A Portable Array-Type Optical Fiber Sensing Instrument for Real-Time Gas Detection
Abstract
:1. Introduction
2. Principle of Fiber Sensing
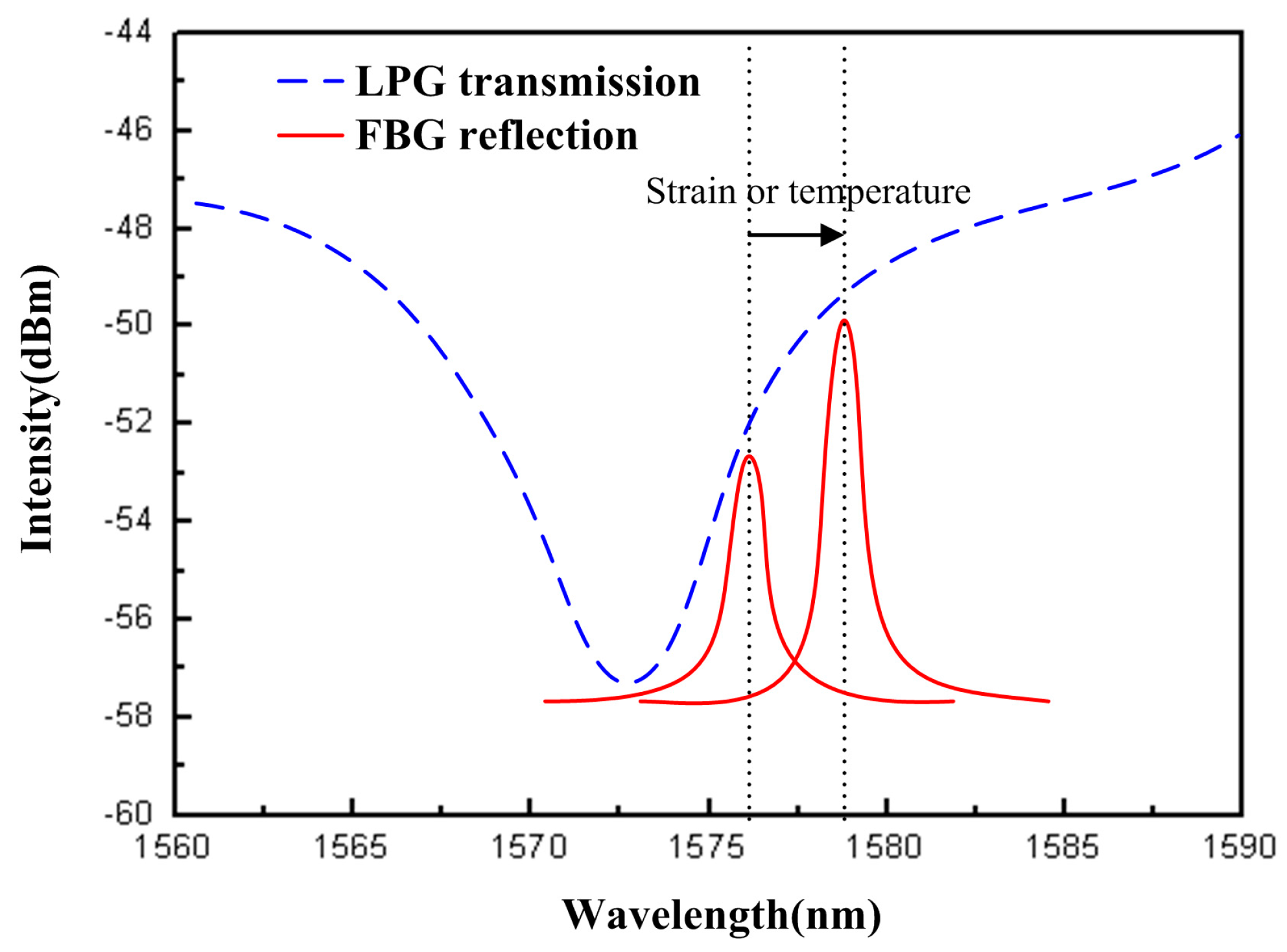
2.1. Principle of Bragg Grating Sensing
2.2. Long-Period Fiber Grating
2.3. Fluorescent Reaction
3. Fabrication of the Gas Sensing Array
3.1. Sol-Gel Method
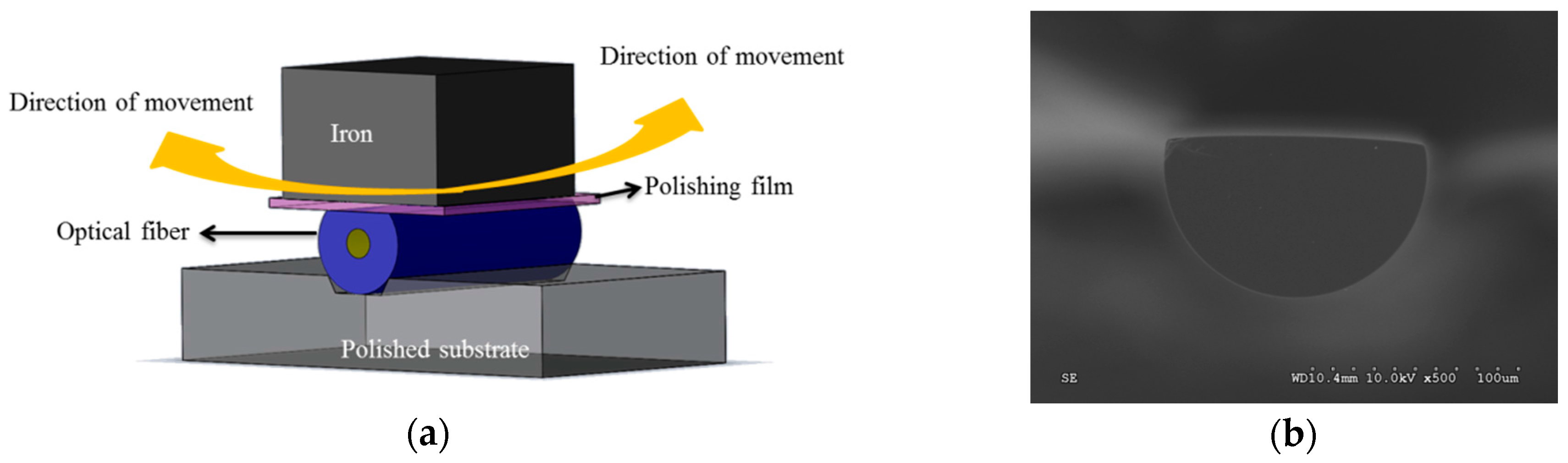
3.2. Nano Film Preparation
3.3. CO2 and O2 Sensors
3.4. Temperature and NH3 Sensors
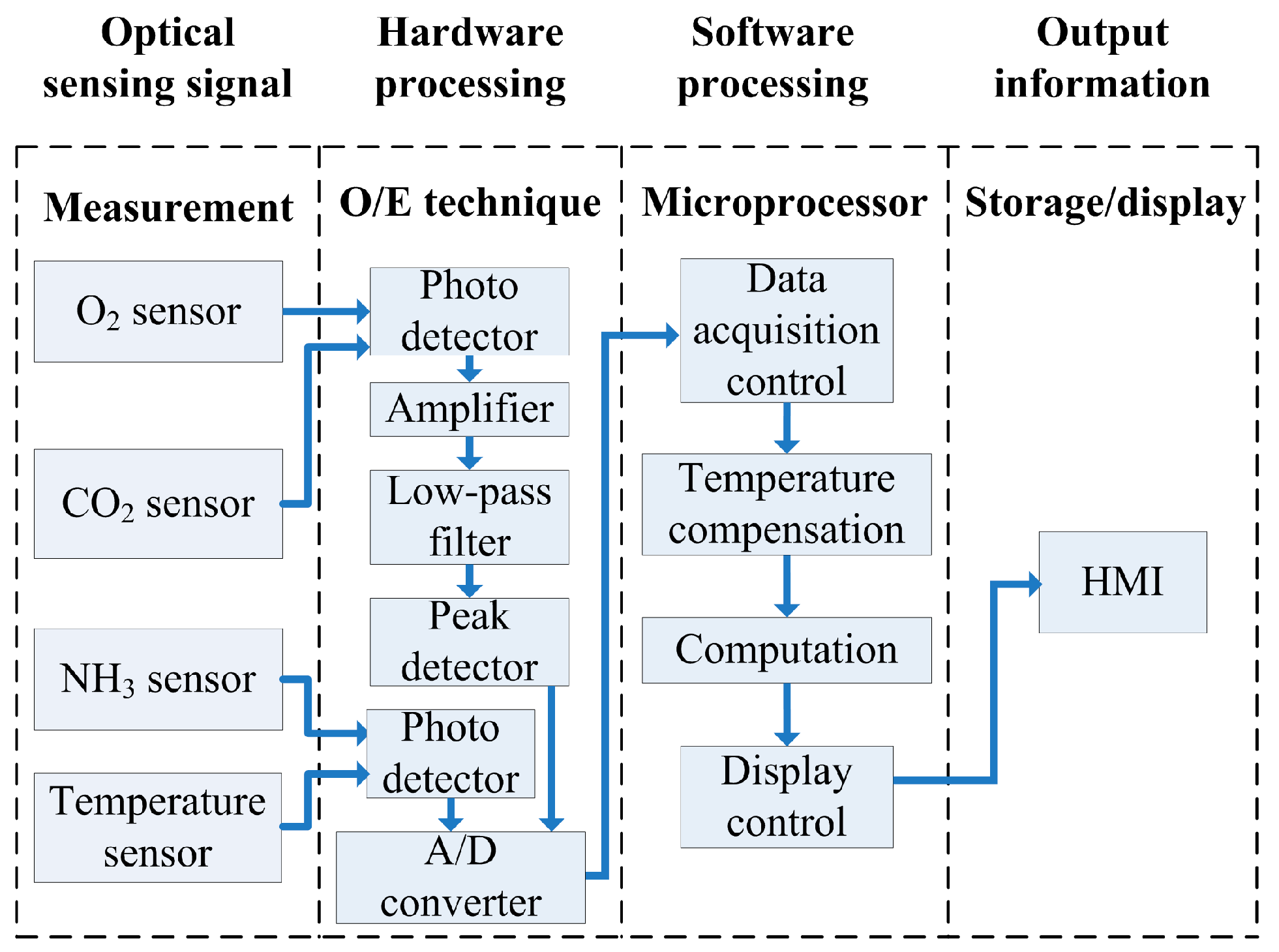
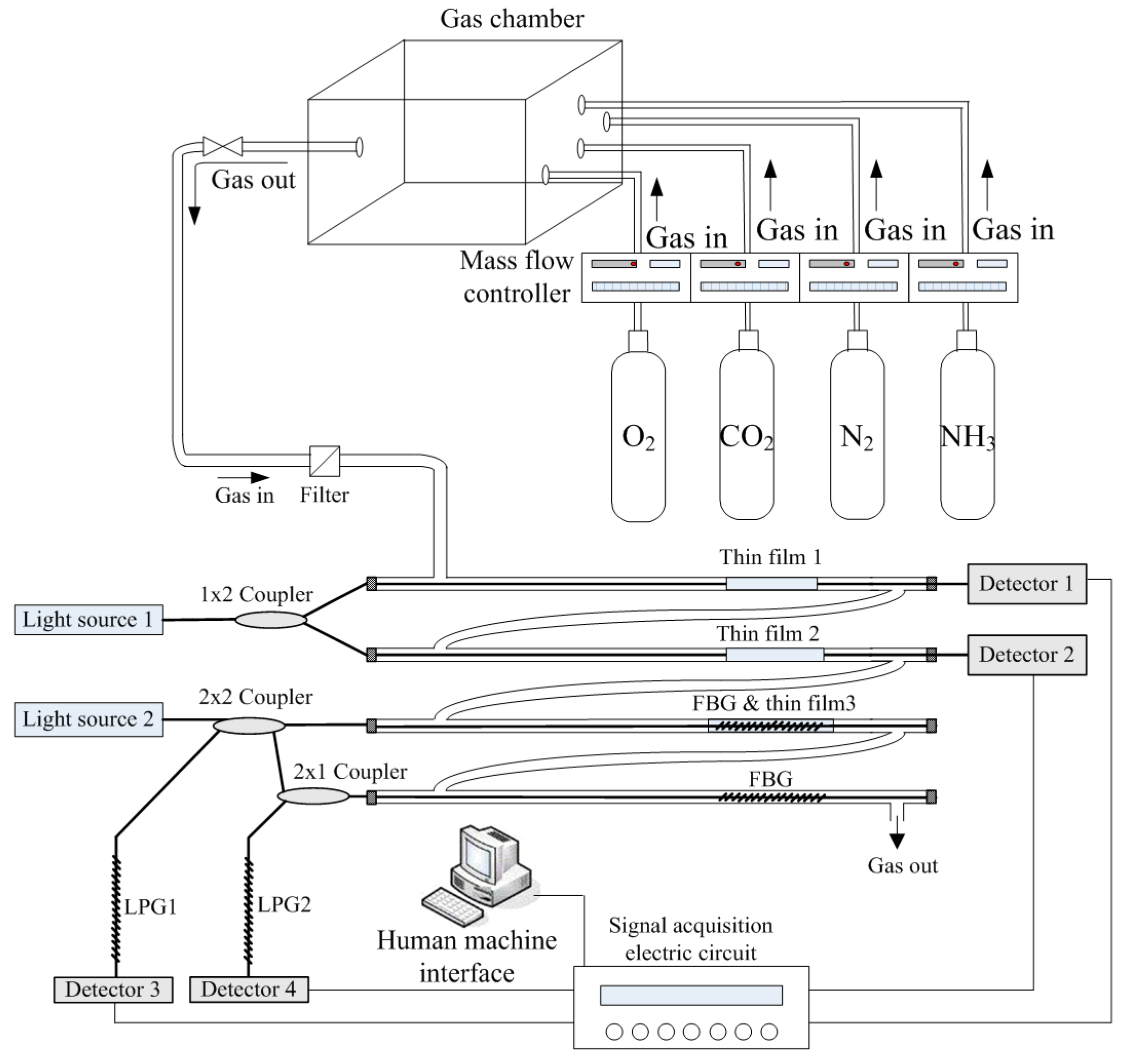
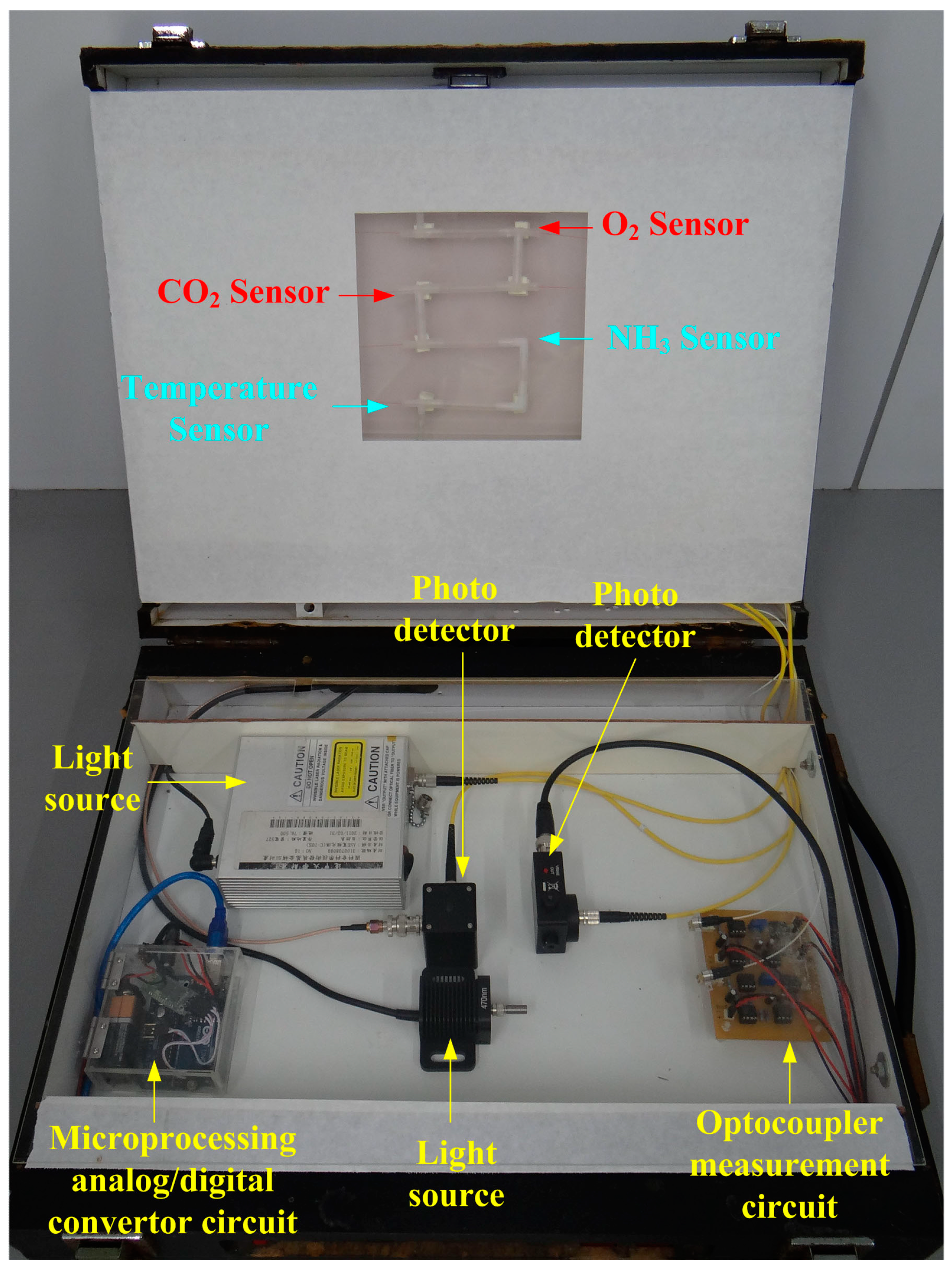
3.5. Design of Measurement System
4. Measurement Analysis and Discussion
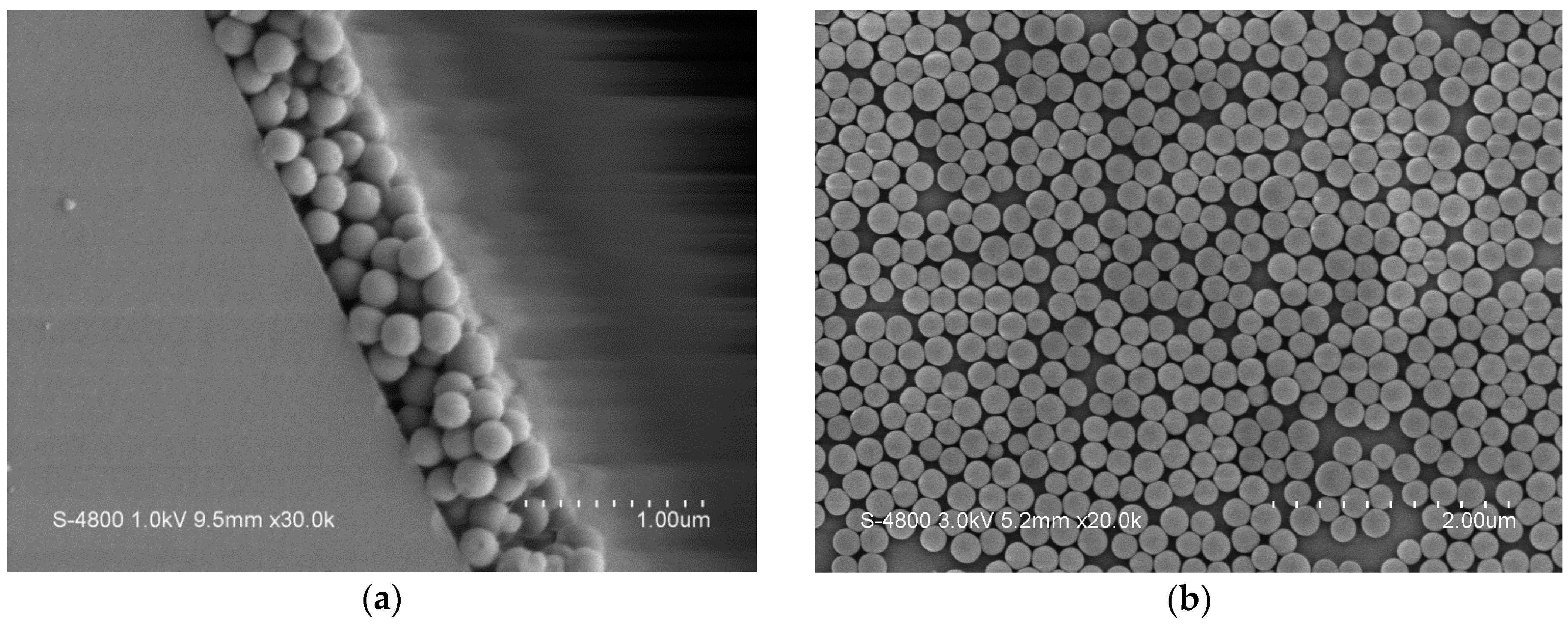
4.1. Analysis on Nano Sensing Structure
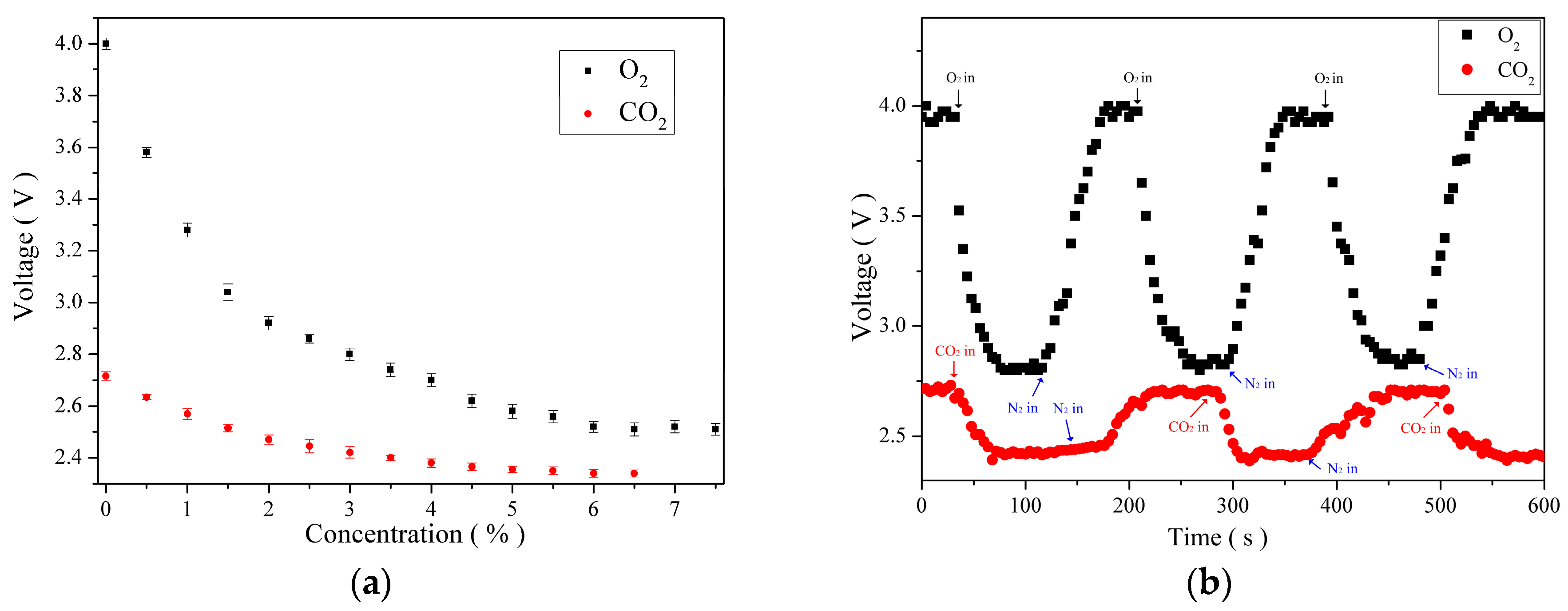
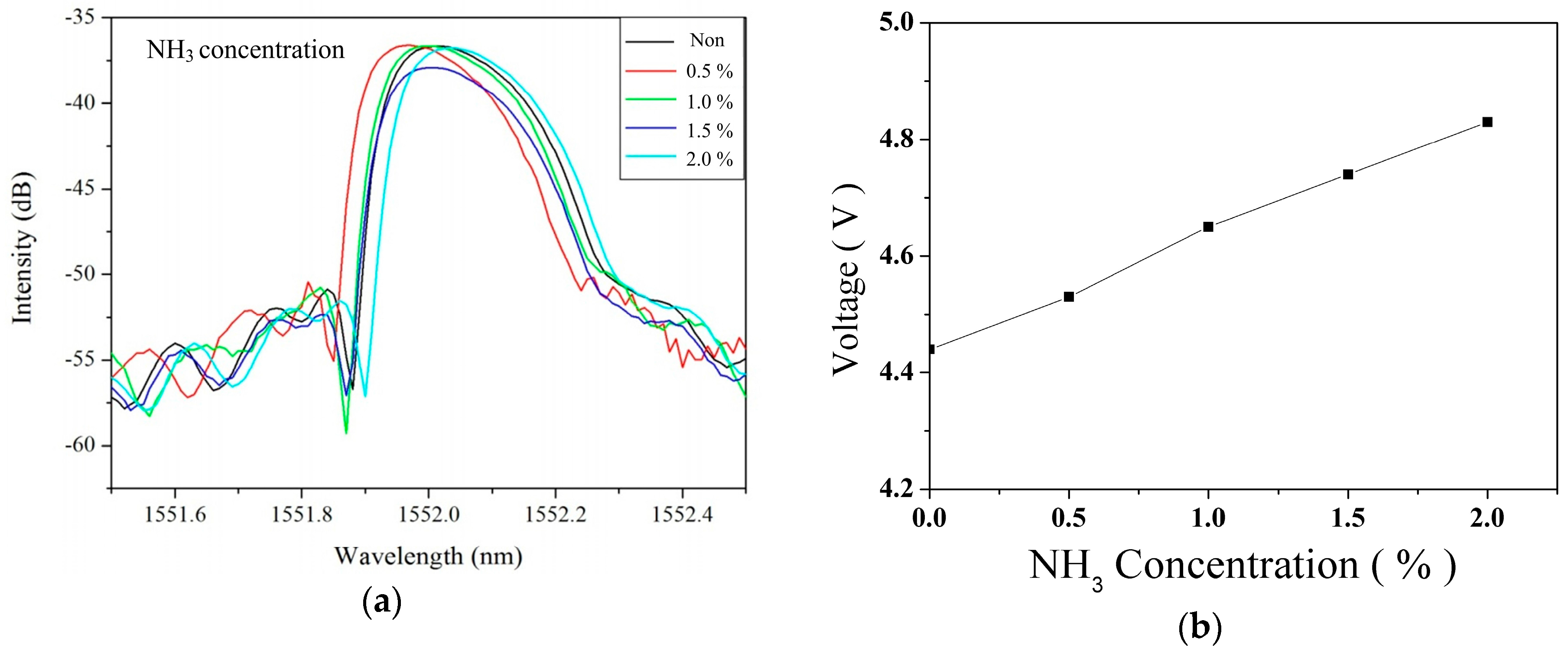
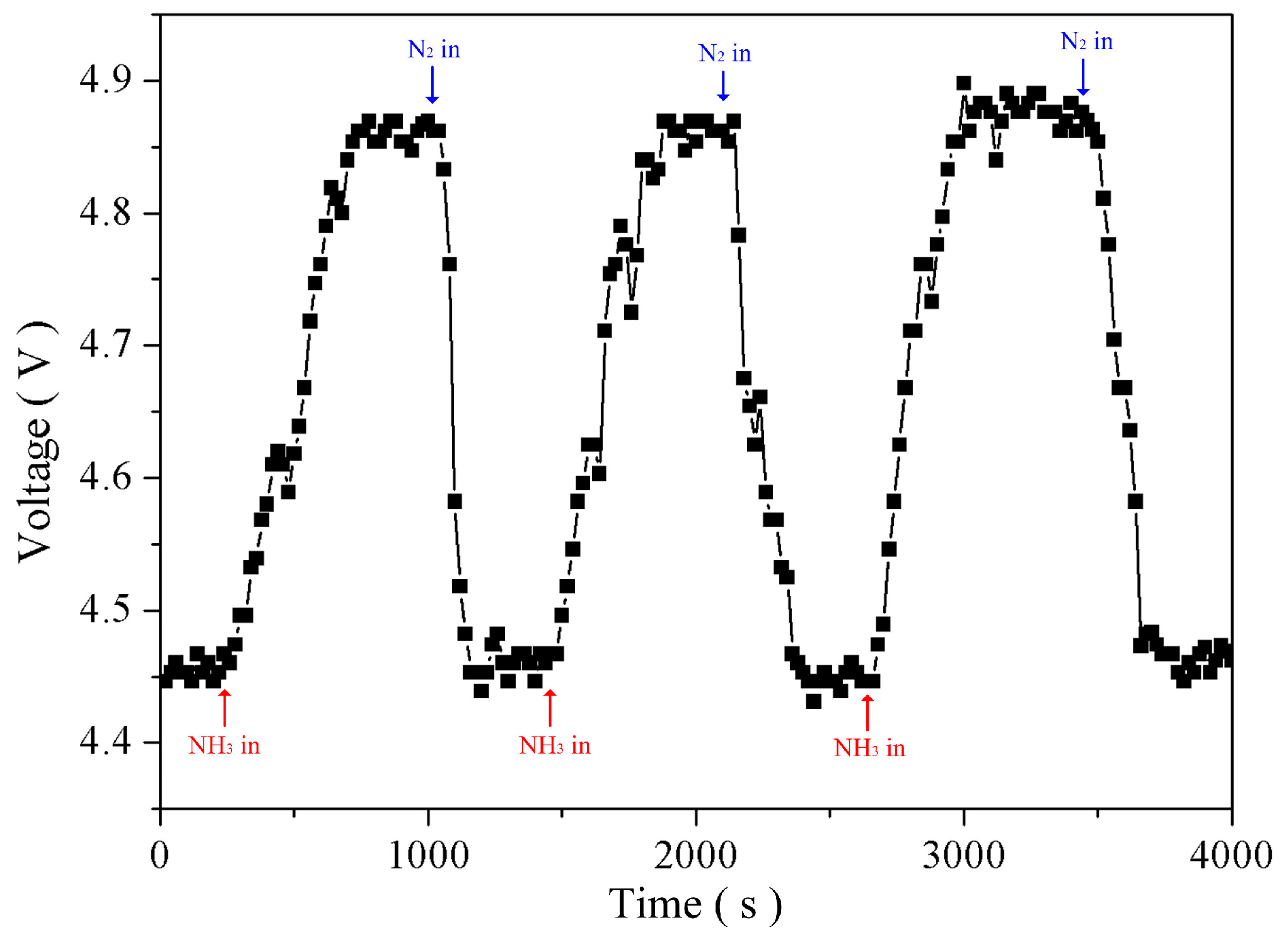
4.2. Gas Sensing Measurement and Analysis
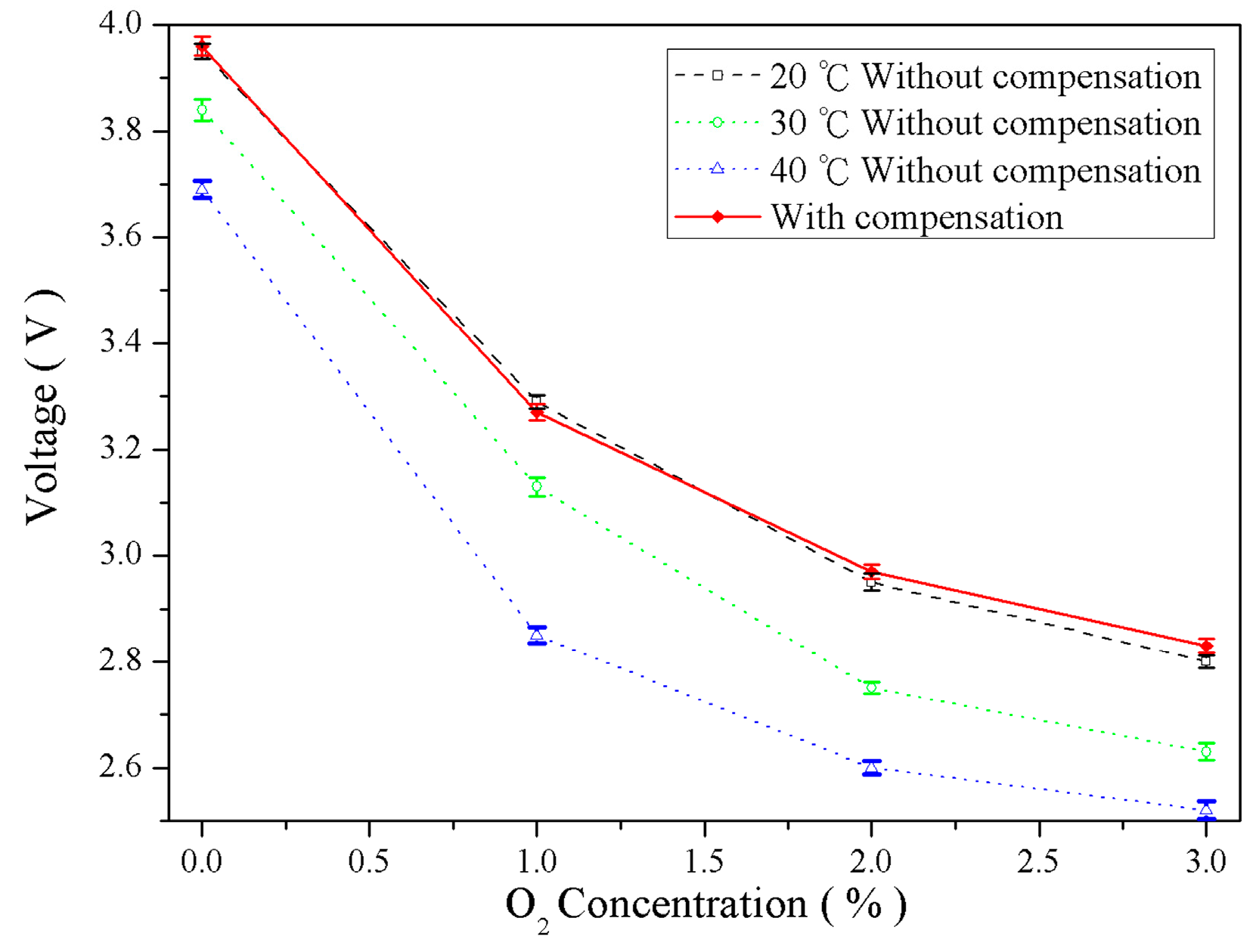
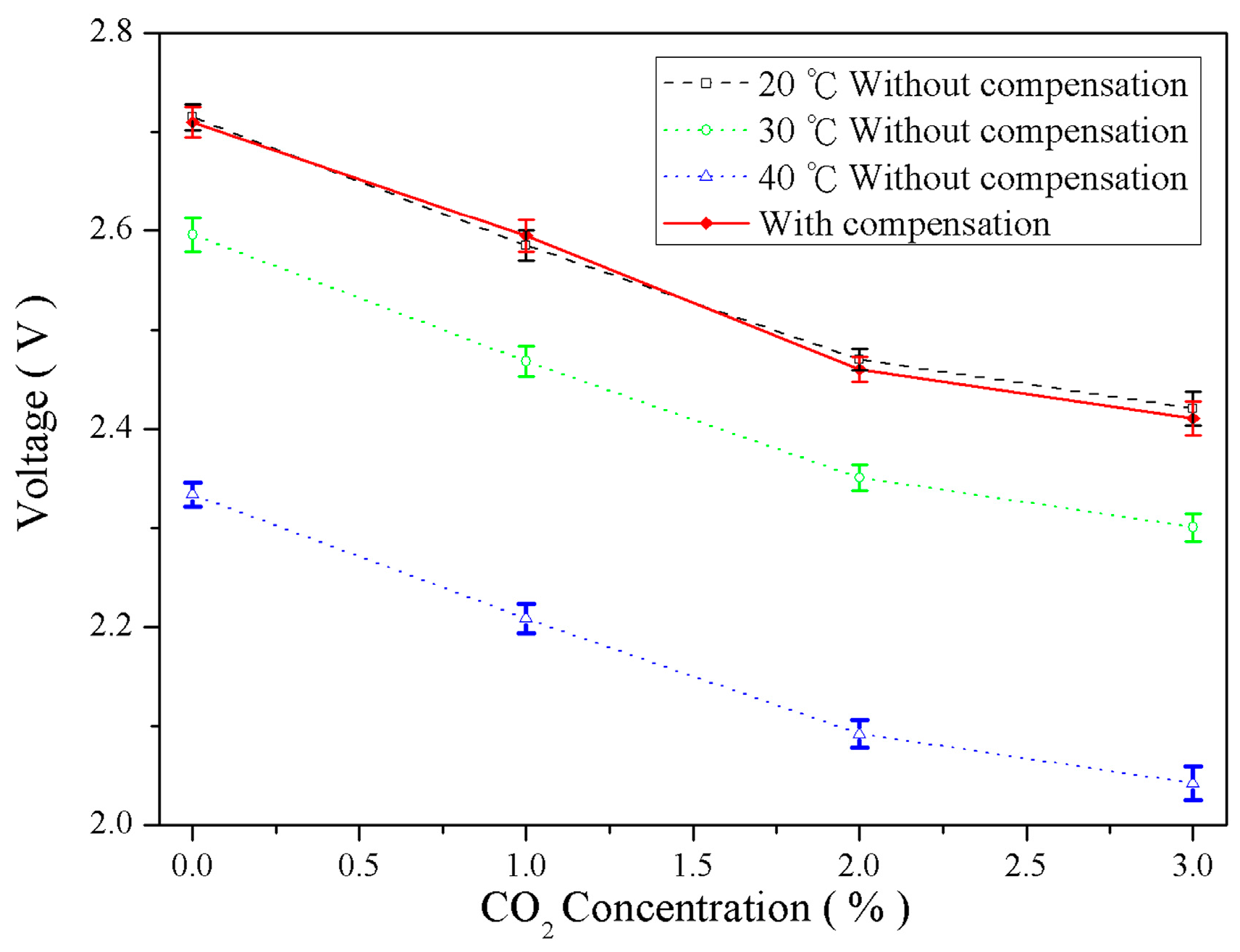
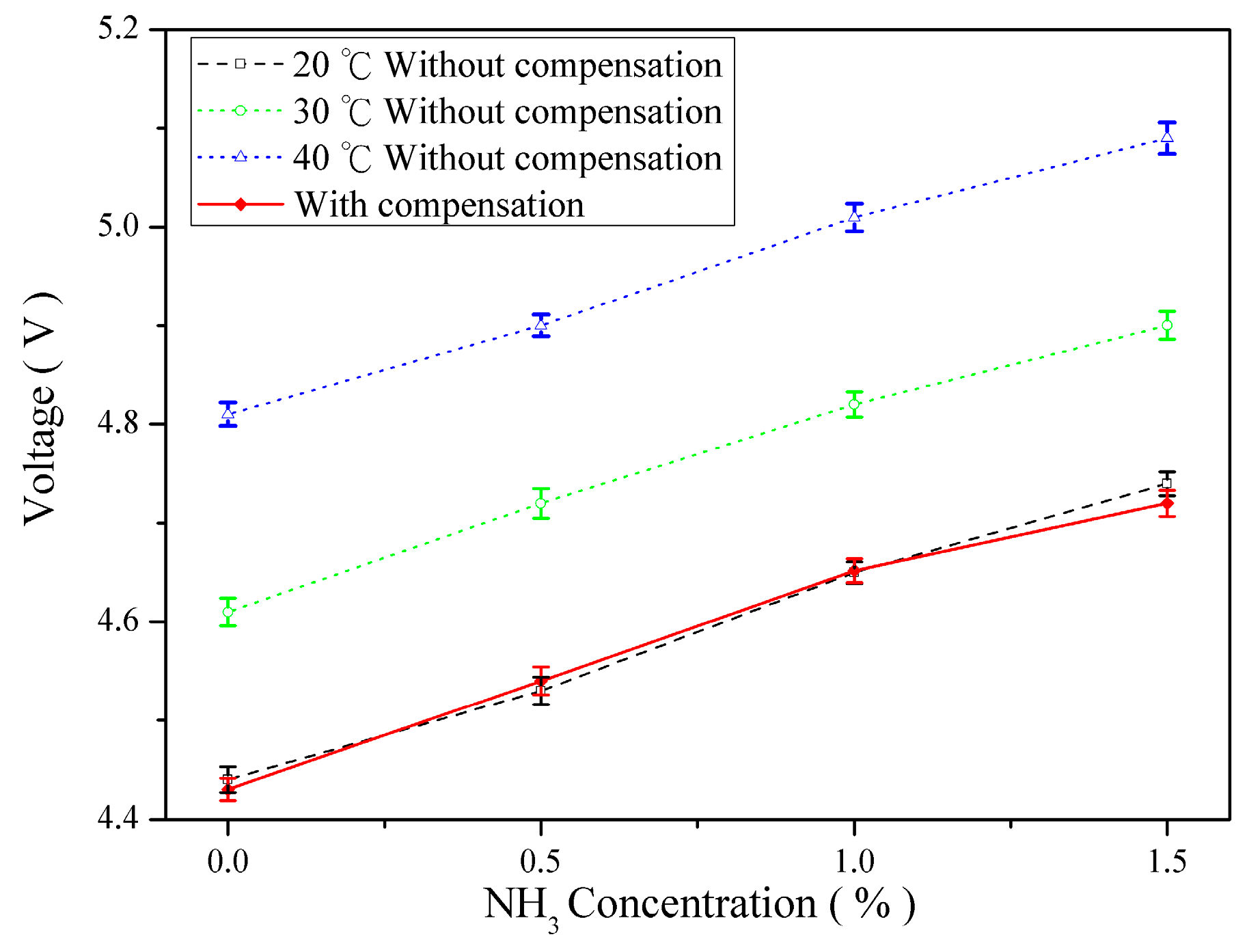
4.3. Temperature Compensation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jane, H.; Ralph, P.T. Optical gas sensor: A review. Meas. Sci. Technol. 2013, 24, 012004. [Google Scholar]
- McDonagh, C.; Kolle, C.; McEvoy, A.K.; Dowling, D.L.; Cafolla, A.A.; Cullen, S.J.; MacCraith, B.D. Phase fluorometric dissolved oxygen sensor. Sens. Actuators B Chem. 2001, 74, 124–130. [Google Scholar] [CrossRef]
- Gu, Z.T.; Xu, Y.P. Design optimization of a long-period fiber grating with sol–gel coating for a gas sensor. Meas. Sci. Technol. 2007, 18, 3530–3536. [Google Scholar] [CrossRef]
