Developing an Efficient and General Strategy for Immobilization of Small Molecules onto Microarrays Using Isocyanate Chemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Proteins
2.2. Preparation of Isocyanate Functionalized Glass Slides
2.3. Fabrication of SMMs on Isocyanate Functionalized Glass Slides
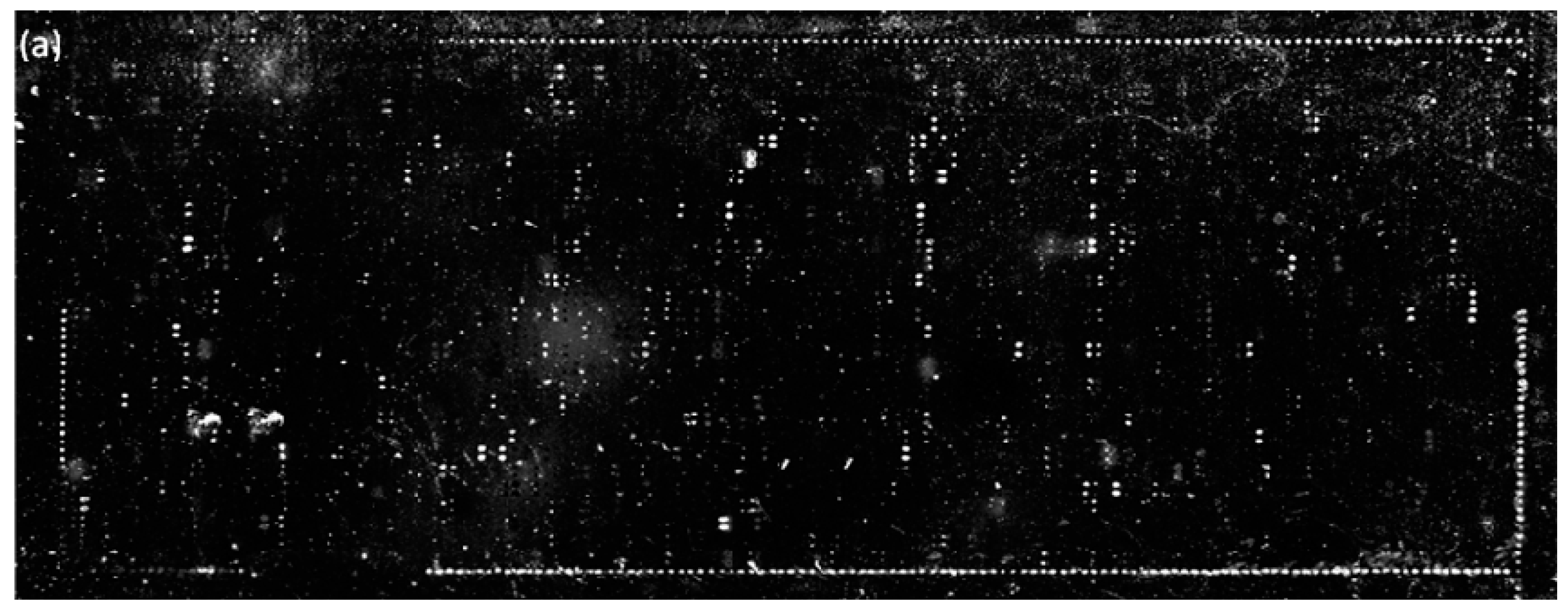
2.4. Detection of SMMs with a Label-Free Ellipsometry-Based Scanning Microscope
3. Results
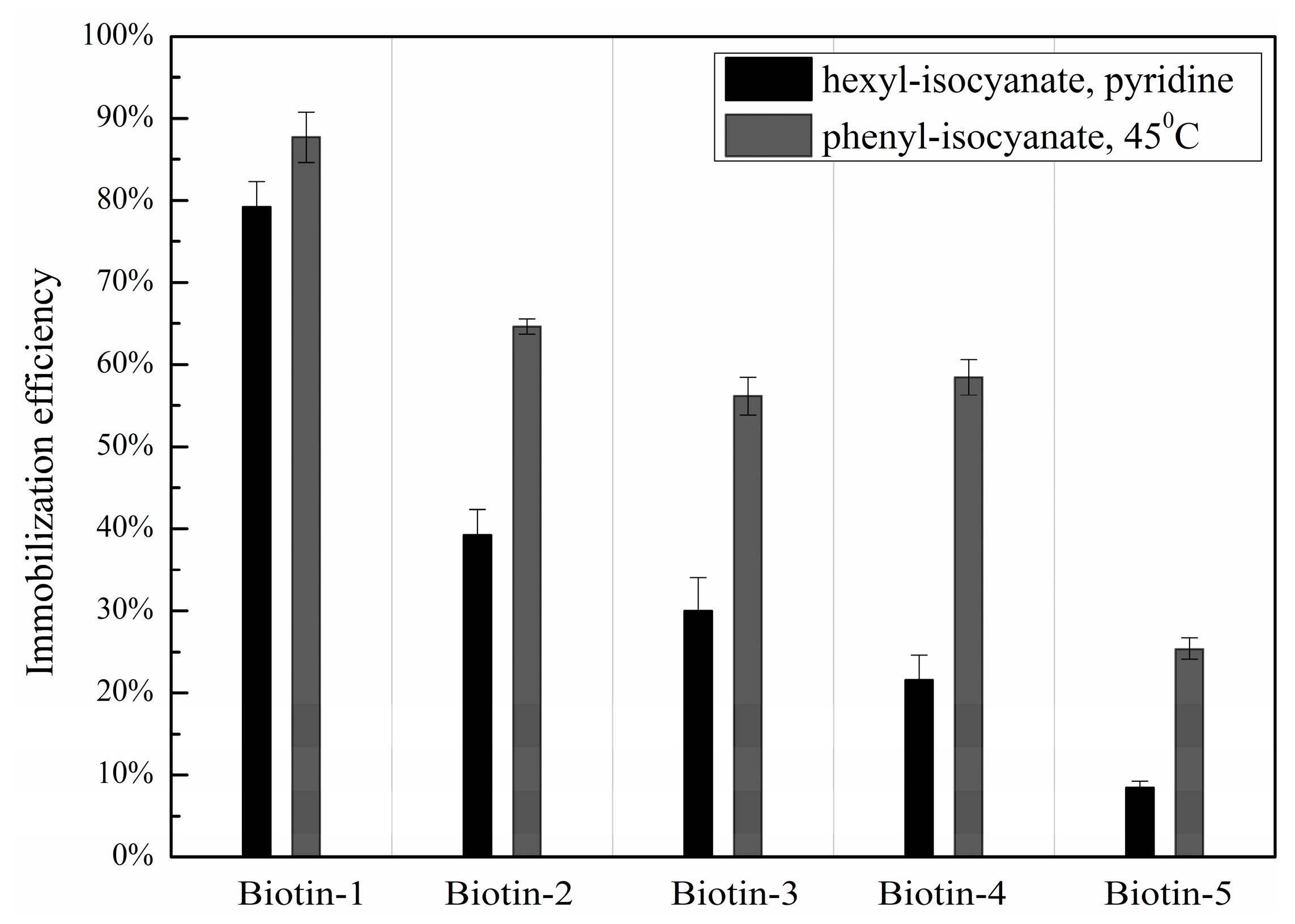
3.1. Immobilization Efficiencies of Compounds with Different Nucleophilic Residues Printed on Hexyl-Isocyanate Functionalized Slides
3.2. Immobilization Efficiencies and Spot Morphology of Printed Compounds vs. the Length of Spacer Molecule on Hexyl-Isocyanate Functionalized Surface
3.3. Dependence of Immobilization Efficiency on the Penultimate Chemical Group to the Isocyanate Residue and Post-Printing Treatment
3.4. Stability of Phenyl-Isocyanate Functionalized Slides in Storage
3.5. Immobilization of a Large Collection of Bioactive Compounds on Isocyanate Functionalized Glass Slides—Confirmation of the Benefit of the Optimal Procedures for SMM Fabrication
3.6. Screening Microarrays of~3000 Compounds for Protein Ligands—Further Confirmation of Improved Immobilization Efficiencies in Terms of Identified Ligands
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, H.Y.; Chen, G.Y.J.; Yao, S.Q. Recent advances in microarray technologies for proteomics. Chem. Biol. 2013, 20, 685–699. [Google Scholar] [CrossRef] [PubMed]
- He, X.Z.G.; Gerona-Navarro, G.; Jaffrey, S.R. Ligand discovery using small molecule microarrays. J. Pharmacol. Exp. Ther. 2005, 313, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.A.; Neel, D.V.; Wassaf, D.; Caballero, F.; Koehler, A.N. Recent discoveries and applications involving small-molecule microarrays. Curr. Opin. Chem. Biol. 2014, 18, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Uttamehandani, M.; Sun, H.Y.; Yao, S.Q. Small molecule microarrays: Applications using specially tagged chemical libraries. Qsar. Comb. Sci. 2006, 25, 1009–1019. [Google Scholar] [CrossRef]
