Investigation of Hemoglobin/Gold Nanoparticle Heterolayer on Micro-Gap for Electrochemical Biosensor Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
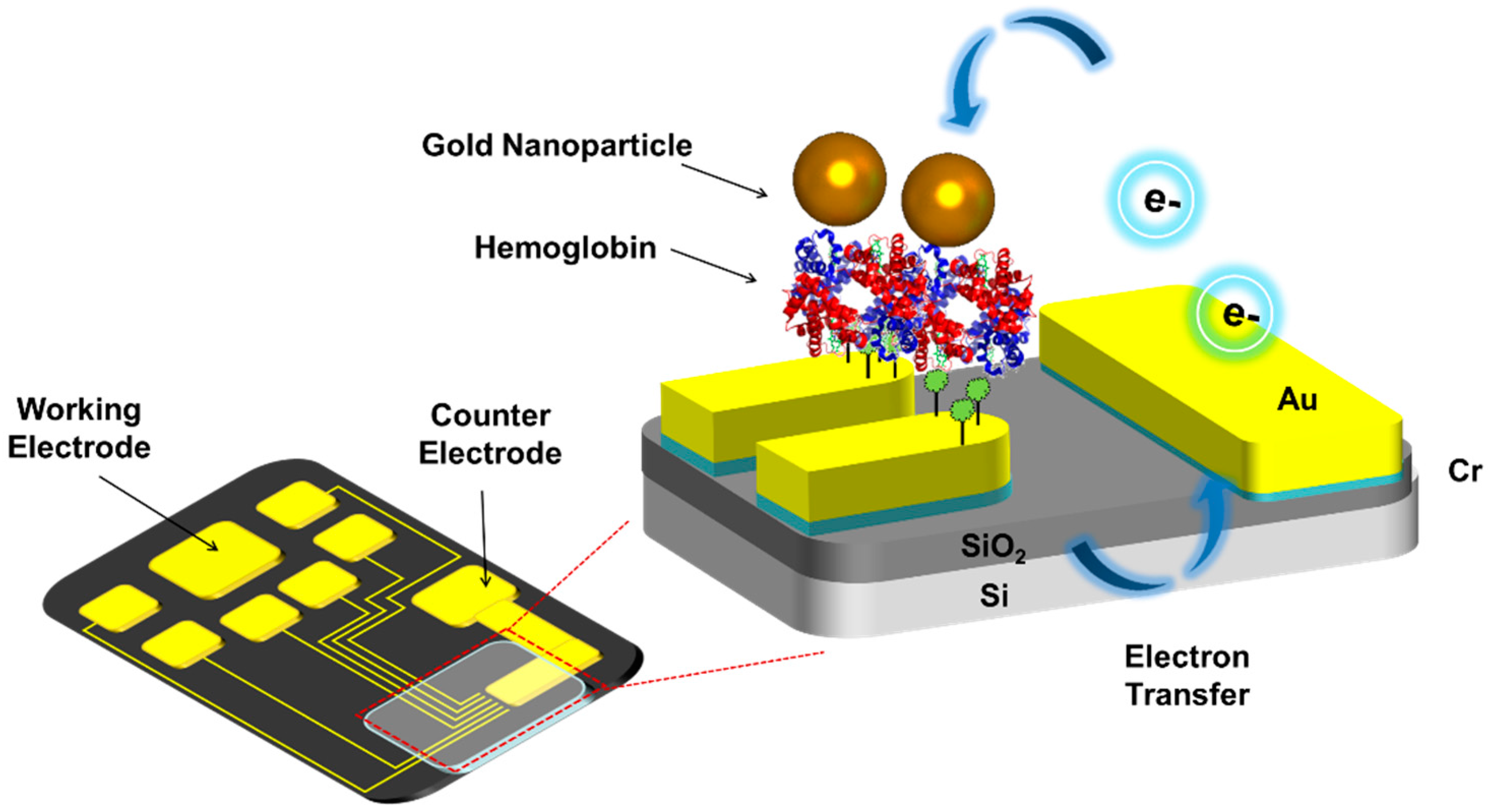
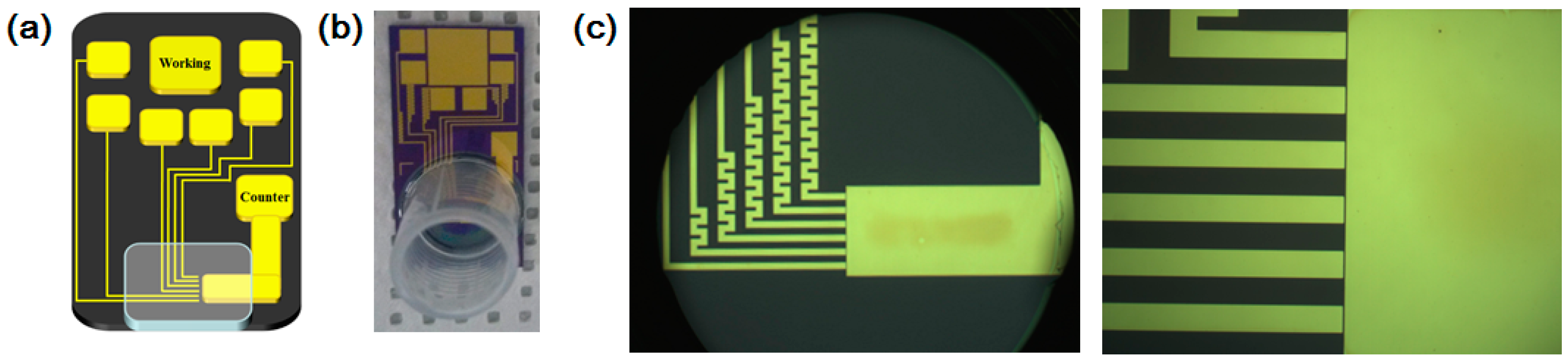
2.2. Fabrication of Micro-Gap
2.3. Fabrication of Hemoglobin/Gold Nanoparticle Heterolayer
2.4. Investigation of Hemoglobin/Gold Nanoparticle Heterolayer Using AFM and SERS
2.5. Electrochemical Analysis of Hemoglobin-Gold Nanoparticle Heterolayer
3. Results and Discussion
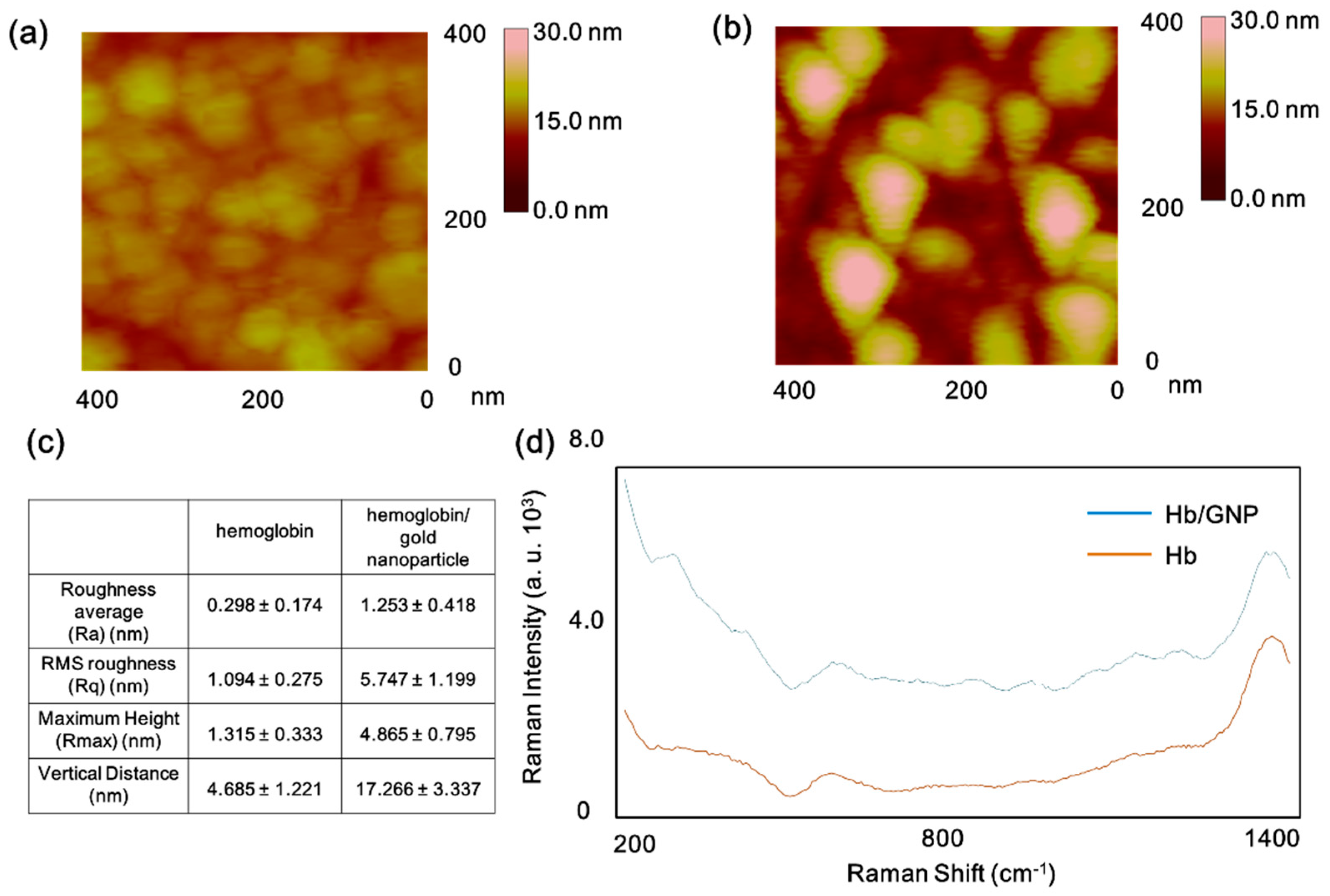
3.1. The Surface Investigation of Hemoglobin/Gold Nanoparticle Hybrid by AFM and SERS
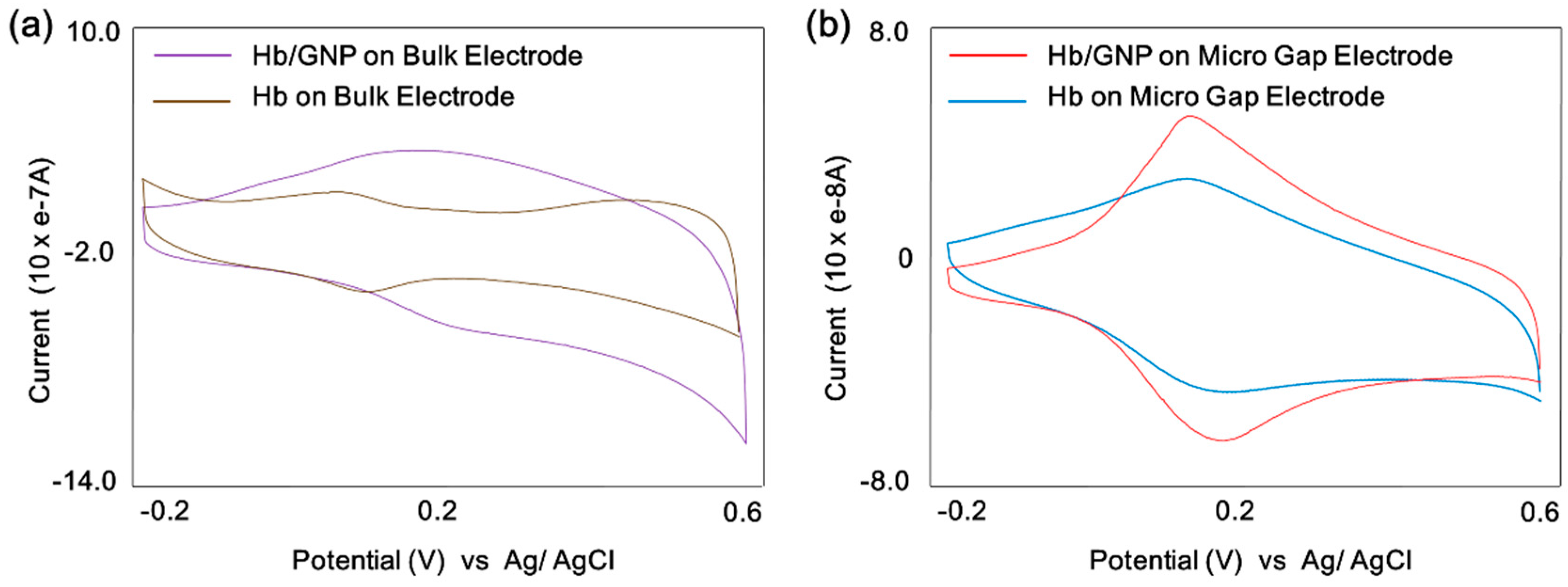
3.2. Cyclic Voltammetric Behavior of Hemoglobin/Gold Nanoparticle
3.3. Electrocatalytic Properties of Hemoglobin/Gold Nanoparticle on the Micro-Gap Electrode
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Hb | Hemoglobin |
| GNP | Gold Nanoparticle |
| AFM | Atomic Force Microscopy |
| SERS | Surface-Enhanced Raman Spectroscopy |
| 6-MHA | 6-Mercaptohexanoic Acid |
| LbL | layer-by-layer |
| CV | Cyclic Voltammetry |
References
- Bartlett, P.N. Bioelectrochemistry: Fundamentals, Experimental Techniques and Applications, 1st ed.; John Wiley & Sons Inc.: West Sussex, UK, 2008; pp. 3–38. [Google Scholar]
- Kulys, J.; Gorton, L.; Domingues, E.; Emnéus, J.; Jarskog, H. Electrochemical characterization of carbon pastes modified with proteins and polycations. J. Electroanal. Chem. 1994, 372, 49–55. [Google Scholar] [CrossRef]
- Bănică, F. Chemical Sensors and Biosensors: Fundamentals and Applications; John Wiley & Sons Inc.: Chichester, UK, 2012; pp. 133–139. [Google Scholar]