- Von Bultzingslowen, C.; McEvoy, A.K.; McDonagh, C.; MacCraith, B.D.; Klimant, I.; Krause, C.; Wolfbeis, O.S. Sol-gel based optical carbon dioxide sensor employing dual luminophore referencing for application in food packaging technology. Analyst 2002, 127, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.S.; Lo, Y.L. Highly sensitive and linear optical fiber carbon dioxide sensor based on sol-gel matrix doped with silica particles and HPTS. Sens. Actuators B Chem. 2009, 143, 205–210. [Google Scholar] [CrossRef]
- Mendes, L.B.; Ogink, N.W.M.; Edouard, N.; van Dooren, H.J.C.; Tinoco, I.D.F.; Mosquera, J. NDIR gas sensor for spatial monitoring of carbon dioxide concentrations in naturally ventilated livestock buildings. Sensors 2015, 15, 11239–11257. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.; MacGregor, C. A novel solid state non-dispersive infrared CO2 gas sensor compatible with wireless and portable deployment. Sensors 2013, 13, 7079–7103. [Google Scholar] [CrossRef] [PubMed]
- Vallon, R.; Soutade, J.; Verant, J.L.; Meyers, J.; Paris, S.; Mohamed, A. A compact tunable diode laser absorption spectrometer to monitor CO2 at 2.7 μm wavelength in hypersonic flows. Sensors 2010, 10, 6081–6091. [Google Scholar] [CrossRef] [PubMed]
- Renganathan, B.; Sastikumar, D.; Gobi, G.; Yogamalar, N.R.; Bose, A.C. Nanocrystalline ZnO coated fiber optic sensor for ammonia gas detection. Opt. Laser Technol. 2011, 43, 1398–1404. [Google Scholar] [CrossRef]
