1393.
- Giuliani, G.; Bozzi-Pietra, S.; Donati, S. Self-mixing laser diode vibrometer. Meas. Sci. Technol. 2003, 14, 24. [Google Scholar] [CrossRef]
- Zabit, U.; Bernal, O.; Bosch, T. Self-mixing laser sensor for large displacements: Signal recovery in the presence of speckle. IEEE Sens. J. 2013, 13, 824–831. [Google Scholar] [CrossRef]
- Riva, C.; Ross, B.; Benedek, G.B. Laser doppler measurements of blood flow in capillary tubes and retinal arteries. Investig. Ophthalmol. Vis. Sci. 1972, 11, 936. [Google Scholar]
- De Mul, F.; Koelink, M.; Weijers, A.; Greve, J.; Aarnoudse, J.; Graaff, R.; Dassel, A. A semiconductor laser used for direct measurement of the blood perfusion of tissue. IEEE Trans. Biomed. Eng. 1993, 40, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Campagnolo, L.; Nikolić, M.; Perchoux, J.; Lim, Y.L.; Bertling, K.; Loubière, K.; Prat, L.; Rakić, A.D.; Bosch, T. Flow profile measurement in microchannel using the optical feedback interferometry sensing technique. Microfluid. Nanofluid. 2013, 14, 113–119. [Google Scholar] [CrossRef]
- Tuchin, V.V. Handbook of Optical Biomedical Diagnostics; SPIE Press: Bellingham, WA, USA, 2002. [Google Scholar]
- Kliese, R.; Lim, Y.L.; Bosch, T.; Rakić, A.D. GaN laser self-mixing velocimeter for measuring slow flows. Opt. Lett. 2010, 35, 814–816. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, M.; Jovanović, D.P.; Lim, Y.L.; Bertling, K.; Taimre, T.; Rakić, A.D. Approach to frequency estimation in self-mixing interferometry: Multiple signal classification. Appl. Opt. 2013, 52, 3345–3350. [Google Scholar] [CrossRef] [PubMed]