- Casalena, D.; Wassaf, D.; Koehler, A. Ligand discovery using small-molecule microarrays. In Chemical proteomics; Drewes, G., Bantscheff, M., Eds.; Humana Press: New York, NY, USA, 2012; pp. 249–263. [Google Scholar]
- Zhang, B.; Jarrell, J.A.; Price, J.V.; Tabakman, S.M.; Li, Y.G.; Gong, M.; Hong, G.S.; Feng, J.; Utz, P.J.; Dai, H.J. An integrated peptide-antigen microarray on plasmonic gold films for sensitive human antibody profiling. PLoS One 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Lesaicherre, M.L.; Uttamchandani, M.; Chen, G.Y.J.; Yao, S.Q. Developing site-specific immobilization strategies of peptides in a microarray. Bioorg. Med. Chem. Lett. 2002, 12, 2079–2083. [Google Scholar] [CrossRef]
- Gao, L.Q.; Uttamchandani, M.; Yao, S.Q. Comparative proteomic profiling of mammalian cell lysates using phosphopeptide microarrays. Chem. Commun. 2012, 48, 2240–2242. [Google Scholar] [CrossRef] [PubMed]
- Uttamchandani, M.; Lee, W.L.; Wang, J.; Yao, S.Q. Quantitative inhibitor fingerprinting of metalloproteases using small molecule microarrays. J. Am. Chem. Soc. 2007, 129, 13110–13117. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Lu, C.H.S.; Shi, H.; Gao, L.Q.; Yao, S.Q. Peptide microarrays for high-throughput studies of ser/thr phosphatases. Nat. Protoc. 2008, 3, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Lee, S.S.; Chen, J.; Sun, H.; Zhao, Y.; Chai, Z.; Hu, Y. High-throughput screening of substrate specificity for protein tyrosine phosphatases (ptps) on phosphopeptide microarrays. Methods Mol. Biol. 2016, 1368, 181–196. [Google Scholar] [PubMed]