- Chu, C.S.; Lo, Y.L. A plastic optical fiber sensor for the dual sensing of temperature and oxygen. IEEE Photonics Technol. Lett. 2008, 20, 63–65. [Google Scholar] [CrossRef]
- Mishra, S.K.; Bhardwaj, S.; Gupta, B.D. Surface plasmon resonance-based fiber optic sensor for the detection of low concentrations of ammonia gas. IEEE Sens. J. 2015, 15, 1235–1239. [Google Scholar] [CrossRef]
- Rodriguez, A.J.; Zamarreno, C.R.; Matias, I.R.; Arregui, F.J.; Cruz, R.F.D.; May-Arrioja, D.A. A fiber optic ammonia sensor using a universal pH indicator. Sensors 2014, 14, 4060–4073. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.O.; Fujii, Y.; Johnson, D.C.; Kawasaki, B.S. Photosensitivity in optical fiber waveguides: Application to reflection filter fabrication. Appl. Phys. Lett. 1987, 32, 647–649. [Google Scholar] [CrossRef]
- Hill, K.O.; Malo, B.; Bilodeau, F.; Johnson, D.C.; Albert, J. Bragg gratings fabricated in monomode photosensitive optical fiber by UV exposure through a phase mask. Appl. Phys. Lett. 1993, 62, 1035–1037. [Google Scholar] [CrossRef]
- Kersey, A.D.; Davis, M.A.; Patrick, H.J.; LeBlanc, M.; Koo, K.P.; Askins, C.G.; Putnam, M.A.; Friebele, E.J. Fiber grating sensors. J. Lightwave Technol. 1997, 15, 1442–1463. [Google Scholar] [CrossRef]
- Rao, Y.J. In-fibre Bragg Grating Sensors. Meas. Sci. Technol. 1997, 8, 355–375. [Google Scholar] [CrossRef]
- Ni, X.J.; Zhao, Y.; Yang, J. Research of a novel fiber Bragg grating underwater acoustic sensor. Sens. Actuators A Phys. 2007, 138, 76–80. [Google Scholar] [CrossRef]