- Bartlett, P.N.; Birkin, P.R.; Wang, J.H.; Palmisano, F.; Benedetto, G.D. An Enzyme Switch Employing Direct Electrochemical Communication between Horseradish Peroxidase and a Poly(aniline) Film. Anal. Chem. 1998, 70, 3685–3694. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, Y.; Chen, L. Organic-phase biosensors for monitoring phenol and hydrogen peroxide in pharmaceutical antibacterial products. Analyst 1993, 118, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Hasebe, Y. DNA–Cu(II) poly(amine) complex membrane as novel catalytic layer for highly sensitive amperometric determination of hydrogen peroxide. Biosens. Bioelectron. 2006, 21, 2121–2128. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yuan, R.; Chai, Y.; Zhang, L.; Wang, N.; Li, X. Amperometric third-generation hydrogen peroxide biosensor based on the immobilization of hemoglobin on multiwall carbon nanotubes and gold colloidal nanoparticles. Biosens. Bioelectron. 2007, 22, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Ding, J.; Cai, J.; Zhang, A. Determination of carbaryl pesticide using amperometric acetylcholinesterase sensor formed by electrochemically deposited chitosan. Colloid Surface B 2007, 58, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Yagati, A.K.; Choi, J.-W. Protein Based Electrochemical Biosensors for H2O2 Detection towards Clinical Diagnostics. Electroanalysis 2014, 26, 1259–1276. [Google Scholar] [CrossRef]
- Rojking, R.; Dominguez-Rosales, J.-A.; Nieto, N.; Greenwel, P. Role of hydrogen peroxide and oxidative stress in healing responses. CMLS Cell. Mol. Life. Sci. 2002, 59, 1872–1891. [Google Scholar] [CrossRef]