- Gao, L.; Sun, H.; Uttamchandani, M.; Yao, S.Q. Phosphopeptide microarrays for comparative proteomic profiling of cellular lysates. Methods Mol. Biol. 2013, 1002, 233–251. [Google Scholar] [PubMed]
- Gao, L.Q.; Sun, H.Y.; Yao, S.Q. Activity-based high-throughput determination of ptps substrate specificity using a phosphopeptide microarray. Biopolymers 2010, 94, 810–819. [Google Scholar] [CrossRef] [PubMed]
- MacBeath, G.; Koehler, A.N.; Schreiber, S.L. Printing small molecules as microarrays and detecting protein-ligand interactions en masse. J. Am. Chem. Soc. 1999, 121, 7967–7968. [Google Scholar] [CrossRef]
- Hergenrother, P.J.; Depew, K.M.; Schreiber, S.L. Small-molecule microarrays: Covalent attachment and screening of alcohol-containing small molecules on glass slides. J. Am. Chem. Soc. 2000, 122, 7849–7850. [Google Scholar] [CrossRef]
- Barnes-Seeman, D.; Park, S.B.; Koehler, A.N.; Schreiber, S.L. Expanding the functional group compatibility of small-molecule microarrays: Discovery of novel calmodulin ligands. Angew. Chem. Int.Edit. 2003, 42, 2376–2379. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Shin, I. Fabrication of chemical microarrays by efficient immobilization of hydrazide-linked substances on epoxide-coated glass surfaces. Angew. Chem. Int.Edit. 2005, 44, 2881–2884. [Google Scholar] [CrossRef] [PubMed]
- Fazio, F.; Bryan, M.C.; Blixt, O.; Paulson, J.C.; Wong, C.H. Synthesis of sugar arrays in microtiter plate. J. Am. Chem. Soc. 2002, 124, 14397–14402. [Google Scholar] [CrossRef] [PubMed]
- Kohn, M.; Wacker, R.; Peters, C.; Schroder, H.; Soulere, L.; Breinbauer, R.; Niemeyer, C.M.; Waldmann, H. Staudinger ligation: A new immobilization strategy for the preparation of small-molecule arrays. Angew. Chem. Int. Edit. 2003, 42, 5830–5834. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.S.; Jaipuri, F.A.; Pohl, N.L. Fluorous-based carbohydrate microarrays. J. Am. Chem. Soc. 2005, 127, 13162–13163. [Google Scholar] [CrossRef] [PubMed]