- Zhao, Y.; Meng, Q.Y.; Chen, K. Novel current measurement method based on fiber Bragg grating sensor technology. Sens. Actuators A Phys. 2006, 126, 112–116. [Google Scholar] [CrossRef]
- Sohn, K.R.; Shim, J.H. Liquid-level monitoring sensor systems using fiber Bragg grating embedded in cantilever. Sens. Actuators A Phys. 2009, 152, 248–251. [Google Scholar] [CrossRef]
- Yam, S.P.; Brodzeli, Z.; Wade, S.A.; Rollinson, C.M.; Baxter, G.W.; Collins, S.F. Use of First-order Diffraction Wavelengths Corresponding to Dual-grating Periodicities in A Single Fibre Bragg Grating for Simultaneous Temperature and Strain Measurement. Meas. Sci. Technol. 2009, 20, 034008. [Google Scholar] [CrossRef]
- Silva, S.; Frazao, O.; Viegas, J.; Ferreira, L.A.; Araujo, F.M.; Malcata, F.X.; Santos, J.L. Temperature and strain-independent curvature sensor based on a singlemode/multimode fiber optic structure. Meas. Sci. Technol. 2011, 22, 085201. [Google Scholar] [CrossRef]
- Chang, H.C.; Chang, I.N.; Chen, Y.H.; Lin, S.L.; Hung, S.S.; Lin, J.C.; Liu, W.F. An optomechatronic curvature measurement array based on fiber Bragg gratings. Meas. Sci. Technol. 2014, 25, 085201. [Google Scholar] [CrossRef]
- Wei, X.T.; Wei, T.; Xiao, H.; Lin, Y.S. Nano-structured Pd-long period fiber gratings integrated optical sensor for hydrogen detection. Sensor Actuators B Chem. 2008, 134, 687–693. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, J.K.; Kim, W.S. Influence of reaction conditions on sol-precipitation process producing silicon oxide particles. Ceram. Int. 2002, 28, 187–194. [Google Scholar] [CrossRef]