- Lean, J.M.; Jagger, C.J.; Kirstein, B.; Fuller, K.; Chambers, T.J. Hydrogen Peroxide is Essential for Estrogen-Deficiency Bone Loss and Osteoclast Formation. Endocrinology 2005, 146, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.-C.; Zhang, J.-L.; Tan, S.-W.; Zhao, D.-D.; Huang, Z.-W.; Mi, Y.; Huang, Z.-Y. Amperometric Hydrogen Peroxide Biosensor Based on Immobilization of Hemoglobin on a Glassy Carbon Electrode Modified with Fe3O4/Chitosan Core-Shell Microspheres. Sensors 2009, 9, 6185–6199. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.-X.; Hu, S.-Q.; Gao, N.; Shen, G.-L.; Yu, R.-Q. An amperometric hydrogen peroxide biosensor based on immobilizing horseradish peroxidase to a nano-Au monolayer supported by sol-gel derived carbon ceramic electrode. Bioelectrochemistry 2004, 65, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization strategies to develop enzymatic biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Aghebati-maleki, L.; Salehi, B.; Behfar, R.; Saeidmanesh, H.; Ahmadian, F.; Sarebanhassanabadi, M.; Negahdary, M. Designing a Hydrogen Peroxide Biosensor Using Catalase and Modified Electrode with Magnesium Oxide Nanoparticles. Int. J. Electrochem. Sci. 2014, 9, 257–271. [Google Scholar]
- Yagati, A.K.; Lee, T.; Min, J.; Choi, J.-W. Electrochemical performance of gold nanoparticle-cytochrome c hybrid interface for H2O2 detection. Colloid. Surf. B 2012, 92, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Yip, R. Significance of an abnormally low or high hemoglobin concentration during pregnancy: Special consideration of iron nutrition. Am. J. Clin. Nutr. 2000, 72, 272–279. [Google Scholar]
- Schechter, A.N. Hemoglobin research and the origins of molecular medicine. Blood 2008, 112, 3927–3938. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Cheng, W.; Zhang, Z.; Dong, S.; Wang, E. Direct Electron Transfer between Hemoglobin and a Glassy Carbon Electrode Facilitated by Lipid-Protected Gold Nanoparticles. Biochim. Biophys. Acta Bioenerg. 2002, 1556, 273–277. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Ramanaviciene, A. Hemoproteins in design of biofuel cells. Fuel Cells 2009, 9, 25–36. [Google Scholar] [CrossRef]