- Urbina, H.D.; Debaene, F.; Jost, B.; Bole-Feysot, C.; Mason, D.E.; Kuzmic, P.; Harris, J.L.; Winssinger, N. Self-assembled small-molecule microarrays for protease screening and profiling. ChemBioChem 2006, 7, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, N.; Kumashiro, S.; Simizu, S.; Kondoh, Y.; Hatakeyama, S.; Tashiro, H.; Osada, H. Immobilization of natural products on glass slides by using a photoaffinity reaction and the detection of protein-small-molecule interactions. Angew. Chem. Int.Edit. 2003, 42, 5584–5587. [Google Scholar] [CrossRef] [PubMed]
- Bradner, J.E.; McPherson, O.M.; Mazitschek, R.; Barnes-Seeman, D.; Shen, J.P.; Dhaliwal, J.; Stevenson, K.E.; Duffner, J.L.; Park, S.B.; Neuberg, D.S.; et al. A robust small-molecule microarray platform for screening cell lysates. Chem. Biol. 2006, 13, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Kurosu, M.; Mowers, W.A. Small-molecule microarrays: Development of novel linkers and an efficient detection method for bound proteins. Bioorg. Med. Chem. Lett. 2006, 16, 3392–3395. [Google Scholar] [CrossRef] [PubMed]
- Bradner, J.E.; McPherson, O.M.; Koehler, A.N. A method for the covalent capture and screening of diverse small molecules in a microarray format. Nat. Protoc. 2006, 1, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Park, S.B. Surface modification for small-molecule microarrays and its application to the discovery of a tyrosinase inhibitor. Mol. Biosys. 2011, 7, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Landry, J.P.; Fei, Y.Y.; Zhu, X.D.; Ke, Y.H.; Yu, G.L.; Lee, P. Discovering small molecule ligands of vascular endothelial growth factor that block VEGF-KDR binding using label-free microarray-based assays. Assay Drug Dev. Technol. 