- Adelkhani, H.; Mellatnavaz, B.; Roohi, H.; Noorbakhsh, M. New sol-gel solution with 45 days stability for preparation silica thin films. Iran. J. Chem. Chem. Eng. 2007, 26, 25–29. [Google Scholar]
- Estella, J.; Echeverria, J.C.; Laguna, M.; Garrido, J.J. Silica xerogels of tailored porosity as support matrix for optical chemical sensors. Simultaneous effect of pH, ethanol: TEOS and water: TEOS molar ratios, and synthesis temperature on gelation time, and textural and structural properties. J. Non-Cryst. Solids 2007, 353, 286–294. [Google Scholar] [CrossRef]
- Ohyama, T.; Maruo, Y.Y.; Tanaka, T.; Hayashi, T. Fluorescence-intensity changes in organic dyes impregnated in porous glass on exposure to NO2. Sens. Actuators B Chem. 1999, 59, 16–20. [Google Scholar] [CrossRef]
- Razek, T.M.A.; Miller, M.J.; Hassan, S.S.M.; Arnold, M.A. Optical sensor for sulfur dioxide based on fluorescence quenching. Talanta 1999, 50, 491–498. [Google Scholar] [CrossRef]
- Cao, W.Q.; Duan, Y.X. Optical fiber-based evanescent ammonia sensor. Sens. Actuators B Chem. 2005, 110, 252–259. [Google Scholar] [CrossRef]
- MacCraith, B.D.; McDonagh, C.M.; O’Keeffe, G.; McEvoy, A.K.; Butler, T.; Sheridan, F.R. Sol-gel coatings for optical chemical sensors and biosensors. Sens. Actuators B Chem. 1995, 29, 51–57. [Google Scholar] [CrossRef]
- Chu, C.S.; Lo, Y.L. Fiber-optic carbon dioxide sensor based on fluorinated xerogels doped with HPTS. Sens. Actuators B Chem. 2008, 129, 120–125. [Google Scholar] [CrossRef]