- Quotb, A.; Ramirez-Miquet, E.; Tronche, C.; Perchoux, J. Optical Feedback Interferometry sensor for flow characterization inside ex-vivo vessel. In Proceedings of the 2014 IEEE SENSORS, Valencia, Spain, 2–5 November 2014; pp. 362–365.
- Norgia, M.; Pesatori, A.; Rovati, L. Low-cost optical flowmeter with analog front-end electronics for blood extracorporeal circulators. IEEE Trans. Instrum. Meas. 2010, 59, 1233–1239. [Google Scholar] [CrossRef]
- Clifford, P.S.; Ella, S.R.; Stupica, A.J.; Nourian, Z.; Li, M.; Martinez-Lemus, L.A.; Dora, K.A.; Yang, Y.; Davis, M.J.; Pohl, U.; et al. Spatial distribution and mechanical function of elastin in resistance arteries: A role in bearing longitudinal stress. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2889–2896. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Kassab, G.S. Assessment of endothelial function of large, medium, and small vessels: A unified myograph. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H94–H100. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, J.O. Fluid Mechanics for Chemical Engineers with Microfluidics and CFD; Pearson Education: Upper Saddle River, NJ, USA, 2006. [Google Scholar]
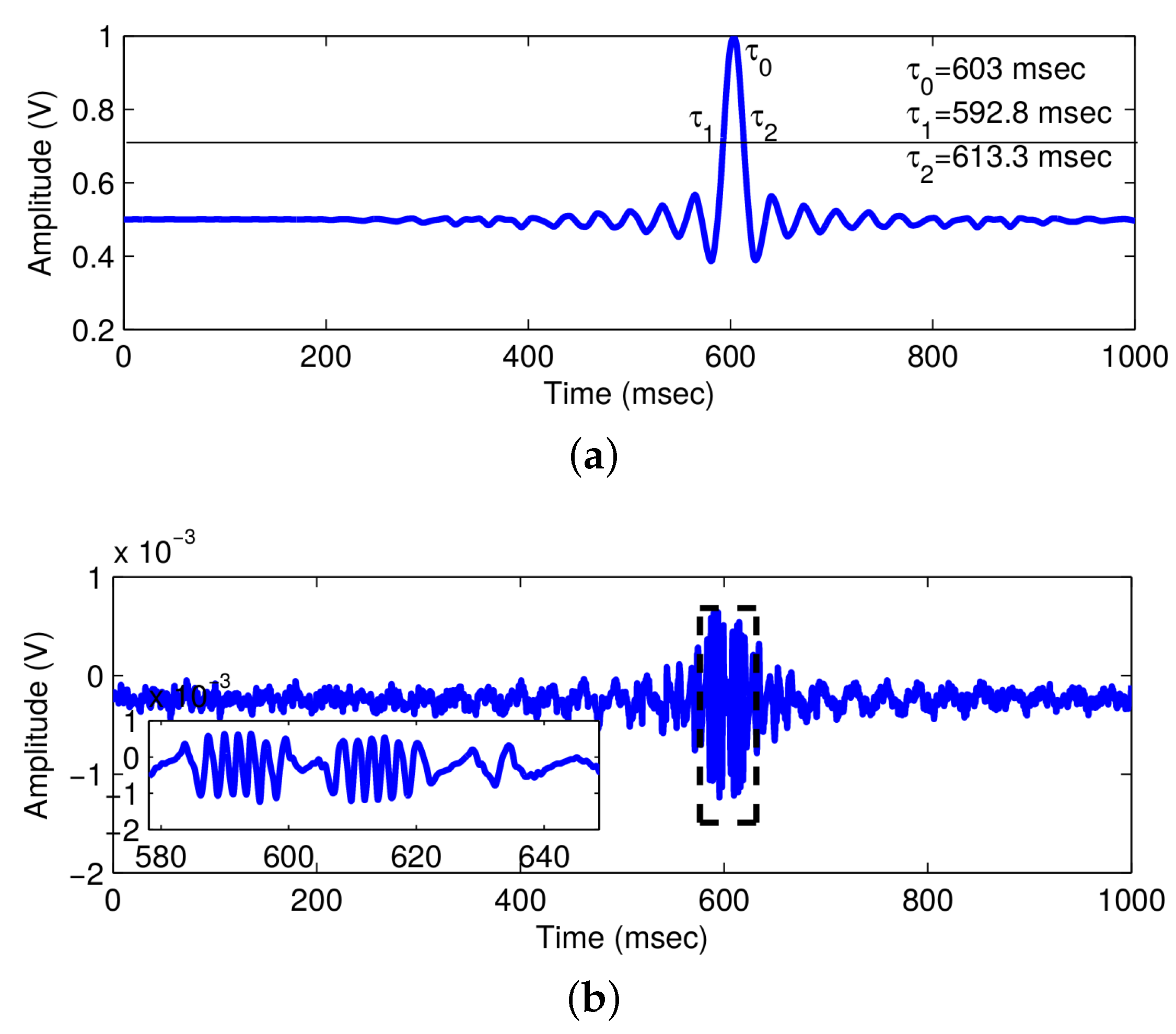
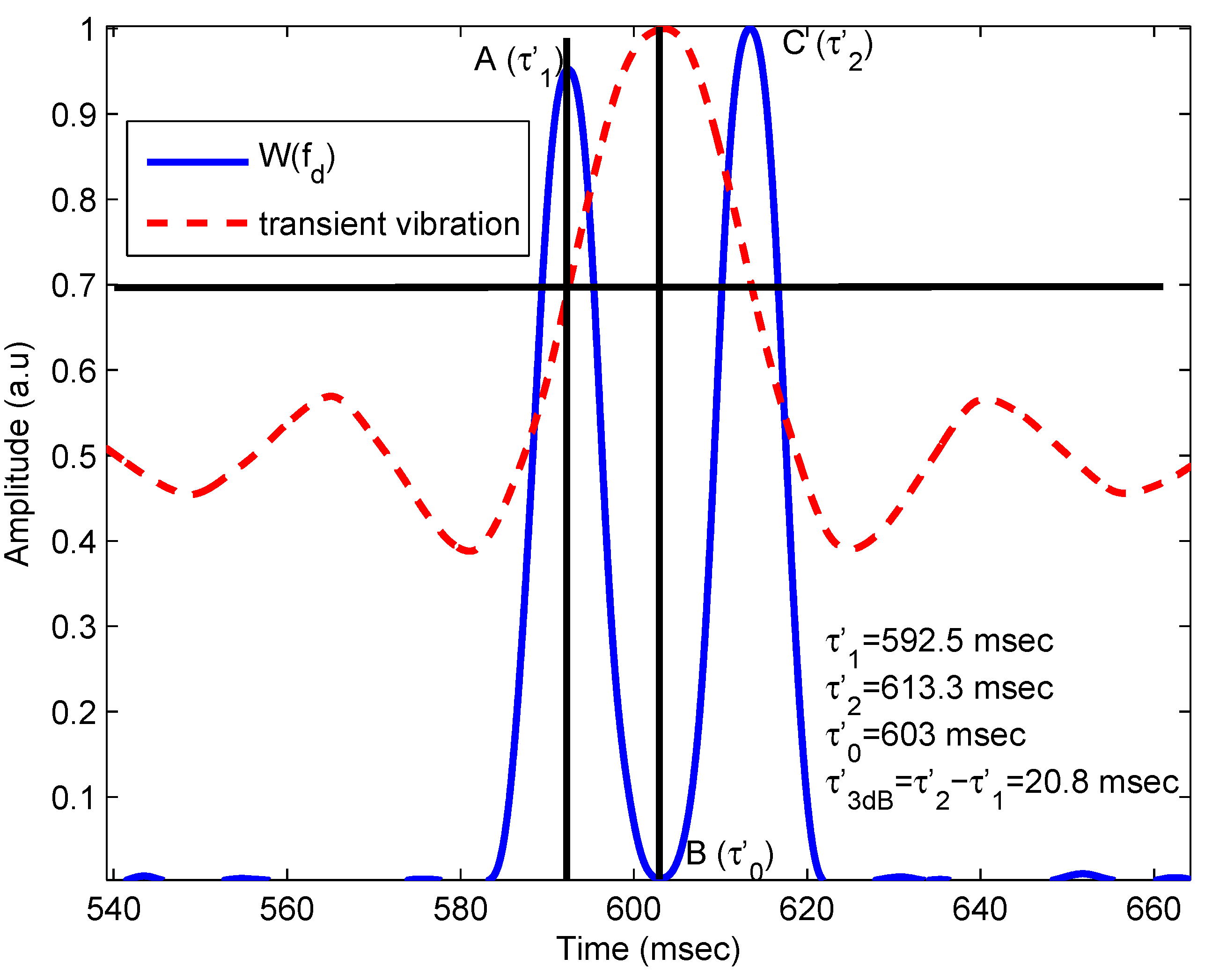



| Parameters | Original Value | Calculated Value | Error |
|---|---|---|---|
| Center pulse time | ms | ms | |
| First 3-dB time | ms | ms | |
| Second 3-dB time | ms | ms | |
| 3-dB pulse duration | ms | ms | |