2013, 11, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Armstrong, A.H.; Koehler, A.N.; Hecht, M.H. Small molecule microarrays enable the discovery of compounds that bind the alzheimer's a beta peptide and reduce its cytotoxicity. J. Am. Chem. Soc. 2010, 132, 17015–17022. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Paz, J.L.; Gonzalez-Martinez, M.A.; Escorihuela, J.; Banuls, M.J.; Puchades, R.; Maquieira, A. Direct and label-free monitoring oligonucleotide immobilization, non-specific binding and DNA biorecognition. Sens. Actuator B-Chem. 2014, 192, 221–228. [Google Scholar] [CrossRef]
- Vigano, M.; Levi, M.; Turri, S.; Chiari, M.; Damin, F. New copolymers of n,n-dimethylacrylamide with blocked isocyancates for oligonucleotide immobilization in DNA microarray technology. Polymer 2007, 48, 4055–4062. [Google Scholar] [CrossRef]
- Xu, Y.L.; Xu, H.J.; Jiang, X.S.; Yin, J. Versatile functionalization of the micropatterned hydrogel of hyperbranched poly(ether amine) based on "thiol-yne" chemistry. Adv. Funct. Mater. 2014, 24, 1679–1686. [Google Scholar] [CrossRef]
- Vigano, M.; Suriano, R.; Levi, M.; Turri, S.; Chiari, M.; Damin, F. Glass silanization with blocked-isocyanate for the fabrication of DNA microarrays. Surf. Sci. 2007, 601, 1365–1370. [Google Scholar] [CrossRef]
- Fei, Y.Y.; Schmidt, A.; Bylund, G.; Johansson, D.X.; Henriksson, S.; Lebrilla, C.; Solnick, J.V.; Boren, T.; Zhu, X.D. Use of real-time, label-free analysis in revealing low-affinity binding to blood group antigens by helicobacter pylori. Anal. Chem. 2011, 83, 6336–6341. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.Y.; Landry, J.P.; Sun, Y.S.; Zhu, X.D.; Luo, J.T.; Wang, X.B.; Lam, K.S. A novel high-throughput scanning microscope for label-free detection of protein and small-molecule chemical microarrays. Rev. Sci. Instrum. 2008, 79. [Google Scholar] [CrossRef] [PubMed]