- MacCraith, B.D.; McDonagh, C. Enhanced fluorescence sensing using sol-gel materials. J. Fluoresc. 2002, 12, 333–342. [Google Scholar] [CrossRef]
- Miliou, A.N.; Srivastava, R.; Ramaswamy, R.V. A 1.3 μm directional coupler polarization splitter by ion exchange. J. Lightwave Technol. 1993, 11, 220–225. [Google Scholar] [CrossRef]
- Chin, M.K.; Youtsey, C.; Zhao, W.; Pierson, T.; Ren, Z.; Wu, S.L.; Wang, L.; Zhao, Y.G.; Ho, S.T. GaAs microcavity channel-dropping filter based on a race-track resonator. IEEE Photonics Technol. Lett. 1999, 11, 1620–1622. [Google Scholar] [CrossRef]
- Choi, S.; Eom, T.J.; Jung, Y.; Lee, B.H.; Lee, J.W.; Oh, K. Broad-band tunable all-fiber bandpass filter based on hollow optical fiber and long-period grating pair. IEEE Photonics Technol. Lett. 2005, 17, 115–117. [Google Scholar] [CrossRef]
- Sakata, H.; Ito, H. Optical fiber temperature sensor using a pair of nonidentical long-period fiber gratings for intensity-based sensing. Opt. Commun. 2007, 280, 87–90. [Google Scholar] [CrossRef]
- Sakata, H.; Suzuki, S.; Ito, H.; Takata, Y. Long-period fiber-grating-based bandpass filter using self-aligned absorptive core mode blocker. Opt. Fiber Technol. 2008, 14, 93–96. [Google Scholar] [CrossRef]
- Khan, M.R.R.; Kang, B.H.; Lee, S.W.; Kim, S.H.; Yeom, S.H.; Lee, S.H.; Kang, S.W. Fiber-optic multi-sensor array for detection of low concentration volatile organic compounds. Opt. Express 2013, 21, 20119–20130. [Google Scholar] [CrossRef] [PubMed]