- Xuan, J.; Jia, X.-D.; Jiang, L.-P.; Abdel-Halim, E.S.; Zhu, J.-J. Gold nanoparticle-assembled capsules and their application as hydrogen peroxide biosensor based on hemoglobin. Bioelectrochemistry 2012, 84, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xu, Y.; Cao, X. Hydrogen peroxide biosensor based on hemoglobin immobilized at graphene, flower-like zinc oxide, and gold nanoparticle nanocomposite modified glassy carbon electrode. Colloids Surf. B 2013, 107, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; El-Safty, S.A.; Khairy, M. Simple and Sensitive Electrochemical Sensor-Based Three-Dimensional Porous Ni-Hemoglobin Composite Electrode. Chemosensors 2014, 2, 235–250. [Google Scholar] [CrossRef]
- Tian, H.; Liang, F.; Jiao, J.; Hu, J. Direct Electrochemistry of Hemoglobin on Nickel Ion Implanted-Modified ITO Electrode and Biosensing for H2O2. J. Electrochem. Soc. 2013, 160, B125–B131. [Google Scholar] [CrossRef]
- Xu, M.-Q.; Wu, J.-F.; Zhao, G.-C. Direct Electrochemistry of Hemoglobin at a Graphene Gold Nanoparticle Composite Film for Nitric Oxide Biosensing. Sensors 2013, 13, 7492–7504. [Google Scholar] [CrossRef] [PubMed]
- German, N.; Ramanavicius, A.; Ramanaviciene, A. Electrochemical Deposition of Gold Nanoparticles on Graphite Rod for Glucose Biosensing. Sens. Actuators B Chem. 2014, 203, 25–34. [Google Scholar] [CrossRef]
- German, N.; Kausaite-Minkstimiene, A.; Ramanavicius, A.; Semashko, T.; Mikhailova, R.; Ramanaviciene, A. The Use of Different Glucose Oxidases for the Development of an Amperometric Reagentless Glucose Biosensor based on Gold Nanoparticles Covered by Polypyrrole. Electrochim. Acta 2015, 169, 326–333. [Google Scholar] [CrossRef]