- Landry, J.P.; Fei, Y.; Zhu, X. Simultaneous measurement of 10,000 protein-ligand affinity constants using microarray-based kinetic constant assays. Assay Drug Dev. Technol. 2012, 10, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Sun, Y.; Li, Y.; Yu, H.; Lau, K.; Landry, J.; Luo, Z.; Baumgarth, N.; Chen, X.; Zhu, X. Characterization of receptor binding profiles of influenza a viruses using an ellipsometry-based label-free glycan microarray assay platform. Biomolecules 2015, 5, 1480–1489. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, J.H.; He, L.P.; Dai, J.; Lu, H.B.; Wu, L.; Jin, K.J.; Yang, G.Z.; Zhu, H. Label-free, real-time detection of the dynamic processes of protein degradation using oblique-incidence reflectivity difference method. Appl. Phys. Lett. 2014, 104. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.S.; Landry, J.P.; Fei, Y.Y.; Zhu, X.D.; Luo, J.T.; Wang, X.B.; Lam, K.S. Macromolecular scaffolds for immobilizing small molecule microarrays in label-free detection of protein-ligand interactions on solid support. Anal. Chem. 2009, 81, 5373–5380. [Google Scholar] [CrossRef] [PubMed]
- Golander, C.G.; Kiss, E. Protein adsorption on functionalized and esca-characterized polymer-films studied by ellipsometry. J. Colloid Interface Sci. 1988, 121, 240–253. [Google Scholar] [CrossRef]
- Sun, Y.S.; Landry, J.P.; Fei, Y.Y.; Zhu, X.D. Effect of fluorescently labeling protein probes on kinetics of protein-ligand reactions. Langmuir 2008, 24, 13399–13405. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Otsuka, H.; Kaneko, M.; Kataoka, K.; Nagasaki, Y. A reactive poly(ethylene glycol) layer to achieve specific surface plasmon resonance sensing with a high s/n ratio: The substantial role of a short underbrushed peg layer in minimizing nonspecific adsorption. Anal. Chem. 2005, 77, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Torres-Lugo, M.; Garcia, M.; Record, R.; Peppas, N.A. Physicochemical behavior and cytotoxic effects of p(methacrylic acid-g-ethylene glycol) nanospheres for oral delivery of proteins. J. Controlled Release 2002, 80, 197–205. [Google Scholar] [CrossRef]

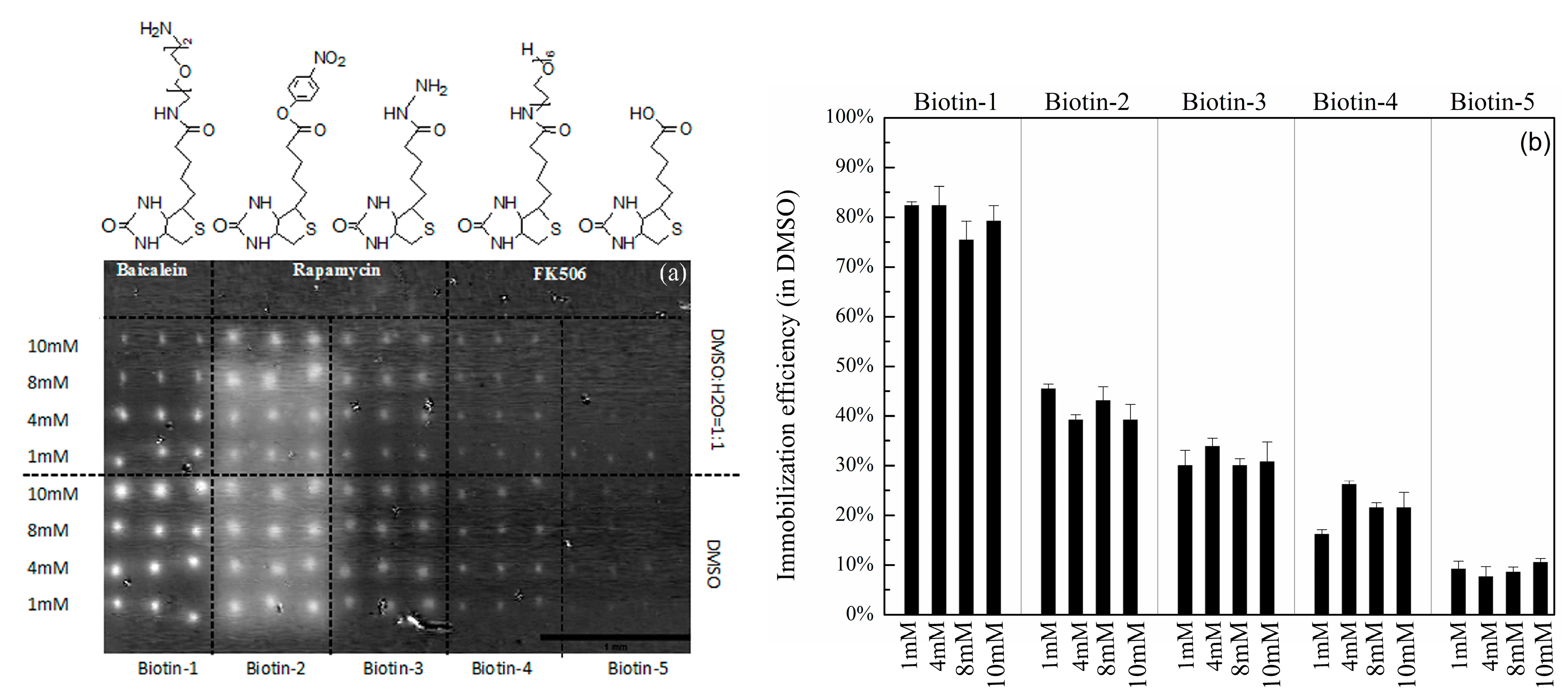
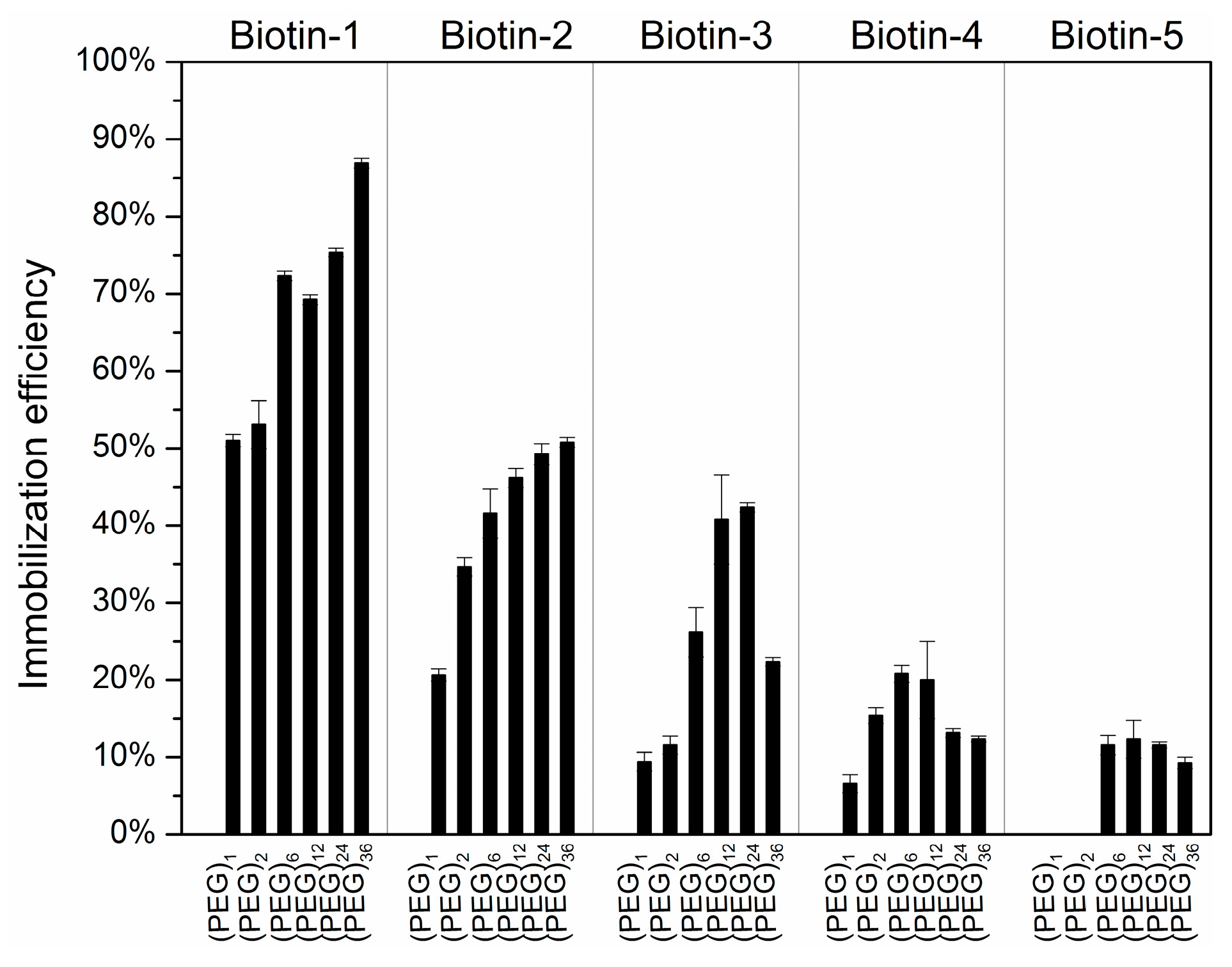
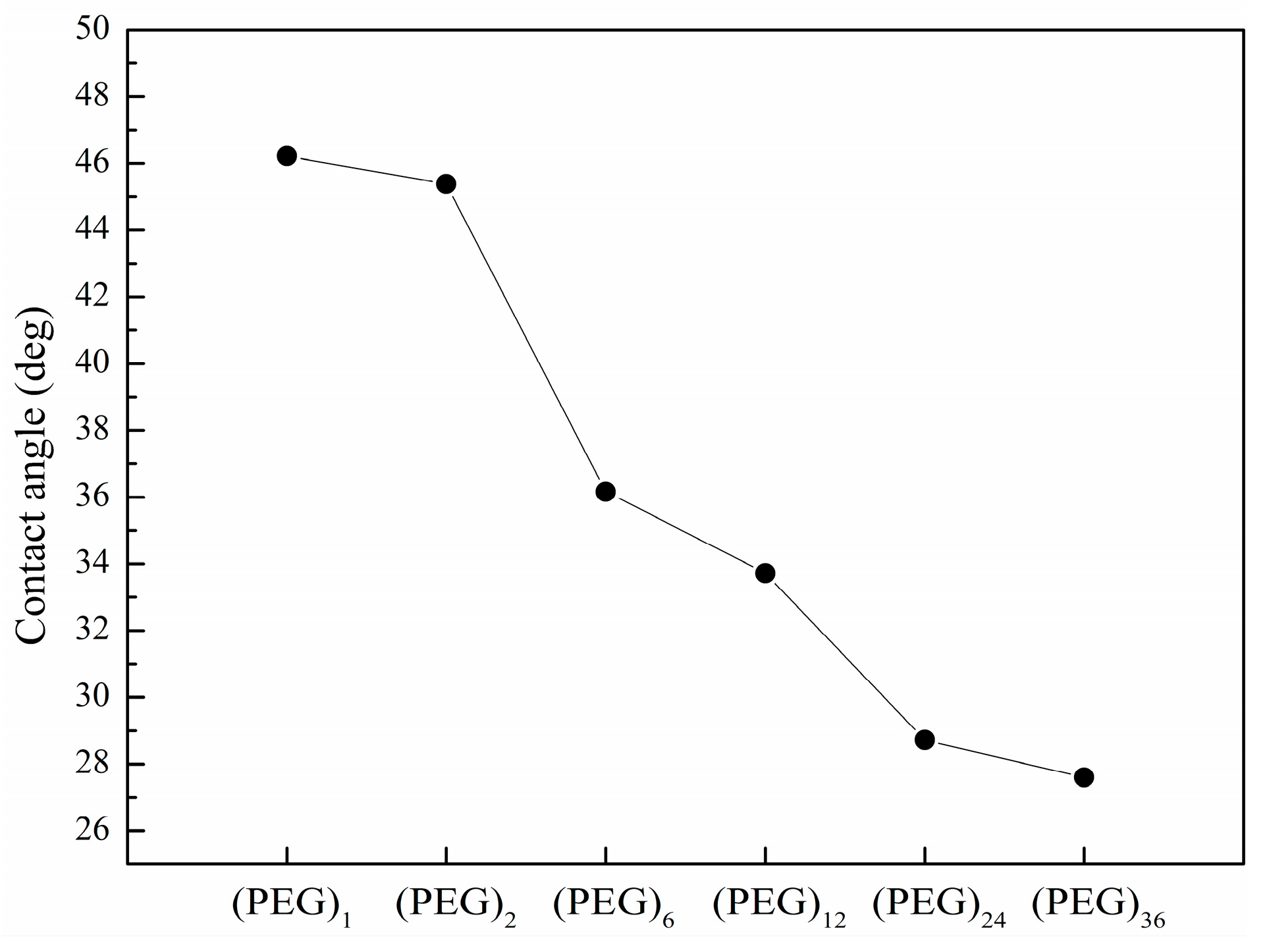
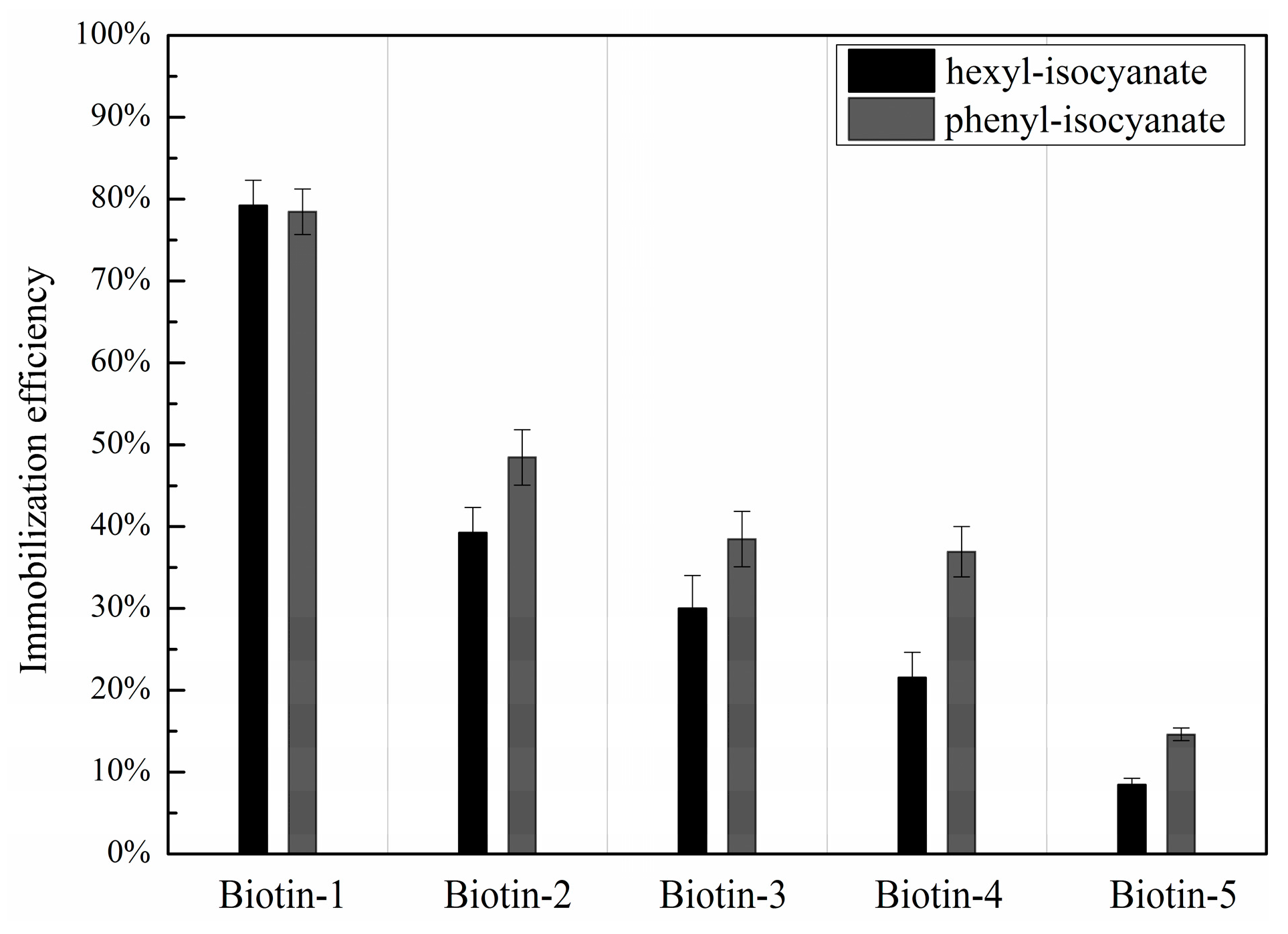


| Compound Names | Compound Structures | Rectangular Color |
|---|---|---|
| NVP-AEW541 | 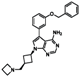 | red |
| WY-14643 | 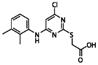 | green |
| Arbidol HCl | 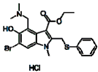 | blue |
| Compound Names | Compound Structures | SAVD Coverage on Phenyl-Isocyanate Surface | SAVD Coverage on Hexyl-Isocyanate Surface |
|---|---|---|---|
| BMS 777607 | 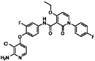 | 0.85 | 0.54 |
| JNJ-7706621 | 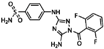 | 0.85 | 0.28 |
| Biotin-NH2 | 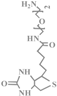 | 0.73 | 0.61 |
| Azelnidipine | 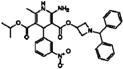 | 0.64 | 0.28 |
| Amfenac sodium monohydrate | 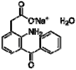 | 0.64 | 0.64 |
| Dryocrassin | 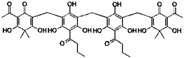 | 0.61 | 0.45 |
| Magnolol | 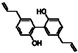 | 0.58 | 0.39 |
| LDN193189 | 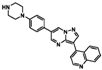 | 0.52 | 0.36 |
| Manidipine | 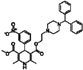 | 0.51 | 0.45 |
| Tyrphostin AG 879 | 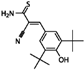 | 0.48 | 0.48 |
| Honokiol | 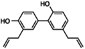 | 0.45 | 0.36 |
| NVP-BSK805 | 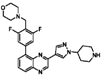 | 0.39 | 0.33 |