- Arkwright, J.W.; Underhill, I.D.; Maunder, S.A.; Jafari, A.; Cartwright, N.; Lemckert, C. Fiber optic pressure sensing arrays for monitoring horizontal and vertical pressures generated by traveling water waves. IEEE Sens. J. 2014, 14, 2739–2742. [Google Scholar] [CrossRef]
- Cazzulani, G.; Cinquemani, S.; Comolli, L.; Gardella, A.; Resta, F. Vibration control of smart structures using an array of Fiber Bragg Grating sensors. Mechatronics 2014, 24, 345–353. [Google Scholar] [CrossRef]
- Lee, J.S.; Yoon, N.R.; Kang, B.H.; Lee, S.W.; Gopalan, S.A.; Jeong, H.M.; Lee, S.H.; Kwon, D.H.; Kang, S.W. Response characterization of a fiber optic sensor array with dye-coated planar waveguide for detection of volatile organic compounds. Sensors 2014, 14, 11659–11671. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Oyadiji, S.O. Theoretical and experimental study of self-reference intensity-modulated plastic fibre optic linear array displacement sensor. Sens. Actuators A Phys. 2015, 222, 67–79. [Google Scholar] [CrossRef]
- Elshafey, A.; Marzouk, H.; Gu, X.; Haddara, M.; Morsy, R. Use of fiber Bragg grating array and random decrement for damage detection in steelbeam. Eng. Struct. 2016, 106, 348–354. [Google Scholar] [CrossRef]
- Kao, H.P.; Yang, N.; Schoeniger, J.S. Enhancement of evanescent fluorescence from fiber-optic sensors by thin-film sol-gel coatings. J. Opt. Soc. Am. A 1998, 15, 2163–2171. [Google Scholar]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Boston, MA, USA, 1990. [Google Scholar]
- Jorge, P.A.S.; Caldas, P.; Rosa, C.C.; Oliva, A.G.; Santos, J.L. Optical fiber probes for fluorescence based oxygen sensing. Sens. Actuators B Chem. 2004, 103, 290–299. [Google Scholar] [CrossRef]