- Seo, S.M.; Kang, T.J.; Kim, Y.; Kim, N.; Ahn, J.; Kim, T.W.; Kim, Y.H.; Ryu, S.H.; Park, Y.J. Electrode Asymmetry Driven Self-gating Effect on the Electrical Detection of Protein. Sens. Actuators B Chem. 2014, 191, 800–805. [Google Scholar] [CrossRef]
- Chornokur, G.; Arya, S.K.; Phelan, C.; Tanner, R.; Bhansali, S. Impedance-BasedMiniaturized Biosensor for Ultrasensitive and Fast Prostate-Specific Antigen Detection. J. Sens. 2011, 983751, 1–7. [Google Scholar] [CrossRef]
- Ben-Yoav, H.; Dykstra, P.H.; Bentley, W.E.; Ghodssi, R. A Microfluidic-Based Electrochemical Biochip for Label-Free Diffusion-Restricted DNA Hybridization Analysis. Biosens. Bioelectron. 2012, 38, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Kim, S.-U.; Min, J.; Choi, J.-W. Multi-Level Biomemory Device Consisting of Recombinant Azurin/ Cytochrome c. Adv. Mater. 2010, 22, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-H.; Lee, T.; Park, H.J.; Yun, W.S.; Min, J.; Choi, J.-W. Nanoscale biomemory composed of recombinant azurin on a nanogap electrode. Nanotechnology 2013, 24, 365301. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Yoo, S.-Y.; Chung, Y.-H.; Min, J.; Choi, J.-W. Signal enhancement of Electrochemical Biomemory Device Composed of Recombinant Azruin/Gold Nanoparticale. Electroanalysis 2011, 23, 2023–2029. [Google Scholar] [CrossRef]
- Lee, T.; Chung, Y.-H.; Yoon, J.; Min, J.; Choi, J.-W. Fusion Protein-based Dual-level Biomemory Device Consisted of Recombinant Azurin-Myoglobin. Appl. Surf. Sci. 2014, 329, 448–454. [Google Scholar] [CrossRef]