| Peak velocity | mm/ms | mm/ms |
| after 5 min | after 25 min | |
|---|---|---|
| Patient A | 186 | 154 |
| Patient B | 165 | 77 |
| Patient C | 89 | 71 |
| Patient D | 53 | −36 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perchoux, J.; Quotb, A.; Atashkhooei, R.; Azcona, F.J.; Ramírez-Miquet, E.E.; Bernal, O.; Jha, A.; Luna-Arriaga, A.; Yanez, C.; Caum, J.; et al. Current Developments on Optical Feedback Interferometry as an All-Optical Sensor for Biomedical Applications. Sensors 2016, 16, 694. https://doi.org/10.3390/s16050694
Perchoux J, Quotb A, Atashkhooei R, Azcona FJ, Ramírez-Miquet EE, Bernal O, Jha A, Luna-Arriaga A, Yanez C, Caum J, et al. Current Developments on Optical Feedback Interferometry as an All-Optical Sensor for Biomedical Applications. Sensors. 2016; 16(5):694. https://doi.org/10.3390/s16050694
Chicago/Turabian StylePerchoux, Julien, Adam Quotb, Reza Atashkhooei, Francisco J. Azcona, Evelio E. Ramírez-Miquet, Olivier Bernal, Ajit Jha, Antonio Luna-Arriaga, Carlos Yanez, Jesus Caum, and et al. 2016. "Current Developments on Optical Feedback Interferometry as an All-Optical Sensor for Biomedical Applications" Sensors 16, no. 5: 694. https://doi.org/10.3390/s16050694
APA StylePerchoux, J., Quotb, A., Atashkhooei, R., Azcona, F. J., Ramírez-Miquet, E. E., Bernal, O., Jha, A., Luna-Arriaga, A., Yanez, C., Caum, J., Bosch, T., & Royo, S. (2016). Current Developments on Optical Feedback Interferometry as an All-Optical Sensor for Biomedical Applications. Sensors, 16(5), 694. https://doi.org/10.3390/s16050694