- Dong, S.Y.; Luo, M.; Peng, G.D.; Cheng, W.H. Broad range pH sensor based on sol-gel entrapped indicators on fibre optic. Sens. Actuators B Chem. 2008, 129, 94–98. [Google Scholar] [CrossRef]
- Alvarado-Méndez, E.; Rojas-Laguna, R.; Andrade-Lucio, J.A.; Hernández-Cruz, D.; Lessard, R.A.; Aviña-Cervantes, J.G. Design and characterization of pH sensor based on sol-gel silica layer on plastic optical fiber. Sens. Actuators B Chem. 2005, 106, 518–522. [Google Scholar]
- Li, W.; Cheng, H.; Xia, M.; Yang, K.C. An experimental study of pH optical sensor using a section of no-core fiber. Sens. Actuators A Phys. 2013, 199, 260–264. [Google Scholar] [CrossRef]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, S.-S.; Chang, H.-C.; Chang, I.-N. A Portable Array-Type Optical Fiber Sensing Instrument for Real-Time Gas Detection. Sensors 2016, 16, 2087. https://doi.org/10.3390/s16122087
Hung S-S, Chang H-C, Chang I-N. A Portable Array-Type Optical Fiber Sensing Instrument for Real-Time Gas Detection. Sensors. 2016; 16(12):2087. https://doi.org/10.3390/s16122087
Chicago/Turabian StyleHung, San-Shan, Hsing-Cheng Chang, and I-Nan Chang. 2016. "A Portable Array-Type Optical Fiber Sensing Instrument for Real-Time Gas Detection" Sensors 16, no. 12: 2087. https://doi.org/10.3390/s16122087
APA StyleHung, S.-S., Chang, H.-C., & Chang, I.-N. (2016). A Portable Array-Type Optical Fiber Sensing Instrument for Real-Time Gas Detection. Sensors, 16(12), 2087. https://doi.org/10.3390/s16122087