- Drescher, D.; Büchner, T.; McNaughton, D.; Kneipp, J. SERS reveals the specific interaction of silver and gold nanoparticles with hemoglobin and red blood cell components. Phys. Chem. Chem. Phys. 2013, 15, 5364–5373. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Si, M.; Zhu, Y.; Miao, L.; Xu, G. Surface-enhanced Raman scattering (SERS) spectra of hemoglobin of mouse and rabbit with self-assembled nano-silver film. Spectrochim. Acta Mol. Biomol. Spectrosc. 2013, 108, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Pingarron, J.M.; Yanez-Sedeno, P.; Gonzalez-Cortes, A. Gold Nanoparticle-Based Electrochemical Biosensors. Electrochimica Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- Laviron, E. General Expression of the Linear Potential Sweep Voltammogram in the Case of Diffusionless Electrochemical Systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.; Kim, T.-H.; Yoon, J.; Chung, Y.-H.; Lee, J.Y.; Choi, J.-W. Investigation of Hemoglobin/Gold Nanoparticle Heterolayer on Micro-Gap for Electrochemical Biosensor Application. Sensors 2016, 16, 660. https://doi.org/10.3390/s16050660
Lee T, Kim T-H, Yoon J, Chung Y-H, Lee JY, Choi J-W. Investigation of Hemoglobin/Gold Nanoparticle Heterolayer on Micro-Gap for Electrochemical Biosensor Application. Sensors. 2016; 16(5):660. https://doi.org/10.3390/s16050660
Chicago/Turabian StyleLee, Taek, Tae-Hyung Kim, Jinho Yoon, Yong-Ho Chung, Ji Young Lee, and Jeong-Woo Choi. 2016. "Investigation of Hemoglobin/Gold Nanoparticle Heterolayer on Micro-Gap for Electrochemical Biosensor Application" Sensors 16, no. 5: 660. https://doi.org/10.3390/s16050660
APA StyleLee, T., Kim, T.-H., Yoon, J., Chung, Y.-H., Lee, J. Y., & Choi, J.-W. (2016). Investigation of Hemoglobin/Gold Nanoparticle Heterolayer on Micro-Gap for Electrochemical Biosensor Application. Sensors, 16(5), 660. https://doi.org/10.3390/s16050660