| PF-04929113 | 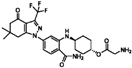 | 0.33 | 0.27 |
| Flupirtine maleate | 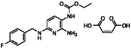 | 0.30 | 0.27 |
| Linifanib (ABT-869) | 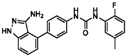 | 0.27 | 0.18 |
| Sciadopitysin | 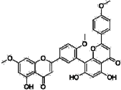 | 0.36 | 0 |
| ABT-263 (Navitoclax) | 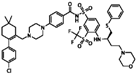 | 0.33 | 0 |
| GW4064 | 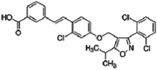 | 0.29 | 0 |
| MLN8054 | 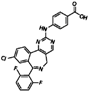 | 0.27 | 0 |
| Flunarizine dihydrochlor |  | 0.26 | 0 |
| Nomilin |  | 0.25 | 0 |
| Liothyronine sodium |  | 0.18 | 0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Zhu, X.; Landry, J.P.; Cui, Z.; Li, Q.; Dang, Y.; Mi, L.; Zheng, F.; Fei, Y. Developing an Efficient and General Strategy for Immobilization of Small Molecules onto Microarrays Using Isocyanate Chemistry. Sensors 2016, 16, 378. https://doi.org/10.3390/s16030378
Zhu C, Zhu X, Landry JP, Cui Z, Li Q, Dang Y, Mi L, Zheng F, Fei Y. Developing an Efficient and General Strategy for Immobilization of Small Molecules onto Microarrays Using Isocyanate Chemistry. Sensors. 2016; 16(3):378. https://doi.org/10.3390/s16030378
Chicago/Turabian StyleZhu, Chenggang, Xiangdong Zhu, James P. Landry, Zhaomeng Cui, Quanfu Li, Yongjun Dang, Lan Mi, Fengyun Zheng, and Yiyan Fei. 2016. "Developing an Efficient and General Strategy for Immobilization of Small Molecules onto Microarrays Using Isocyanate Chemistry" Sensors 16, no. 3: 378. https://doi.org/10.3390/s16030378
APA StyleZhu, C., Zhu, X., Landry, J. P., Cui, Z., Li, Q., Dang, Y., Mi, L., Zheng, F., & Fei, Y. (2016). Developing an Efficient and General Strategy for Immobilization of Small Molecules onto Microarrays Using Isocyanate Chemistry. Sensors, 16(3), 378. https://doi.org/10.3390/s16030378






